Abstract
Here, we present evidence that the tumor-like growth of mouse embryonic stem cells (mESCs) is suppressed by short-term serum-free culture, which is reversed by pharmacological inhibition of Gsk3β. Mouse ESCs maintained under standard conditions using fetal bovine serum (FBS) were cultured in a uniquely formulated chemically-defined serum-free (CDSF) medium, namely ESF7, for three passages before being subcutaneously transplanted into immunocompromised mice. Surprisingly, the mESCs failed to produce teratomas for up to six months, whereas mESCs maintained under standard conditions generated well-developed teratomas in five weeks. Mouse ESCs cultured under CDSF conditions maintained the expression of Oct3/4, Nanog, Sox2 and SSEA1, and differentiated into germ cells in vivo. In addition, when mESCs were cultured under CDSF conditions supplemented with FBS, or when the cells were cultured under CDSF conditions followed by standard culture conditions, they consistently developed into teratomas. Thus, these results validate that the pluripotency of mESCs was not compromised by CDSF conditions. Mouse ESCs cultured under CDSF conditions proliferated significantly more slowly than mESCs cultured under standard conditions, and were reminiscent of Eras-null mESCs. In fact, their slower proliferation was accompanied by the downregulation of Eras and c-Myc, which regulate the tumor-like growth of mESCs. Remarkably, when mESCs were cultured under CDSF conditions supplemented with a pharmacological inhibitor of Gsk3β, they efficiently proliferated and developed into teratomas without upregulation of Eras and c-Myc, whereas mESCs cultured under standard conditions expressed Eras and c-Myc. Although the role of Gsk3β in the self-renewal of ESCs has been established, it is suggested with these data that Gsk3β governs the tumor-like growth of mESCs by means of a mechanism different from the one to support the pluripotency of ESCs.
Introduction
Embryonic stem cells (ESCs) [1], [2], [3] and induced pluripotent stem cells (iPSCs) [4], [5], [6], [7] are very promising tools for use in drug screening and customized tissue replacement [8] because they are capable of self-renewal that sustains pluripotency. The self-renewal and pluripotency of mouse stem cells (ESCs and iPSCs) are maintained by extrinsic factors, such as supplementing basal culture medium with leukemia inhibitory factor (LIF) [9], [10], [11], [12], [13] and fetal bovine serum (FBS). FBS further facilitates their self-renewal by offering other factors, such as bone morphogenetic protein 4 (Bmp4) [14], retinoids [15], [16], [17], threonine [18] and glutathione [19]. However, FBS also provides cultures with many other uncharacterized components that may affect the capability of ESCs and iPSCs to self-renew and differentiate. Undefined culture conditions using animal sera may have contributed to results finding contradictory roles of the Wnt signaling pathway in ESCs [20], [21], [22], [23]. However, it is now firmly established that pharmacological inhibition of glycogen synthase kinase 3β (Gsk3β) promotes the self-renewal of both mouse [22], [24] and human ESCs [23], and derivation of mouse ESCs [25].
To eliminate the effects of unknown components in animal sera as well as the contamination of animal products, chemically-defined serum-free culture methods have been established [14], [26], [27], [28]. Typically, defined culture media are composed of critical growth factors (e.g., LIF and/or Bmp4) and other factors present in animal sera, such as hormones (e.g., insulin and transferrin), vitamins, fatty acids and minerals. Commercially-made serum replacements that may contain these components in animal sera [29] are often used to maintain ESC culture (e.g., [30]), although the exact components cannot be disclosed by their patents [28]. The maintenance of the undifferentiated state of mouse ESCs (mESCs) using defined culture media has been well documented [31], [32]. Furthermore, the pluripotency of these mESCs has been validated by their differentiation in vitro [26], [32] or by the development of chimeric mice [14].
Another way to validate the pluripotency of ESCs and iPSCs is to examine the ability of these cells to develop into tumors called teratomas after their transplantation into immunocompromised mice [33], [34], [35]. Such teratoma formation assays have validated the pluripotency of mESCs maintained in the presence of a Gsk3β inhibitor [23], [25]. This method, which requires no special technique or equipment and reduces the use of experimental animals, is particularly useful and widely accepted for the validation of pluripotency in human ESCs and iPSCs [13], [36]. However, their tumor-like growth hampers the therapeutic application of human iPSCs [37]. Little is known about the inherent tumorigenic property of ESCs, except that the oncogene Eras regulates the tumor-like growth of mESCs via activation of Akt1 [38], which may result in inactivation of Gsk3β [24], [39], [40]. However, human ESCs do not express human ERAS [41], [42], but grow into teratomas [3]. Therefore, the underlying mechanism involved in the tumor-like growth of ESCs remains unknown.
On the other hand, mESCs contribute to the development of normal chimeras, instead of forming teratomas, when mixed with mouse preimplantation embryos. This finding indicates that mESCs require proper extrinsic signals or niches [43] to differentiate normally and to contribute to the development of chimeras. In contrast, mESCs exhibit cell death when they are cultured without LIF [26], [44]. This result raises the question of whether mESCs inherently possess the tumor-like property or are provided with extrinsic signals that promote their tumorigenesis. In this report, we present experimental evidence that short-term serum-free culture reduces the tumorigenicity of mESCs, which is reversed by pharmacological inhibition of Gsk3β. It is suggested with these data that the activity of Gsk3β orchestrates the tumor-like growth of ESCs, which may support a novel mechanism independent from the one regulating the pluripotency of ESCs.
