Abstract
Antimicrobial peptides (AMPs) are an essential and multifunctional element for immune defense of the skin during infection and injury. In this issue, Ahrens et al. characterize the response of β-defensins, a class of AMPs, following acute and chronic challenges to the permeability barrier of the skin. Their findings suggest that the antimicrobial and permeability barriers of the skin are closely linked.
The multiple defensive functions of human skin depend on its ability to detect danger from a broad range of physical, chemical, and microbiological challenges, and they are interconnected to minimize the potential for damage. The skin's defensive functions are often thought of as acting through two simultaneously acting barriers, the immune antimicrobial barrier and the physical permeability barrier. Following physical injury to the skin, a cascade of events occurs to restore the breached skin barrier and reestablish homeostasis. In contrast, during infection, microbes encounter both the complex lipid and protein structures of the stratum corneum and an array of antimicrobial molecules that are already present or may be triggered by a set of pattern recognition receptors. In combination, these barriers typically act to facilitate the elimination of pathogens. In recent years, it has been shown that the pathways that generate and regulate the antimicrobial barrier of the skin are closely tied to pathways that modulate permeability barrier function (Dorschner et al., 2001; Schauber et al., 2007; Aberg et al., 2007, 2008). In this issue, Ahrens et al. report that both acute and chronic skin barrier disruption lead to increased expression of murine β-defensins (mBDs)-1, -3, and -14 and that this increase in expression is diminished when the barrier is artificially restored. Their data contribute to the concept that the antimicrobial and permeability barriers of the skin are closely linked.
Antimicrobial nature of the skin
The integrity of the skin barrier is essential for it to properly serve its purpose as a shield from the environment. Keratinocytes are at the forefront of this defense because they make up the majority of epidermal cells and are in constant contact with the outside world. Keratinocytes are responsible for producing the stratum corneum, the terminally differentiated outer layer of the epidermis composed of rigid, anucleate corneocytes cemented by hydrophobic, lipid-rich lamellar bilayers that impede water loss and protect from pathogenic organisms (reviewed in Candi et al., 2005). Although keratinocytes serve as a physical barrier, they also express an array of molecules that contribute to the antimicrobial properties of skin (reviewed in Elias, 2007). A wide arsenal of weapons combats possible invaders via the skin. Constant desquamation of the skin makes it difficult for organisms to establish permanent residence. The surface of the skin is an acidic environment (pH ~ 5.5) uninhabitable to many microorganisms. Additionally, it has been suggested that the microflora that normally inhabit human skin can contribute to barrier defenses by competing for nutrients and niches that more pathogenic organisms require, by expressing antimicrobial molecules that kill or inhibit the growth of pathogenic microbes (Cogen et al., 2010; Nakatsuji et al., 2010), and by modulating the inflammatory response (Lai et al., 2009).
Over the past decade, it has become increasingly apparent that keratinocytes and other resident skin cells produce a number of antimicrobial molecules important for maintaining homeostasis (Gallo and Huttner, 1998). Studies of antimicrobial peptides (AMPs) in many organ systems have shown them to engage in a wide range of activities including direct microbial killing, chemotaxis, modification of inflammatory responses, angiogenesis, and wound healing (reviewed in Lai and Gallo, 2009). More than 1,200 AMPs have been identified or predicted. They are generally small in size (12–50 amino acids), are positively charged, and have amphipathic structures. They contain common secondary structures that vary from α-helical to β-sheets, and their unifying characteristic is the ability to kill microbes or inhibit them from growing. Cathelicidins and defensins are two classes of AMPs that have been well characterized and studied in the skin. The cathelicidin protein hCAP18, as well as the human β-defensins (hBDs)-1, -2, and -3, are produced in keratinocytes and packaged in lamellar bodies prior to extrusion to the stratum corneum (Braff et al., 2005). hBD2 and hBD3 are induced following activation with bacteria or cytokines (Liu et al., 2002; Lai et al., 2010). hCAP18 can be induced by vitamin D and is activated proteolytically to several peptide forms, the most commonly studied being LL-37, a form that is produced predominantly by neutrophils. Although these two groups of AMPs dominate the AMP literature, over 20 other AMPs have been identified in the skin. In addition to their antimicrobial activity, some AMPs exhibit protease/enzyme activity, chemotactic activity, and neuropeptide activity (reviewed in Braff and Gallo, 2006). Other skin resident cells such as sebocytes, eccrine glands, and mast cells also produce AMPs, whereas neutrophils and natural killer cells can be recruited to the skin, where they deposit additional AMPs following wounding or infection (Dorschner et al., 2001). Elucidation of the multifunctional roles of AMPs in skin has made the regulation of their expression a focus of research in recent years.
