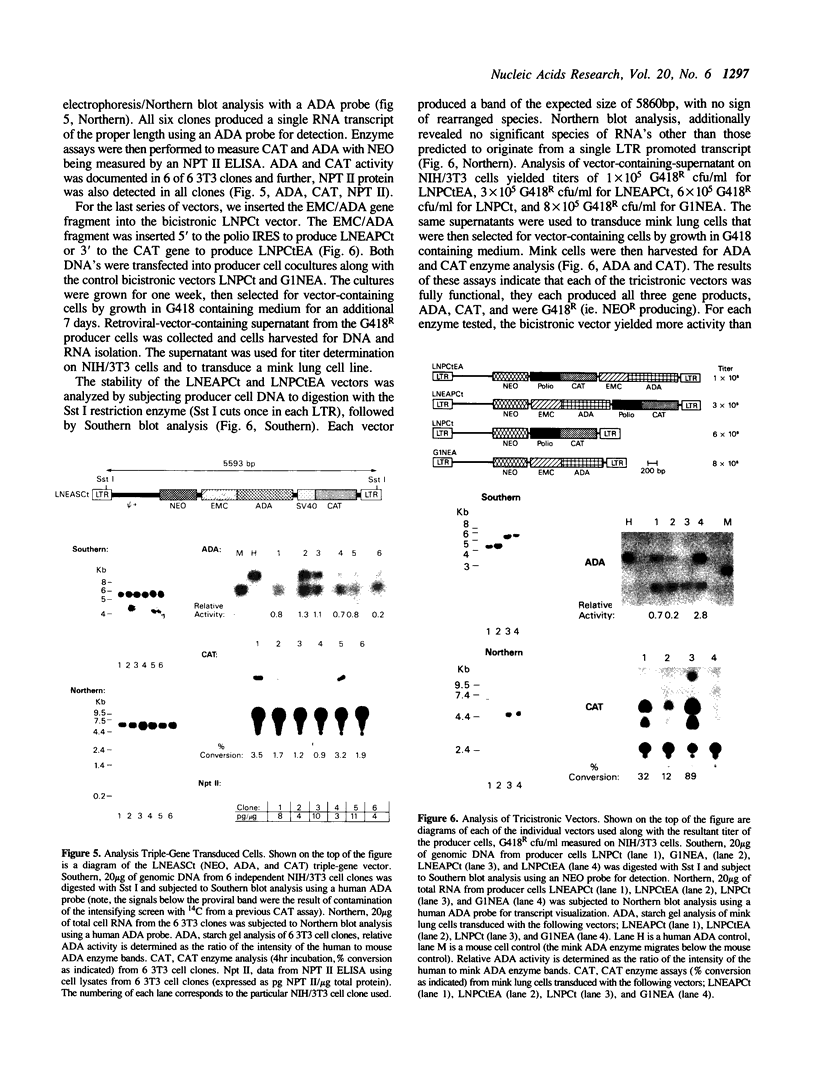
Abstract
Recombinant retroviral vectors producing multicistronic mRNAs were constructed. Picornavirus putative internal ribosome entry sites (IRES) were used to confer cap-independent translation of an internal cistron. Internal cistrons were engineered by ligation of various lengths of the IRES of encephalomyocarditis (EMC) virus or polio virus to the E. coli chloramphenicol acetyltransferase (CAT) gene. The IRES/CAT fusions were introduced into retroviral vectors 3' to the translation stop codon of the neomycin phosphotransferase (NEO) gene, and the molecular constructs transfected into retroviral vector packaging lines. Retroviral vector producer cells efficiently express the internal CAT gene product only when the full length IRES is used. Both the EMC/CAT and polio/CAT retroviral vectors produced high titer vector supernatant capable of productive transduction of target cells. To test the generality of this gene transfer system, a retroviral vector containing an IRES fusion to the human adenosine deaminase (ADA) gene was constructed. Producer cell supernatant was used to transduce NIH/3T3 cells, and transduced cells were shown to express NEO, and ADA. Novel three-gene-containing retroviral vectors were constructed by introducing the EMC/ADA fusion into either an existing internal-promoter-containing vector, or a polio/CAT bicistronic vector. Producer cell clones of the three-gene vectors synthesize all three gene products, were of high titer, and could productively transduce NIH/3T3 cells. By utilizing cap-independent translation units, IRES vectors can produce polycistronic mRNAs which enhance the ability of retroviral-mediated gene transfer to engineer cells to produce multiple foreign proteins.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam M. A., Ramesh N., Miller A. D., Osborne W. R. Internal initiation of translation in retroviral vectors carrying picornavirus 5' nontranslated regions. J Virol. 1991 Sep;65(9):4985–4990. doi: 10.1128/jvi.65.9.4985-4990.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. French, Blaese R. Michael, Culver Kenneth. The ADA human gene therapy clinical protocol: Points to Consider response with clinical protocol, July 6, 1990. Hum Gene Ther. 1990 Fall;1(3):331–362. doi: 10.1089/hum.1990.1.3-331. [DOI] [PubMed] [Google Scholar]
- Belmont J. W., MacGregor G. R., Wager-Smith K., Fletcher F. A., Moore K. A., Hawkins D., Villalon D., Chang S. M., Caskey C. T. Expression of human adenosine deaminase in murine hematopoietic cells. Mol Cell Biol. 1988 Dec;8(12):5116–5125. doi: 10.1128/mcb.8.12.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick R. K., Kozak S. L., Kabat D. Overcoming interference to retroviral superinfection results in amplified expression and transmission of cloned genes. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5404–5408. doi: 10.1073/pnas.85.15.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowtell D. D., Cory S., Johnson G. R., Gonda T. J. Comparison of expression in hemopoietic cells by retroviral vectors carrying two genes. J Virol. 1988 Jul;62(7):2464–2473. doi: 10.1128/jvi.62.7.2464-2473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P., Gilboa E., Anderson W. F. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985 Dec 20;230(4732):1395–1398. doi: 10.1126/science.2999985. [DOI] [PubMed] [Google Scholar]