Results
Mouse embryonic stem cells reduced their tumorigenicity but maintained their pluripotency under chemically-defined serum-free culture
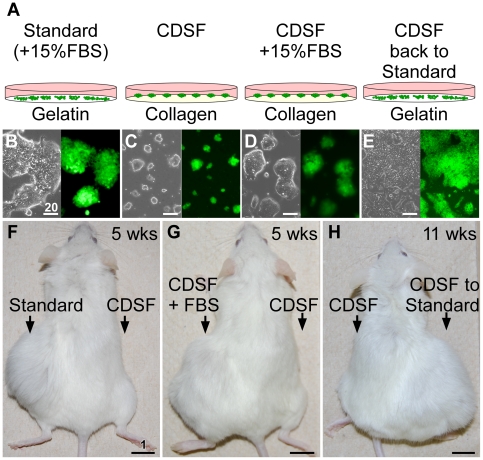
To examine whether mESCs are determined to grow as teratomas, we cultured mESCs in chemically-defined serum-free medium with LIF (referred to as “CDSF”) [26] for three passages (Fig. 1A, 1C) and subcutaneously transplanted them into non-obese diabetic mice with severe combined immunodeficiency disease (NOD-SCID mice) [45]. We used a uniquely formulated serum-free medium, ESF7 (see Materials and Methods), because components in this medium is fully disclosed [26]. This medium has been used to maintain mESCs by other studies (e.g., [46], [47]). However, the pluripotency of mESCs cultured under this medium has not been tested by teratoma formation assays. Surprisingly, these mESCs failed to grow as teratomas (Fig. 1F-1H), whereas mESCs maintained under standard conditions (Fig. 1A, 1B) grew into a well-developed teratoma in 5 weeks (Fig. 1F, 2A–2E, and supporting information Fig. S1A). When mESCs were cultured in CDSF supplemented with 15% FBS (referred to as “CDSF+FBS”; Fig. 1A, 1D), they formed a well-developed teratoma in 5 weeks (Fig. 1G, 2F–2J, and supporting information Fig. S1B). Thus, tumorigenesis in mESCs is not simply inhibited by CDSF conditions. When the injections were properly performed, we did not observe blood coming out of the injection sites. Only properly performed injections were counted in the present study.
Figure 1. The tumorigenicity of mouse embryonic stem cells can be reduced by short-term serum-free culture.
(A): A mouse embryonic stem cell (mESC) line harboring an EGFP reporter driven by the Oct3/4 promoter (Oct3/4::EGFP) was maintained in either standard or chemically-defined serum-free (CDSF) medium as indicated. (B–E): Phase contrast (left) and fluorescence (right) images of the ESC line under the conditions indicated above are shown. Bars, 20 µm. (F–H): After the mESCs were cultured as indicated, they were transplanted into NOD-SCID mice subcutaneously. Teratoma formation was observed by week 11. Bars, 1 cm.
Figure 2. Identification of three germ layers in teratomas.
(A–E): Teratomas developed from mESCs cultured under standard conditions. (F–J): Teratomas developed from mESCs cultured in CDSF supplemented with 15% FBS (CDSF+FBS). (K–O): Teratomas developed from mESCs cultured in CDSF followed by transfer to standard conditions (CDSF to Stand). Ectoderm is represented by neural tissue and keratin pearl, mesoderm is represented by cartilage and striated muscle, and endoderm is represented by ciliated epithelium. Bars, 20 µm.
We conclude that CDSF did not compromise the pluripotency of mESCs per se for the following reasons. First, throughout the culture period (three passages, 9–12 days), the transcriptional activity of the master regulator of pluripotency, Oct3/4 (Pou5f1) [48], [49], [50], was validated using a mESC line that expresses the enhanced green fluorescent protein (EGFP) under the Oct3/4 promoter [51] (Fig. 1B, 1C). Unless otherwise noted, this mESC line was used throughout the present study. Next, immunofluorescence microscopy confirmed that the mESC line cultured under CDSF conditions maintained the expression of Nanog, Sox2, and SSEA1 (Fig. 3A–3C). In addition, when the mESC line cultured under CDSF conditions was aggregated with wild-type morulae, resulting chimeric blastocysts exhibited green fluorescence in the inner cell mass (Fig. 3E). Eleven days after these blastocysts were transferred to pseudopregnant females, fluorescent cells were detected in a nascent male gonad of an embryo at embryonic day 13.5 (Fig. 3F, 3G).
Figure 3. Mouse embryonic stem cells cultured under CDSF conditions are pluripotent.
(A–D): Mouse ESCs cultured under CDSF conditions were immunostained (right) with either specific antibodies against Nanog (A), Sox2 (B) and SSEA1 (C), or normal mouse serum (D). Also, phase contrast (left), and DAPI-stained (middle) images of these mESCs are shown. Bars, 20 µm. (E): Phase contrast (left) and fluorescence (right) images of a chimeric blastocyst aggregated with the Oct3/4::EGFP mESC line. Bar, 20 µm. (F): A phase contrast image of a male gonad dissected from a chimeric E13.5 embryo. The rectangle indicates the area shown in G. Bar, 100 µm. (G): Enlarged phase contrast (left) and fluorescence (right) images of the male gonad shown in F. Bar, 50 µm.
Furthermore, when the mESC line was maintained in either CDSF+FBS (n = 4) or CDSF for three passages and brought back to the standard medium for one or two passages (referred to as “CDSF-Standard”, n = 4; Fig. 1A), EGFP expression was maintained (Fig. 1D, 1E) and the cells became teratomas (Fig. 1G, 1H, and 2F–2O; p<0.00016, see supporting information Table S1 and Fig. S1B, S1C). Similar results were obtained using four different lots of FBS. These data demonstrate that tumorigenesis in mESCs can be suppressed by short-term culture in the serum-free medium. The plasticity of tumorigenicity in mESCs appears to be unique; embryonic carcinomas (F9) [52], which are also pluripotent stem cells, formed teratomas when cultured in CDSF and transplanted (supporting information Fig. S1D). Likewise, germline-incompetent mESCs, D3, grew into teratomas (data not shown).
Mouse embryonic stem cells cultured under serum-free conditions exhibited longer doubling time while maintained expression of genes associated with cellular pluripotency
When NOD-SCID mice transplanted with mESCs reached their end points, they were sacrificed and examined for teratomas. This procedure usually yielded teratomas of about 30 mm in diameter (bars in Fig. 4A). However, the number of days needed for the experimental animals to reach their end points varied (stars in Fig. 4A). Interestingly, it took 47±3.1 days for mESCs cultured in CDSF+FBS to grow into 30 mm teratomas, whereas it took 37±1.8 days for mESCs cultured in CDSF followed by standard conditions for two passages to reach 30 mm in diameter. However, when mESCs cultured in CDSF were passaged only once into standard conditions, it took 76 days for these mESCs to grow into a 30 mm teratoma. Although this sample size is too small to be statistically significant, it is suggested with these data that CDSF may even suppress the growth of teratomas.