AMPs fulfill a wide range of functions, some yet to be characterized. First and foremost, AMPs exert direct antimicrobial activity by binding of the cationically charged AMP to negatively charged phospholipid head groups present in many bacteria in the form of lipopolysaccharide, teichoic acids, lipoteichoic acids, and lysophosphat idylglycerol (reviewed in Lai and Gallo, 2009). This binding results in membrane destabilization and produces a physical disruption of the membrane or cell wall of the microbe, leading to decreased growth or death. AMPs also play an important role in modulating the host immune response following infection or injury. They can recruit leukocytes directly or stimulate cells to release IL-8, MCP-1, and IFN-α, thereby indirectly recruiting other effector cells, including neutrophils, macrophages, monocytes, immature dendritic cells, and T cells, to sites of injury/infection. These functions can also influence wound healing: some AMPs have been shown to stimulate migration, proliferation, and tube formation by endothelial cells, stimulate cell proliferation, and re-epithelialize skin (Heilborn et al., 2003).
Interdependence of the antimicrobial and permeability barriers
Because AMPs play such a wide range of roles in skin, it is not hard to imagine that they would also impact the permeability barrier. Several papers reporting studies of the expression of endogenous AMPs in keratinocytes have suggested that their expression coincides with the presence of a number of epidermal structural components (involucrin, loricrin, keratin-1 and -10, transglutaminase-1 and -3, specialized lipids, and other processing enzymes) that may become part of the permeability barrier. Importantly, studies by Aberg et al. (2007, 2008) have shown that murine cathelin-related antimicrobial peptide CRAMP (the murine ortholog of LL37) and mBD-3 (the murine ortholog of hBD-2) are essential for permeability barrier homeostasis. This work also demonstrates that acute and chronic disruption of the physical barrier leads to induction of CRAMP and mBD-3.
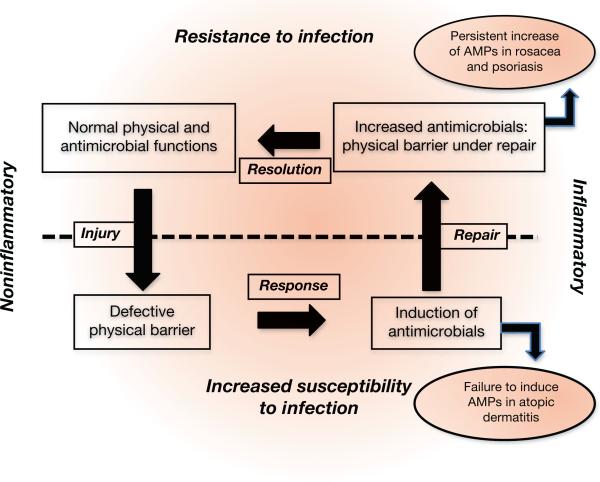
In this issue, Ahrens et al. (2011) further characterize the response of AMP expression to barrier disruption. They show that mBD-1, -3, and -14 (orthologs of hBD-1, -2, and -3) are all upregulated following acute barrier disruption methods that include tape stripping and acetone treatment, as well as a metabolically induced chronic barrier disruption achieved by maintaining mice on an essential fatty acid deficient diet. These methods of barrier disruption all led to increased levels of mBD mRNA and protein. Artificial restoration of the barrier by occlusion moderately inhibited the increases in mBD expression following acute barrier disruption or drastically inhibited the increases following chronic barrier disruption. The authors also show that the growth factor TGF-α modulated the mBD-14 response and that TNF-α modulates the mBD-3 response. These studies highlight the importance of AMPs to the permeability barrier of the skin and provide further evidence of a dynamic interplay between the physical barrier and the chemical shield provided by AMPs against infection (Figure 1).
Figure 1. Homeostasis of the physical and antimicrobial barrier of the skin.
Counterclockwise from upper left: Under resting conditions, the multiple elements of the physical permeability barrier and the antimicrobial defense shield combine to resist microbial invasion in the absence of inflammation. Following injury, a defect in barrier function triggers a response that includes induction of antimicrobial peptide production. The increase in antimicrobials is deficient in patients with atopic dermatitis. Under normal conditions, the repair process results in increased antimicrobial expression in a setting of a decreased barrier, thus restoring resistance to microbial invasion. Patients with rosacea and psoriasis have persistent elevated expression of antimicrobials that perpetuates inflammation. Upon resolution of the repair process, the physical and antimicrobial barriers regain a state of homeostasis. AMP, antimicrobial peptide.
