Abstract
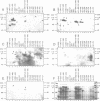
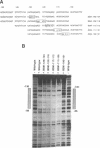
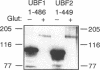
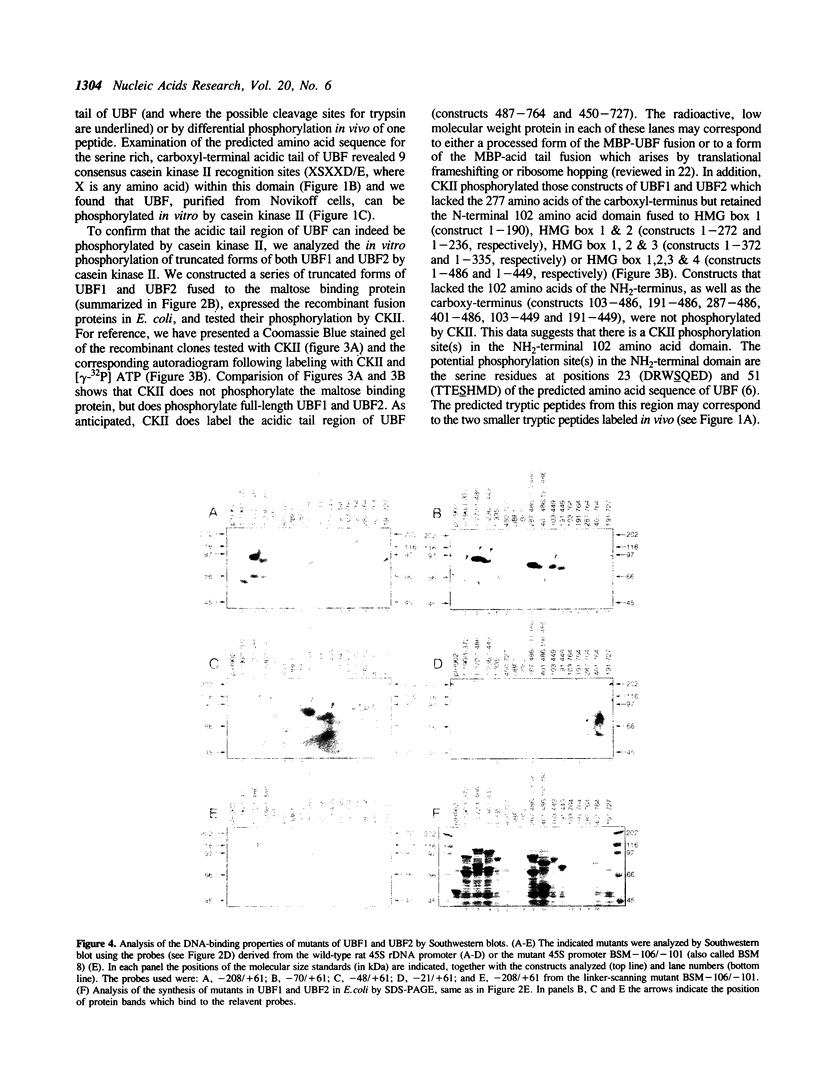
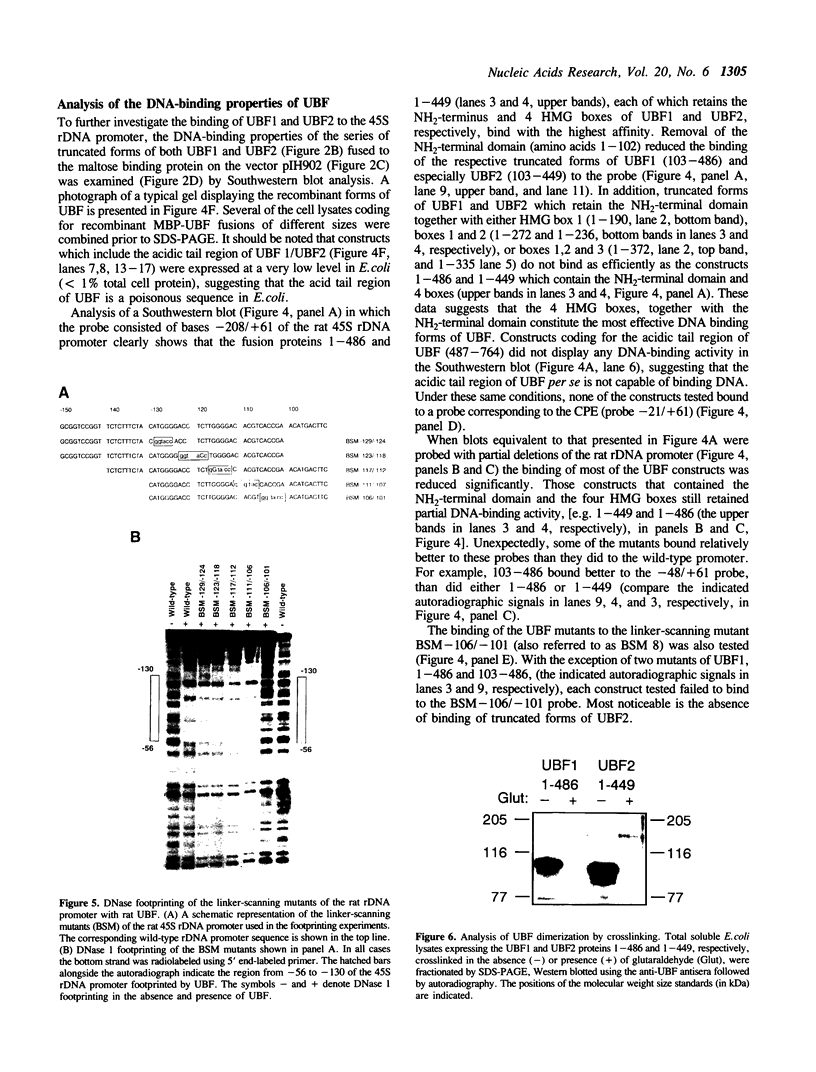
The phosphorylation, DNA-binding and dimerization properties of both forms of the RNA polymerase I transcription factor UBF were studied and compared. Tryptic peptide maps of in vivo 32P-labeled UBF contained four phospho-peptides. Two of these peptides are predicted to derive from the serine-rich, carboxyl-terminal of UBF. This region contains nine consensus phosphorylation sites for casein kinase II, and is one of the regions phosphorylated in vitro by casein kinase II. Analysis of the DNA-binding properties of recombinant forms of UBF1 and UBF2 by Southwestern blots revealed: (1) a role for the NH2-terminal 102 amino acid domain of UBF1/UBF2 in DNA-binding; (2) the importance of the bases from -106 to -101 of the rat ribosomal DNA promoter for the binding of UBF; and (3) functional differences between UBF1 and UBF2. Glutaraldehyde cross-linking and overlay assays using recombinant forms of UBF1 and UBF2 demonstrated that the molecules can form both homodimers and heterodimers. These assays also demonstrated that the NH2-terminal 102 amino acids of UBF plays a significant role in dimerization and that other domains contribute to dimerization. The dimerization properties of recombinant forms of UBF1 and UBF2 were different, suggesting that the HMG box 2 of UBF1, which is partially deleted in UBF2, also contributes to UBF dimerization.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman P., Osheroff N. Regulation of casein kinase II activity by epidermal growth factor in human A-431 carcinoma cells. J Biol Chem. 1989 Jul 15;264(20):11958–11965. [PubMed] [Google Scholar]
- Atkins J. F., Weiss R. B., Gesteland R. F. Ribosome gymnastics--degree of difficulty 9.5, style 10.0. Cell. 1990 Aug 10;62(3):413–423. doi: 10.1016/0092-8674(90)90007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachvarov D., Moss T. The RNA polymerase I transcription factor xUBF contains 5 tandemly repeated HMG homology boxes. Nucleic Acids Res. 1991 May 11;19(9):2331–2335. doi: 10.1093/nar/19.9.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachvarov D., Normandeau M., Moss T. Heterogeneity in the Xenopus ribosomal transcription factor xUBF has a molecular basis distinct from that in mammals. FEBS Lett. 1991 Aug 19;288(1-2):55–59. doi: 10.1016/0014-5793(91)81002-p. [DOI] [PubMed] [Google Scholar]
- Bell S. P., Jantzen H. M., Tjian R. Assembly of alternative multiprotein complexes directs rRNA promoter selectivity. Genes Dev. 1990 Jun;4(6):943–954. doi: 10.1101/gad.4.6.943. [DOI] [PubMed] [Google Scholar]