Figure 4. Phenotypic changes observed in mouse embryonic stem cells cultured under CDSF conditions.
(A): The sizes of the teratomas formed (orange bar, left axis) and the number of days required for the experimental NOD-SCID mice to reach their end points (blue stars, right axis) were compared among mESCs cultured under the conditions indicated (see Fig. 1A). Parentheses indicate the number of biological replicates (i.e., mESCs prepared at different passages) per culture condition. Standard errors of the means are indicated by bars. a: Only one passage in standard conditions followed CDSF culture. b: Two out of seven transplantations showed no sign of teratoma formation when paired with the standard and CDSF-Standard conditions, whereas five out of seven transplantations showed no sign of teratoma formation for 6 months. (B, top): Cell doublings were measured every 48 hours after plating 1×106 cells per well onto 6-well plates (see Materials and Methods for the formula). Only CDSF conditions produced statistically-significant differences compared to standard conditions. Parentheses indicate the number of biological replicates per condition. Standard errors of the means are indicated by bars. (B, bottom): Phase contrast (top) and fluorescence (bottom) images are shown for the Oct3/4::EGFP mESC line (Fig. 1A) grown under the conditions indicated below 1 and 2 days after plating 0.1×106 cells per well in 6-well plates. Bars, 50 µm. (C): Abundance of each transcript indicated above was examined in mESCs cultured under each condition on the right by 25 cycles of PCR. Ef1α was used as a reference.
Among the experimental NOD-SCID mice examined in this study, two out of the seven mice had mESCs cultured in CDSF injected into one side of the animal and mESCs cultured in media containing FBS injected into the other side. Therefore, the formation of teratomas from mESCs cultured in CDSF could not be examined beyond the end point of the animals (Fig. 4A). However, in the other five animals, we were able to determine that transplanted mESCs under CDSF did not generate teratomas for up to 6 months.
At one injection site, we were able to identify a tiny mass of mESCs that had been cultured under CDSF-Standard conditions one week after transplantation (supporting information Fig. S1E). The mESCs in this mass had the appearance of cells undergoing initial differentiation (supporting information Fig. S2). However, we did not observe any cellular mass at the injection sites that were derived from mESCs cultured in CDSF at either one week or six months after transplantation.
Mouse ESCs cultured in CDSF exhibited a significantly longer doubling time (∼28 hrs) than ones cultured under standard conditions (∼17 hrs, p<0.005) during the first two passages, whereas ones cultured in CDSF+FBS or under CDSF-Standard conditions took ∼19 and ∼20 hrs to divide, respectively (Fig. 4B, top). Similar results were obtained with another mESC line, W4 [53], [54] (data not shown). The differences in doubling times of mESCs were evident as soon as two days after transfer to CDSF conditions (1.91 doublings ± S.E.M. of 0.0967 vs. 2.72 doublings ± S.E.M. of 0.0923 for the standard condition in 48 hrs, p<0.005). Despite the longer doubling time, mESCs cultured in CDSF did proliferate steadily (Fig. 4B, bottom). Additionally, transcripts associated with cellular pluripotency, Sox2, Esg1/Dppa5 and Oct3/4, were expressed in mESCs cultured in CDSF (Fig. 4C). Interestingly, Eras, which regulates the tumorigenic growth of mESCs [38], was downregulated in these mESCs (Fig. 4C), whereas it became upregulated when mESCs were maintained in CDSF+FBS or under CDSF-Standard conditions (Fig. 4C, see also supporting information Fig. S5). Collectively, the loss of tumor-like potential in mESCs cultured under CDSF is associated with a slower growth rate and the reduced expression of Eras. It is interesting to note that Eras-null mESCs can contribute to the germline in chimeric animals but show significantly reduced growth rate [38].
Pharmacological inhibition of Gsk3β reversed the effect of serum-free culture on the tumor-like growth of mouse embryonic stem cells
To identify a potential serum factor responsible for inducing the tumor-like growth in mESCs, initially we focused on molecules known to sustain the self-renewing growth of mESCs, such as LIF [9], [10], Bmp4 [14], vitamin A derivatives (all-trans retinoic acid, RA [15], and retinol [16], [17]), and simultaneous inhibition of Erk and Gsk3β [31]. Pharmacological inhibition of Gsk3β alone promotes self-renewal of both mouse and human ESCs [23]. We excluded LIF from screening because CDSF contains LIF [26]. In the absence of LIF, mESCs undergo differentiation or cell death [26], [44]. We also ruled out an inhibitor of Erk [31], which acts downstream of FGF receptors, because inhibition of Erk cannot promote the growth of mESCs [31]. Therefore, we focused on testing other molecules such as Bmp4, RA, retinol with or without retinol binding protein (RBP) [55], and an inhibitor of Gsk3β (CIHR99021) [31].
Addition of RA in CDSF induced differentiation of mESCs as evidenced by the reduced expression level of EGFP (supporting information Fig. S3A). Addition of retinol with or without RBP in CDSF did not induce differentiation of mESCs (supporting information Fig. S3B, S3C), but failed to accelerate their growth and to induce teratoma formation (Fig. 5A, 5B). In contrast, mESCs cultured in CDSF with Bmp4 or the Gsk3β inhibitor maintained Oct3/4 expression (supporting information Fig. S3D, S3E), increased the number of cell doublings (Fig. 5A) and formed teratomas in 17% or 67% of transplantations by 7 months, respectively (Fig. 5B, 5C and supporting information Fig. S1F, S1G). When cultured in other established CDSF media supplemented with N2 [56], B27 [57], and either Bmp4 and LIF [14], or pharmacological inhibitors of Erk and Gsk3β [22], mESCs grew into teratomas more efficiently (see “N2B27-BL” and “N2B27-2i” in supporting information Table S1 and Fig. S4). Also, W4 mESCs exhibited similar phenotypic changes when maintained in CDSF with the Gsk3β inhibitor (Fig. 5B). Gsk3β is known to regulate the activity of the c-Myc protein in mESCs [24], [58]. However, RT-PCR analysis showed that inhibition of Gsk3β did not result in upregulation of Eras and c-Myc in mESCs cultured in CDSF, whereas Bmp4 induced upregulation of c-Myc (Fig. 5D and supporting information Fig. S5).
Figure 5. Screening of factors responsible for the tumor-like growth of mouse embryonic stem cells.