- Elroy-Stein O., Fuerst T. R., Moss B. Cap-independent translation of mRNA conferred by encephalomyocarditis virus 5' sequence improves the performance of the vaccinia virus/bacteriophage T7 hybrid expression system. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6126–6130. doi: 10.1073/pnas.86.16.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elroy-Stein O., Moss B. Cytoplasmic expression system based on constitutive synthesis of bacteriophage T7 RNA polymerase in mammalian cells. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6743–6747. doi: 10.1073/pnas.87.17.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Genes with promoters in retrovirus vectors can be independently suppressed by an epigenetic mechanism. Cell. 1984 Dec;39(3 Pt 2):449–467. [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Quantitative analysis of gene suppression in integrated retrovirus vectors. Mol Cell Biol. 1986 Mar;6(3):792–800. doi: 10.1128/mcb.6.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghattas I. R., Sanes J. R., Majors J. E. The encephalomyocarditis virus internal ribosome entry site allows efficient coexpression of two genes from a recombinant provirus in cultured cells and in embryos. Mol Cell Biol. 1991 Dec;11(12):5848–5859. doi: 10.1128/mcb.11.12.5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett M. J., Rose J. K., Baltimore D. 5'-terminal structure of poliovirus polyribosomal RNA is pUp. Proc Natl Acad Sci U S A. 1976 Feb;73(2):327–330. doi: 10.1073/pnas.73.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock R. A., Miller A. D., Osborne W. R. Expression of human adenosine deaminase from various strong promoters after gene transfer into human hematopoietic cell lines. Blood. 1989 Aug 1;74(2):876–881. [PubMed] [Google Scholar]
- Jang S. K., Davies M. V., Kaufman R. J., Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5' nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989 Apr;63(4):1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Kräusslich H. G., Nicklin M. J., Duke G. M., Palmenberg A. C., Wimmer E. A segment of the 5' nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988 Aug;62(8):2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. K., Wimmer E. Cap-independent translation of encephalomyocarditis virus RNA: structural elements of the internal ribosomal entry site and involvement of a cellular 57-kD RNA-binding protein. Genes Dev. 1990 Sep;4(9):1560–1572. doi: 10.1101/gad.4.9.1560. [DOI] [PubMed] [Google Scholar]