This field remains open to discovery and promises to continue to advance our understanding of many aspects of skin biology. AMP dysfunction has been implicated in a number of skin diseases, including psoriasis, rosacea, and atopic dermatitis. In psoriasis, AMPs, including LL37, hBD-2, and hBD-3, are all upregulated and are believed to contribute to inflammation and to the pathogenesis of the disease (Gilliet and Lande, 2008; Lande et al., 2007). In rosacea, LL37 is also highly upregulated and contributes to the progression of the disease (Yamasaki et al., 2007). On the other hand, LL37, hBD-2, and hBD-3 are all downregulated in atopic dermatitis, leaving patients susceptible to infection (Ong et al., 2002). Based on the current report, it is intriguing to speculate that the decrease in AMPs after injury could also explain increased levels of transepidermal water loss observed in both lesional and nonlesional skin (Werner and Lindberg, 1985). By gaining a better understanding of the processes that regulate AMP expression and their interactions with the barrier properties of the epidermis, it may be possible to develop novel and more effective therapeutics for diseases associated with disruption of physical and immunological homeostatic mechanisms in the skin.
Clinical Implications.
Antimicrobial peptides play a multifunctional role in the body, protecting from infection, modulating immune responses, and contributing to wound repair.
Control of the antimicrobial and permeability barriers of the skin occurs simultaneously following disruption of the skin barrier, strengthening the concept that they are closely linked.
Antimicrobial peptide expression is dysregulated in a number of inflammatory skin diseases including psoriasis, rosacea, and atopic dermatitis.
Footnotes
CONFLICT OF INTEREST The authors state no conflict of interest.
REFERENCES
- Aberg KM, Radek KA, Choi EH, et al. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007;117:3339–49. doi: 10.1172/JCI31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg KM, Man MQ, Gallo RL, et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008;128:917–25. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens K, Schunck M, Podda G-F, et al. Mechanical and metabolic injury to the skin barrier leads to increased expression of murine β-defensin-1, -3, and -14. J Invest Dermatol. 2011;131:443–52. doi: 10.1038/jid.2010.289. [DOI] [PubMed] [Google Scholar]
- Braff MH, Di Nardo A, Gallo RL. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J Invest Dermatol. 2005;124:394–400. doi: 10.1111/j.0022-202X.2004.23443.x. [DOI] [PubMed] [Google Scholar]
- Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006;306:91–110. doi: 10.1007/3-540-29916-5_4. [DOI] [PubMed] [Google Scholar]
- Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol. 2005;4:328–40. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- Cogen AL, Yamasaki K, Sanchez KM, et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J Invest Dermatol. 2010;130:192–200. doi: 10.1038/jid.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorschner RA, Pestonjamasp VK, Tamakuwala S, et al. Cutaneous injury induces the release of cathelicidin anti-microbial peptides active against group A Streptococcus. J Invest Dermatol. 2001;117:91–7. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- Elias PM. The skin barrier as an innate immune element. Semin Immunopathol. 2007;29:3–14. doi: 10.1007/s00281-007-0060-9. [DOI] [PubMed] [Google Scholar]
- Gallo RL, Huttner KM. Antimicrobial peptides: an emerging concept in cutaneous biology. J Invest Dermatol. 1998;111:739–43. doi: 10.1046/j.1523-1747.1998.00361.x. [DOI] [PubMed] [Google Scholar]
- Gilliet M, Lande R. Antimicrobial peptides and self-DNA in autoimmune skin inflammation. Curr Opin Immunol. 2008;20:401–7. doi: 10.1016/j.coi.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Heilborn JD, Nilsson MF, Kratz G, et al. The cathelicidin anti-microbial peptide LL-37 is involved in re-epithelialization of human skin wounds and is lacking in chronic ulcer epithelium. J Invest Dermatol. 2003;120:379–89. doi: 10.1046/j.1523-1747.2003.12069.x. [DOI] [PubMed] [Google Scholar]
- Lai Y, Di Nardo A, Nakatsuji T, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009;15:1377–82. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Gallo RL. AMPed up immunity: how antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009;30:131–41. doi: 10.1016/j.it.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Cogen AL, Radek KA, et al. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol. 2010;130:2211–21. doi: 10.1038/jid.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R, Gregorio J, Facchinett V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–9. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- Liu AY, Destoumieux D, Wong AV, et al. Human beta-defensin 2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J Invest Dermatol. 2002;118:275–81. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- Nakatsuji T, Kao MC, Zhang L, et al. Sebum free fatty acids enhance the innate immune defense of human sebocytes by upregulating beta-defensin-2 expression. J Invest Dermatol. 2010;130:985–94. doi: 10.1038/jid.2009.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;247:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Coda AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner Y, Lindberg M. Transepidermal water loss in dry and clinically normal skin in patients with atopic dermatitis. Acta Derm Venereol. 1985;65:102–5. [PubMed] [Google Scholar]
- Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;8:975–80. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]