(A): Cumulative numbers of mESCs were compared among ESCs cultured under each condition indicated for 3 passages. Cell counts were normalized to CDSF conditions. A value for CDSF conditions is normalized to 1. Parentheses indicate the number of biological replicates per condition. Standard errors of the means are indicated by bars. +RA, CDSF with retinoic acid; +RL, CDSF with retinol; +RL+RBP, CDSF with retinol and retinol binding protein; +Bmp4, CDSF with Bmp4; +iGsk3β, CDSF with the Gsk3β inhibitor. (B): Total numbers of biological replicates that resulted in formation of teratomas were compared among mESCs cultured under each condition indicated. Orange and yellow boxes indicate the number of biological replicates that developed into teratomas within 6 months and in more than 6 months, respectively. Blue bars indicate the number of biological replicates that failed to form teratomas for more than 6 months. Data for the Gsk3β inhibitor include results obtained with R1 and W4 mESCs. For those biological replicates indicated as (i), (ii) and (iii) in the bar chart, the number of technical replicates, the number of days needed for the experimental animals to reach their end points, and the sizes of the longest axis of the teratomas are shown per technical replicate. Asterisks (*) indicate that W4 mESCs were transplanted. n.d., not determined. (C, top): NOD-SCID mice that received transplantation of mESCs cultured in CDSF supplemented with Bmp4 (right) or the Gsk3β inhibitor (left) are shown. Bars, 1 cm. (C, bottom): Representative histological images are shown. The presence of three germ layers (see the legend for Fig. 2) is evident in teratomas developed from mESCs cultured in CDSF supplemented with the Gsk3β inhibitor. Bars, 20 µm. (D): Abundance of each transcript indicated above was examined in mESCs cultured under each condition on the right by 24 cycles of PCR. Ef1α was used as a reference. White arrowheads indicate the PCR product of c-Myc.
Discussion
In this report, we present experimental evidence to suggest that short-term CDSF culture reduces the tumor-like growth of mESCs, which is reversed by pharmacological inhibition of Gsk3β. Our present study indicates that downstream of Gsk3β is primarily responsible for tumorigenesis in mESCs, which may involve uncharacterized gene products. Although the exact mechanism currently remains unknown, our present study provides a basis for further study to establish the signaling pathway responsible for the tumor-like property of ESCs.
In general, serum provides hormones, growth factors, and steroids to cultured cells. It also contains remnants of plasma components used for the activation and processing of blood clots and substances that do not normally pass through the endothelial barrier [59], [60], [61]. Therefore, serum is similar but not identical to the interstitial fluid (i.e., lymph) that surrounds cells in vivo [62]. We were unable to observe any cellular mass at the injection sites that were derived from mESCs cultured in CDSF at either one week or six months after transplantation. Therefore, it is suggested with our data that interstitial fluid will not support the tumor-like growth of mESCs.
Perhaps mESCs cultured under short-term CDSF conditions became more susceptible to LIF and exhibited cell death after transplantation due to the absence of a continuous supply of LIF [26]. However, because each mESC line shows a different degree of LIF dependency [54], other mouse pluripotent stem cells cultured in the serum-free medium may exhibit a capability to continuously grow after transplantation, as we have seen in embryonic carcinomas (F9; supporting information Fig. S1D) and germline-incompetent mESCs, D3 (data not shown). In addition, we found that the formation of teratomas became sporadic when we transplanted 0.5×106 mESCs cultured under standard conditions for a period of 6 months. Collectively, our study demonstrated that one or more extrinsic factors or niche [43] plays an important role in the formation of a teratoma. This idea is further supported by the fact that two mESCs were sufficiently able to grow into a teratoma only when mixed with 2×106 non-tumorigenic fibroblasts (MRC-5) prior to transplantation into immunocompromised mice [63]. Interestingly, the slow growth observed in Eras-null mESCs became more evident when they were cultured without feeder cells [38].
Our present data indicate that animal sera contain one or more factors that inhibit the activity of the Gsk3β protein (Fig. 1, 4 and 5). Gsk3β is involved in the canonical Wnt signaling pathway [64], [65], [66] and interacts with other biologically important signaling pathways such as phosphoinositide 3-kinase (PI3K)-Akt1 [40], [67], [68], Bmp4 [69] and hedgehog [70], [71], [72] signaling pathways. Although the secreted protein Wnt eventually inhibits the activity of Gsk3β, the role of Wnt in the maintenance of self-renewal and pluripotency of ESCs remains elusive [20], [21], [22], [23]. On the other hand, pharmacological inhibition of Gsk3β supports the self-renewal and pluripotency of ESCs [22], [23], [24], [25]. Now, the question is what Gsk3β upstream and downstream genes are in self-renewing ESCs.
Both LIF-Stat3 [73], [74] and insulin pathways activate the PI3K-Akt1 signaling pathway [24], [68], which mediates the inactivation of Gsk3β [24], [40], [64]. However, CDSF includes LIF and insulin [26], and failed to support the tumor-like growth of mESCs (Fig. 1 and 4). Thus, LIF and insulin are not the upstream of Gsk3β. Because Eras activates Akt1 [38], the downregulation of Eras in mESCs cultured in CDSF (Fig. 4C and 5D) may have resulted in the activation of Gsk3β. However, human ESCs do not express human ERAS but they do grow into teratomas [41], [42]. Because this signaling cascade is not evolutionarily conserved, Eras is not the upstream of Gsk3β.
Based on our results, Bmp4 poorly promoted proliferation and teratoma formation of mESCs cultured under CDSF conditions (Fig. 5A, 5B). It is well known that the Bmp4 and Wnt signals interact with each other in many morphogenetic events, which could result in either synergistic or antagonistic effects depending on cell types [69]. Because of the following two observations, we consider the effect of Bmp4 on Gsk3β or the tumor-like growth of mESCs antagonistic or indirect. First, our RT-PCR results revealed that mESCs cultured in CDSF supplemented with Bmp4 upregulated c-Myc, which was not the case in mESCs treated with the Gsk3β inhibitor (Fig. 5D and supporting information Fig. S5). Second, in one set of our experiments, Bmp4 efficiently induced formation of teratomas when ESCs at the earlier passage number (passage 7) were used for the culture (see (ii) in Fig. 5B). In contrast, when mESCs at passage 9 or later were cultured in CDSF with Bmp4 and transplanted, they sporadically developed into teratomas. Further investigation is required to determine the upstream of Gsk3β in ESCs.