- Bell S. P., Learned R. M., Jantzen H. M., Tjian R. Functional cooperativity between transcription factors UBF1 and SL1 mediates human ribosomal RNA synthesis. Science. 1988 Sep 2;241(4870):1192–1197. doi: 10.1126/science.3413483. [DOI] [PubMed] [Google Scholar]
- Bell S. P., Pikaard C. S., Reeder R. H., Tjian R. Molecular mechanisms governing species-specific transcription of ribosomal RNA. Cell. 1989 Nov 3;59(3):489–497. doi: 10.1016/0092-8674(89)90032-9. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C. Anionic regions in nuclear proteins. J Cell Biol. 1987 Oct;105(4):1479–1482. doi: 10.1083/jcb.105.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier-Rouvière C., Basset M., Blanchard J. M., Cavadore J. C., Fernandez A., Lamb N. J. Casein kinase II induces c-fos expression via the serum response element pathway and p67SRF phosphorylation in living fibroblasts. EMBO J. 1991 Oct;10(10):2921–2930. doi: 10.1002/j.1460-2075.1991.tb07842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisatake K., Nishimura T., Maeda Y., Hanada K., Song C. Z., Muramatsu M. Cloning and structural analysis of cDNA and the gene for mouse transcription factor UBF. Nucleic Acids Res. 1991 Sep 11;19(17):4631–4637. doi: 10.1093/nar/19.17.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen H. M., Admon A., Bell S. P., Tjian R. Nucleolar transcription factor hUBF contains a DNA-binding motif with homology to HMG proteins. Nature. 1990 Apr 26;344(6269):830–836. doi: 10.1038/344830a0. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Ha I., Maldonado E., Reinberg D., Green M. R. Binding of general transcription factor TFIIB to an acidic activating region. Nature. 1991 Oct 10;353(6344):569–571. doi: 10.1038/353569a0. [DOI] [PubMed] [Google Scholar]
- McStay B., Frazier M. W., Reeder R. H. xUBF contains a novel dimerization domain essential for RNA polymerase I transcription. Genes Dev. 1991 Nov;5(11):1957–1968. doi: 10.1101/gad.5.11.1957. [DOI] [PubMed] [Google Scholar]
- McStay B., Hu C. H., Pikaard C. S., Reeder R. H. xUBF and Rib 1 are both required for formation of a stable polymerase I promoter complex in X. laevis. EMBO J. 1991 Aug;10(8):2297–2303. doi: 10.1002/j.1460-2075.1991.tb07766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony D. J., Rothblum L. I. Identification of two forms of the RNA polymerase I transcription factor UBF. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3180–3184. doi: 10.1073/pnas.88.8.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony D. J., Xie W. Q., Smith S. D., Singer H. A., Rothblum L. I. Differential phosphorylation and localization of the transcription factor UBF in vivo in response to serum deprivation. In vitro dephosphorylation of UBF reduces its transactivation properties. J Biol Chem. 1992 Jan 5;267(1):35–38. [PubMed] [Google Scholar]