- Kantoff P. W., Kohn D. B., Mitsuya H., Armentano D., Sieberg M., Zwiebel J. A., Eglitis M. A., McLachlin J. R., Wiginton D. A., Hutton J. J. Correction of adenosine deaminase deficiency in cultured human T and B cells by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6563–6567. doi: 10.1073/pnas.83.17.6563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim B., Williams D. A., Orkin S. H. Retrovirus-mediated gene transfer of human adenosine deaminase: expression of functional enzyme in murine hematopoietic stem cells in vivo. Mol Cell Biol. 1987 Oct;7(10):3459–3465. doi: 10.1128/mcb.7.10.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIvor R. S. Deletion in a recombinant retroviral vector resulting from a cryptic splice donor signal in the Moloney leukemia virus envelope gene. Virology. 1990 Jun;176(2):652–655. doi: 10.1016/0042-6822(90)90039-t. [DOI] [PubMed] [Google Scholar]
- McLachlin J. R., Cornetta K., Eglitis M. A., Anderson W. F. Retroviral-mediated gene transfer. Prog Nucleic Acid Res Mol Biol. 1990;38:91–135. doi: 10.1016/s0079-6603(08)60709-6. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Morgan R. A., Looney D. J., Muenchau D. D., Wong-Staal F., Gallo R. C., Anderson W. F. Retroviral vectors expressing soluble CD4: a potential gene therapy for AIDS. AIDS Res Hum Retroviruses. 1990 Feb;6(2):183–191. doi: 10.1089/aid.1990.6.183. [DOI] [PubMed] [Google Scholar]
- Moss B., Elroy-Stein O., Mizukami T., Alexander W. A., Fuerst T. R. Product review. New mammalian expression vectors. Nature. 1990 Nov 1;348(6296):91–92. doi: 10.1038/348091a0. [DOI] [PubMed] [Google Scholar]
- Muenchau D. D., Freeman S. M., Cornetta K., Zwiebel J. A., Anderson W. F. Analysis of retroviral packaging lines for generation of replication-competent virus. Virology. 1990 May;176(1):262–265. doi: 10.1016/0042-6822(90)90251-l. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Lee Y. F., Wimmer E. The 5' end of poliovirus mRNA is not capped with m7G(5')ppp(5')Np. Proc Natl Acad Sci U S A. 1976 Feb;73(2):375–380. doi: 10.1073/pnas.73.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overell R. W., Weisser K. E., Cosman D. Stably transmitted triple-promoter retroviral vectors and their use in transformation of primary mammalian cells. Mol Cell Biol. 1988 Apr;8(4):1803–1808. doi: 10.1128/mcb.8.4.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal binding of eucaryotic ribosomes on poliovirus RNA: translation in HeLa cell extracts. J Virol. 1989 Jan;63(1):441–444. doi: 10.1128/jvi.63.1.441-444.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988 Jul 28;334(6180):320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Aebersold P., Cornetta K., Kasid A., Morgan R. A., Moen R., Karson E. M., Lotze M. T., Yang J. C., Topalian S. L. Gene transfer into humans--immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990 Aug 30;323(9):570–578. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- Xu L., Yee J. K., Wolff J. A., Friedmann T. Factors affecting long-term stability of Moloney murine leukemia virus-based vectors. Virology. 1989 Aug;171(2):331–341. doi: 10.1016/0042-6822(89)90600-4. [DOI] [PubMed] [Google Scholar]
- van der Werf S., Bradley J., Wimmer E., Studier F. W., Dunn J. J. Synthesis of infectious poliovirus RNA by purified T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2330–2334. doi: 10.1073/pnas.83.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]