We showed that a CDSF medium supplemented with Bmp4 and LIF supported the proliferation of mESCs that maintained transcriptional activity of Oct3/4 (supporting information Fig. S3D). In contrast, Hayashi et al. demonstrated that a CDSF medium supplemented with Bmp4 supported the differentiation of trophoblasts from mESCs [47]. However, Hayashi et al. used a basal CDSF medium that lacked oleic acid and LIF, and plated mESCs on laminin-coated dishes, but not on collagen IA-coated dishes. In addition, the presence of LIF under these CDSF conditions inhibited the differentiation of trophoblasts [47]. Therefore, it is likely that the different chemical composition of a basal medium contributed to the differentiation of trophoblasts when Bmp4 was supplemented in the culture.
It is interesting to note that in concert with LIF, Bmp4 suppresses differentiation of the neural lineage in mESCs maintained under serum-free conditions [14] supplemented with N2 and B27, which were originally developed to culture a neuroblastoma cell line [56] and hippocampal neurons [57], respectively. Mouse ESCs maintained under serum-free conditions supplemented with LIF, Bmp4, N2 and B27 are pluripotent and can contribute to the germline in chimeric animals [14], and grew into teratomas (supporting information Table S1 and Fig. S4). On the other hand, we showed that both the CDSF medium used in the present study, which contains LIF, and the CDSF medium supplemented with Bmp4 poorly sustained the tumor-like growth of mESCs. Taken together, it is suggested with these data that the cellular pluripotency and the tumor-like growth of ESCs may be regulated by different mechanisms, and that extrinsic factors play significant roles in cell fate decisions of mESCs. It will be interesting to investigate whether ESCs need unique substrate stiffness to grow into teratomas, because our previous study showed that stiffer substrates promoted differentiation of mESCs [75].
Gsk3β inhibits the activity of its target c-Myc [24], [76], which is involved in the self-renewal of mESCs [58] and responsible for an age-associated incidence of tumorigenesis in chimeric mice generated with mouse iPSCs [77], [78]. In contrast, our RT-PCR results showed that mESCs cultured under CDSF conditions with the Gsk3β inhibitor failed to significantly upregulate transcription of Eras and c-Myc (Fig. 5D) but efficiently developed into teratomas (Fig. 5B, 5C). Thus, we propose that Gsk3β downstream genes other than c-Myc may regulate the tumor-like growth of ESCs, which play independent roles from maintaining the self-renewal of ESCs. In addition, candidate Gsk3β downstream genes responsible for tumorigenesis in mESCs may act independently from ERAS in human ESCs [41], [42]. Currently, our investigation is ongoing. Further study to identify the candidate genes and to test their roles in human ESCs and iPSCs will lead us to establishing a strategy to significantly improve the safety of human iPSCs.
Materials and Methods
Ethics statement
Use of animals was approved by the Illinois Institutional Animal Care and Use Committee (protocol # 10093, approved on 6/18/10).
Cell culture
A mouse embryonic stem cell (mESC) line of R1 [79], which expresses EGFP under the Oct3/4 promoter [80], was kindly provided by Dr. William L. Stanford [51]. This Oct3/4::EGFP mESC line was thawed on feeders and maintained under standard [81] or chemically-defined serum-free (CDSF) [26] conditions at 37°C, 5% CO2. The exact number of passages that this mESC line has gone through is unknown. However, when these ESCs were brought to our laboratory, they were passaged on feeders two more times and frozen as a stock. When these ESCs were thawed on feeders, more frozen stocks were made at passage 5–7. These stocks were used for the current study. Under standard conditions, mESCs were maintained on 0.1% gelatin (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com)-coated tissue culture dishes in high glucose-Dulbecco's modified Eagle's medium (DMEM; Invitrogen, Carlsbad, CA, http://www.invitrogen.com) supplemented with 15% fetal bovine serum (FBS; Invitrogen), 0.1 mM non-essential amino acids (Invitrogen), 2 mM GlutaMax I (Invitrogen), 1 mM sodium pyruvate (Invitrogen), 100 U/ml penicillin and 0.1 mg/ml streptomycin (Sigma-Aldrich), 0.1 mM 2-mercaptoethanol (Sigma-Aldrich), and 1,000 U/ml LIF (Millipore, Billerica, MA, http://www.millipore.com). When the mESCs reached 80% to 100% confluence, they were routinely passaged at a ratio of 1∶6 every two days using TrypLETM Express (Invitrogen). The cells were discarded after being passaged 10 times onto gelatin-coated dishes. Approximately 1×105/cm2 mESCs maintained under standard conditions were plated onto tissue culture dishes coated with 0.15 mg/ml type IA collagen (Nitta Gelatin Co., Osaka, Japan, http://www.nitta-gelatin.co.jp), which contain the ESF7 medium (Cell Science & Technology Institute Inc. Miyagi, Japan, http://www.cstimedia.com) [26] supplemented with 1,000 U/ml LIF. This was counted as passage 1 under CDSF conditions. Mouse ESCs at passage 7–12 were used to start CDSF culture. Mouse ESCs grown under CDSF conditions were split every 3 or 4 days with 0.02% EDTA (Sigma-Aldrich). Similarly, mESCs of W4 (129S6, purchased at passage 9; Taconic, Hudson, NY, http://www.taconic.com) and D3 (129S2/SvPas, CRL-1934, ATCC, Manassas, VA) were used to test CDSF conditions. When CDSF culture was supplemented with FBS, 0.02% EDTA was used for passaging cells. Following serum lots were used to supplement CDSF culture: Lot. 1359246 and 726570, Invitrogen; Lot. L0228, Atlanta Biologicals (Lawrenceville, GA, http://www.atlantabio.com); Lot. A74B00Z, Gemini Bio-Products (West Sacramento, CA, http://www.gembio.com). Images of cell morphology and fluorescence were taken under the same conditions using an inverted microscope equipped with an epi-fluorescence lamp (DMI4000B, Leica Microsystems, Wetzlar, Germany, http://www.leica-microsystems.com) [82], [83]. To measure the frequency of cell doubling, the mESCs were plated at 1×106 per one well of 6-well plates. Two days after plating, the number of cells was counted for the second and third passages. Cell doubling was calculated based on the following formula: Cell doubling = log2[(the number of cells 2 days after plating)/(1×106)]. Statistical tests were performed using the Mann-Whitney's U-test.