- Pikaard C. S., McStay B., Schultz M. C., Bell S. P., Reeder R. H. The Xenopus ribosomal gene enhancers bind an essential polymerase I transcription factor, xUBF. Genes Dev. 1989 Nov;3(11):1779–1788. doi: 10.1101/gad.3.11.1779. [DOI] [PubMed] [Google Scholar]
- Pikaard C. S., Smith S. D., Reeder R. H., Rothblum L. rUBF, an RNA polymerase I transcription factor from rats, produces DNase I footprints identical to those produced by xUBF, its homolog from frogs. Mol Cell Biol. 1990 Jul;10(7):3810–3812. doi: 10.1128/mcb.10.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach P. J. Multisite and hierarchal protein phosphorylation. J Biol Chem. 1991 Aug 5;266(22):14139–14142. [PubMed] [Google Scholar]
- Rothblum L. I., Reddy R., Cassidy B. Transcription initiation site of rat ribosomal DNA. Nucleic Acids Res. 1982 Nov 25;10(22):7345–7362. doi: 10.1093/nar/10.22.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley A., Singer R. A., Johnston G. C. CDC68, a yeast gene that affects regulation of cell proliferation and transcription, encodes a protein with a highly acidic carboxyl terminus. Mol Cell Biol. 1991 Nov;11(11):5718–5726. doi: 10.1128/mcb.11.11.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Singer H. A., Oren J. W., Benscoter H. A. Myosin light chain phosphorylation in 32P-labeled rabbit aorta stimulated by phorbol 12,13-dibutyrate and phenylephrine. J Biol Chem. 1989 Dec 15;264(35):21215–21222. [PubMed] [Google Scholar]
- Smith S. D., Oriahi E., Lowe D., Yang-Yen H. F., O'Mahony D., Rose K., Chen K., Rothblum L. I. Characterization of factors that direct transcription of rat ribosomal DNA. Mol Cell Biol. 1990 Jun;10(6):3105–3116. doi: 10.1128/mcb.10.6.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson M. S., Carlson M., Winston F. SPT6, an essential gene that affects transcription in Saccharomyces cerevisiae, encodes a nuclear protein with an extremely acidic amino terminus. Mol Cell Biol. 1990 Sep;10(9):4935–4941. doi: 10.1128/mcb.10.9.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W. Q., O'Mahony D. J., Smith S. D., Rothblum L. Complementary in vivo and in vitro analyses of the interactions between the cis-acting elements of the rat rDNA promoter. 1991 May 29-Jun 12Mol Cell Biochem. 104(1-2):127–135. doi: 10.1007/BF00229812. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Rothblum L. I. Purification and characterization of a high-mobility-group-like DNA-binding protein that stimulates rRNA synthesis in vitro. Mol Cell Biol. 1988 Aug;8(8):3406–3414. doi: 10.1128/mcb.8.8.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I. J., Spector D. L., Bae Y. S., Marshak D. R. Immunocytochemical localization of casein kinase II during interphase and mitosis. J Cell Biol. 1991 Sep;114(6):1217–1232. doi: 10.1083/jcb.114.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]