To compare the incidence of teratoma formation, another basal culture medium was prepared by mixing the NeurobasalTM medium supplemented with B27 (Invitrogen) 1:1 with DMEM/F12 (Invitrogen) supplemented with N2 (Invitrogen) and 50 µg/ml bovine serum albumin as described already [84]. The resulting basal medium was supplemented with either 10 ng/ml LIF and 10 ng/ml Bmp4 (R & D systems) [14], or 1 µM Stemolecule TM PD0325901 and 3 µM Stemolecule TM CHIR99021 (2i; Stemgent, Cambridge, MA, https://www.stemgent.com/) [22], and used to culture mESCs for three passages, which needed seven days before subcutaneous injection into NOD-SCID mice.
Cell transplantation
At the fourth passage under standard or CDSF conditions, the mESCs were trypsinized and counted. TrypLETM Express was inactivated with an equal volume of 1 mg/ml soybean trypsin inhibitor (Sigma-Aldrich). For this purpose, no culture medium with animal serum was used. One to two million cells were centrifuged at 1,000 g for 5 min and resuspended into 25 µl PBS, which was mixed with 25 µl of 0.3 mg/ml type IA collagen. Mouse ESCs were kept on ice before being injected into NOD-SCID mice (the Jackson Laboratory, Bar Harbor, ME, http://www.jax.org) subcutaneously. Animal health was monitored routinely until the diameter of the tumors reached several few centimeters at which time the animals reached their end points and were euthanized. This procedure was approved by the Illinois Institutional Animal Care and Use Committee. The incidence of teratoma formation was statistically validated using the Fisher's exact probability test.
Histology
Before dissecting the teratomas, pictures of the experimental animals were taken. The sizes of the longest axis of the teratomas were measured. Then, the teratomas were surgically dissected out, cut into smaller pieces, and fixed in 4% paraformaldehyde (Sigma-Aldrich) at 4°C overnight, followed by dehydration and embedding in Paraplast plus (Sigma-Aldrich). Sections of 8 µm thickness were cut and processed for standard hematoxylin and eosin staining.
Immunofluorescence microscopy
The Oct3/4::EGFP ESC line was immunostained essentially as described previously [75], [82]. Goat anti-mouse Nanog (AF2729, R & D systems) and anti-human Sox2 (sc-17320, Santa Cruz Biotechnology, Santa Cruz, CA) polyclonal antibodies and a mouse anti-SSEA1 monoclonal antibody (Developmental Studies Hybridoma Bank, University of Iowa) were used as primary antibodies. Alexa Fluor 488 goat anti-mouse IgG (H+L) and Alexa Fluor 546 donkey anti-goat IgG (H+L) polyclonal antibodies (Invitrogen) were used as secondary antibodies. Fluorescence images were taken using the same exposure time (750 msec for 505 nm and 850 msec for 595 nm) and enhanced in the same way.
Aggregation chimeras
Mouse ESCs cultured under CDSF conditions for 10–12 days were aggregated with zona-free morulae, which were obtained from superovulated C57BL/6 females (the Jackson Laboratory) mated with C57BL/6 males. Aggregated embryos were cultured in the KSOM medium with 1/2 amino acids (Millipore) for 14–15 hrs as described previously [79]. Chimeric blastocysts were transferred to pseudopregnant females at the transgenic mouse facility on campus.
RT-PCR
A total of 1.6 µg of total RNA extracted from mESCs cultured under each condition was used to synthesize the first cDNA strand as previously described [83], [85]. PCR mixtures were prepared using Phusion DNA polymerase (New England Biolab, Ipswich, MA, http://www.neb.com) according to the manufacturer's instructions. The PCR conditions were as follows: initial denaturing at 98°C for 1 min followed by 19, 21, 23, 24 or 25 cycles of denaturing at 98°C for 10 sec, annealing at 65°C for 30 sec, extension at 72°C for 30 sec, and a final extension at 72°C for 7.5 min. The primer pairs have previously been described: Eras [38], Oct4 [86], Esg1 and Ef1α [87], Sox2 [88], and c-Myc [89].
Screening of factors
A day after mESCs were plated under CDSF conditions, these cultures were supplemented with 10 nM retinol (Sigma-Aldrich) in 100% EtOH with or without 1 nM retinol binding protein (RBP) from human urine (Sigma-Aldrich), 10 nM all-trans retinoic acid (Sigma-Aldrich), 10 ng/ml Bmp4 (R & D Systems, Minneapolis, MN, http://www.rndsystems.com), or 3 µM Stemolecule TM CHIR99021 in DMSO (Stemgent). Before applying RBP to culture, buffer exchange was carried out with Amicon Ultra (Millipore) to remove a preservative. Mouse ESCs grown under these conditions were split with 0.02% EDTA for 3 passages when they reached confluence. At the fourth passage, these mESCs were injected into NOD-SCID mice as described above.
Supporting Information
Anatomical images of NOD-SCID mice transplanted with mouse embryonic stem cells. (A-D, F and G): Teratomas developed from mouse embryonic stem cells (mESCs) and embryonic carcinomas (F9 in D) cultured under the conditions indicated are shown. Also, the number of weeks (wks) needed for the experimental animals to reach their end points are shown. Bars, 1cm. CDSF+FBS, CDSF culture supplemented with fetal bovine serum; CDSF-Standard, CDSF conditions followed by standard conditions; CDSF (F9), embryonic carcinoma cells F9 maintained under CDSF conditions; CDSF+Bmp4, CDSF culture supplemented with Bmp4; CDSF+iGsk3β, CDSF culture supplemented with the Gsk3β inhibitor. (E): This animal was sacrificed one week after mESCs cultured under CDSF conditions followed by standard conditions were transplanted. The rectangle indicates the area shown in the inset. Bar, 1cm. The inset shows an enlarged image of a tiny mass of the mESCs depicted by dashed lines with a scale bar of 0.1 cm. See supporting information Fig. S2 for detail.
(TIF)
Mouse embryonic stem cells exhibited initial differentiation as early as one week after transplantation when cultured under CDSF-Standard conditions. Mouse ESCs were cultured in CDSF for three passages followed by transfer to standard conditions for two passages prior to transplantation. (A): An epithelialized cellular mass has aggregates formed among the collagen fibers (stained pale pink) used to transplant the mESCs. Rectangles indicate the areas shown in B, C and D. Bar, 500 µm. (B-D): Two types of cells are prominent, one of which is reminiscent of keratin pearls (B and D), and the other that resembles cartilage (C and D). Bars, 20 µm.
(TIF)
Identification of factors that support the tumor-like growth of mouse embryonic stem cells maintained under CDSF conditions. (A-E): Phase contrast (top) and fluorescence (bottom) images of mESCs under CDSF conditions supplemented with each factor indicated above are shown. Bars, 20 µm. CDSF+RA, CDSF with retinoic acid; CDSF+RL, CDSF with retinol; CDSF+RL+RBP, CDSF with retinol and retinol binding protein; CDSF+Bmp4, CDSF with Bmp4; CDSF+iGsk3β, CDSF with the Gsk3β inhibitor.
(TIF)
The incidence of teratoma formation was compared among different CDSF conditions. The sizes of the teratomas formed (orange bar, left axis) and the number of days required for the experimental NOD-SCID mice to reach their end points (blue stars, right axis) were compared among mESCs cultured under the conditions indicated. Parentheses indicate the number of biological replicates (i.e., mESCs prepared at different passages) per culture condition. Standard errors of the means are indicated by bars. Two transplantations for ESF7 showed no sign of teratoma formation when paired with the standard conditions. Standard, Standard conditions; CDSF, CDSF conditions; BL, other established CDSF conditions supplemented with N2, B27, Bmp4 and LIF; 2i, other established CDSF conditions supplemented with N2, B27, and pharmacological inhibitors of Erk and Gsk3β.
(TIF)
Expression levels of markers were compared among different culture conditions. Abundance of each transcript indicated above was examined in mESCs cultured under each condition on the right by 19, 21 and 23 cycles of PCR (indicated by triangles). Ef1α was used as a reference. White arrowheads indicate the PCR product of c-Myc.
(TIF)
Effects of serum on the tumorigenicity of mouse embryonic stem cells.
(DOCX)
Acknowledgments
We are grateful to Drs. Matthew B. Wheeler, Miho Kusuda-Furue, Yuki Kimura, and William L. Stanford for the critical reading of this manuscript. YL was a recipient of AYRE International Research and Learning Fellowships, UIUC. The anti-SSEA1 monoclonal antibody developed by Drs. Solter and Knowles was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the USDA Hatch Project (ILLU-538-323), University of Illinois and the Illinois Regenerative Medicine Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 2.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 5.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, et al. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Park IH, Zhao R, West JA, Yabuuchi A, Huo H, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 8.Gurdon JB, Melton DA. Nuclear reprogramming in cells. Science. 2008;322:1811–1815. doi: 10.1126/science.1160810. [DOI] [PubMed] [Google Scholar]
- 9.Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, et al. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 10.Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 11.Niwa H. How is pluripotency determined and maintained? Development. 2007;134:635–646. doi: 10.1242/dev.02787. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsuka S, Dalton S. Molecular and biological properties of pluripotent embryonic stem cells. Gene Ther. 2008;15:74–81. doi: 10.1038/sj.gt.3303065. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka TS. Transcriptional heterogeneity in mouse embryonic stem cells. Reprod Fertil Dev. 2009;21:67–75. doi: 10.1071/rd08219. [DOI] [PubMed] [Google Scholar]
- 14.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 15.Wang R, Liang J, Yu HM, Liang H, Shi YJ, et al. Retinoic acid maintains self-renewal of murine embryonic stem cells via a feedback mechanism. Differentiation. 2008;76:931–945. doi: 10.1111/j.1432-0436.2008.00272.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen L, Khillan JS. Promotion of feeder-independent self-renewal of embryonic stem cells by retinol (vitamin A). Stem Cells. 2008;26:1858–1864. doi: 10.1634/stemcells.2008-0050. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Khillan JS. A novel signaling by vitamin A/retinol promotes self renewal of mouse embryonic stem cells by activating PI3K/Akt signaling pathway via insulin-like growth factor-1 receptor. Stem Cells. 2010;28:57–63. doi: 10.1002/stem.251. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Alexander P, Wu L, Hammer R, Cleaver O, et al. Dependence of mouse embryonic stem cells on threonine catabolism. Science. 2009;325:435–439. doi: 10.1126/science.1173288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanes O, Clark J, Wong DM, Patti GJ, Sanchez-Ruiz A, et al. Metabolic oxidation regulates embryonic stem cell differentiation. Nat Chem Biol. 2010;6:411–417. doi: 10.1038/nchembio.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development. 2006;133:3787–3796. doi: 10.1242/dev.02551. [DOI] [PubMed] [Google Scholar]
- 21.Dravid G, Ye Z, Hammond H, Chen G, Pyle A, et al. Defining the role of Wnt/beta-catenin signaling in the survival, proliferation, and self-renewal of human embryonic stem cells. Stem Cells. 2005;23:1489–1501. doi: 10.1634/stemcells.2005-0034. [DOI] [PubMed] [Google Scholar]
- 22.Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63. doi: 10.1038/nm979. [DOI] [PubMed] [Google Scholar]
- 24.Bechard M, Dalton S. Subcellular localization of glycogen synthase kinase 3beta controls embryonic stem cell self-renewal. Mol Cell Biol. 2009;29:2092–2104. doi: 10.1128/MCB.01405-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umehara H, Kimura T, Ohtsuka S, Nakamura T, Kitajima K, et al. Efficient Derivation of Embryonic Stem Cells by Inhibition of Glycogen Synthase Kinase-3. Stem Cells. 2007;25:2705–2711. doi: 10.1634/stemcells.2007-0086. [DOI] [PubMed] [Google Scholar]
- 26.Furue M, Okamoto T, Hayashi Y, Okochi H, Fujimoto M, et al. Leukemia inhibitory factor as an anti-apoptotic mitogen for pluripotent mouse embryonic stem cells in a serum-free medium without feeder cells. In Vitro Cell Dev Biol Anim. 2005;41:19–28. doi: 10.1290/0502010.1. [DOI] [PubMed] [Google Scholar]
- 27.Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 28.Furue MK, Na J, Jackson JP, Okamoto T, Jones M, et al. Heparin promotes the growth of human embryonic stem cells in a defined serum-free medium. Proc Natl Acad Sci U S A. 2008;105:13409–13414. doi: 10.1073/pnas.0806136105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldsborough MD, Price PJ, Lobo-Alfonso J, Morrison JR, Stevens ME, et al. Serum-free culture of murine embryonic stem (ES) cells. Focus. 1998;20:8–12. [Google Scholar]
- 30.Suemori H, Tada T, Torii R, Hosoi Y, Kobayashi K, et al. Establishment of embryonic stem cell lines from cynomolgus monkey blastocysts produced by IVF or ICSI. Dev Dyn. 2001;222:273–279. doi: 10.1002/dvdy.1191. [DOI] [PubMed] [Google Scholar]
- 31.Ying Q-L, Wray J, Nichols J, Batlle-Morera L, Doble B, et al. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi Y, Furue MK, Okamoto T, Ohnuma K, Myoishi Y, et al. Integrins Regulate Mouse Embryonic Stem Cell Self-Renewal. Stem Cells. 2007;25:3005–3015. doi: 10.1634/stemcells.2007-0103. [DOI] [PubMed] [Google Scholar]
- 33.Solter D. From teratocarcinomas to embryonic stem cells and beyond: a history of embryonic stem cell research. Nat Rev Genet. 2006;7:319–327. doi: 10.1038/nrg1827. [DOI] [PubMed] [Google Scholar]
- 34.Chambers I, Smith A. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene. 2004;23:7150–7160. doi: 10.1038/sj.onc.1207930. [DOI] [PubMed] [Google Scholar]
- 35.Jaenisch R, Young R. Stem Cells, the Molecular Circuitry of Pluripotency and Nuclear Reprogramming. Cell. 2008;132:567–582. doi: 10.1016/j.cell.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu J, Thomson JA. Pluripotent stem cell lines. Genes Dev. 2008;22:1987–1997. doi: 10.1101/gad.1689808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–17. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi K, Mitsui K, Yamanaka S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. Nature. 2003;423:541–545. doi: 10.1038/nature01646. [DOI] [PubMed] [Google Scholar]
- 39.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 41.Kameda T, Thomson JA. Human ERas gene has an upstream premature polyadenylation signal that results in a truncated, noncoding transcript. Stem Cells. 2005;23:1535–1540. doi: 10.1634/stemcells.2005-0054. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka Y, Ikeda T, Kishi Y, Masuda S, Shibata H, et al. ERas is expressed in primate embryonic stem cells but not related to tumorigenesis. Cell Transplant. 2009;18:381–389. doi: 10.3727/096368909788809794. [DOI] [PubMed] [Google Scholar]
- 43.Voog J, Jones DL. Stem cells and the niche: a dynamic duo. Cell Stem Cell. 2010;6:103–115. doi: 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duval D, Reinhardt B, Kedinger C, Boeuf H. Role of suppressors of cytokine signaling (Socs) in leukemia inhibitory factor (LIF) -dependent embryonic stem cell survival. FASEB J. 2000;14:1577–1584. doi: 10.1096/fj.14.11.1577. [DOI] [PubMed] [Google Scholar]
- 45.Larochelle A, Vormoor J, Hanenberg H, Wang JCY, Bhatia M, et al. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: Implications for gene therapy. Nat Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 46.Hayashi Y, Furue MK, Okamoto T, Ohnuma K, Myoishi Y, et al. Integrins regulate mouse embryonic stem cell self-renewal. Stem Cells. 2007;25:3005–3015. doi: 10.1634/stemcells.2007-0103. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi Y, Furue MK, Tanaka S, Hirose M, Wakisaka N, et al. BMP4 induction of trophoblast from mouse embryonic stem cells in defined culture conditions on laminin. In Vitro Cell Dev Biol Anim. 2010;46:416–430. doi: 10.1007/s11626-009-9266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scholer HR, Ruppert S, Suzuki N, Chowdhury K, Gruss P. New type of POU domain in germ line-specific protein Oct-4. Nature. 1990;344:435–439. doi: 10.1038/344435a0. [DOI] [PubMed] [Google Scholar]
- 49.Okamoto K, Okazawa H, Okuda A, Sakai M, Muramatsu M, et al. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990;60:461–472. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- 50.Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirie F, et al. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990;345:686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- 51.Walker E, Ohishi M, Davey RE, Zhang W, Cassar PA, et al. Prediction and testing of novel transcriptional networks regulating embryonic stem cell self-renewal and commitment. Cell Stem Cell. 2007;1:71–86. doi: 10.1016/j.stem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Bernstine EG, Hooper ML, Grandchamp S, Ephrussi B. Alkaline Phosphatase Activity in Mouse Teratoma. Proc Natl Acad Sci U S A. 1973;70:3899–3903. doi: 10.1073/pnas.70.12.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Auerbach W, Dunmore JH, Fairchild-Huntress V, Fang Q, Auerbach AB, et al. Establishment and chimera analysis of 129/SvEv- and C57BL/6-derived mouse embryonic stem cell lines. Biotechniques. 2000;29:1024–1028, 1030, 1032. doi: 10.2144/00295st04. [DOI] [PubMed] [Google Scholar]
- 54.Raz R, Lee CK, Cannizzaro LA, d'Eustachio P, Levy DE. Essential role of STAT3 for embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 1999;96:2846–2851. doi: 10.1073/pnas.96.6.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soprano DR, Blaner WS, editors. New York: Raven Press Ltd; 1994. Plasma retinol-binding protein. 2nd ed. pp. 257–281. [Google Scholar]
- 56.Bottenstein JE, Sato GH. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 58.Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, et al. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development. 2005;132:885–896. doi: 10.1242/dev.01670. [DOI] [PubMed] [Google Scholar]
- 59.Holliday MA. Extracellular fluid and its proteins: dehydration, shock, and recovery. Pediatr Nephrol. 1999;13:989–995. doi: 10.1007/s004670050741. [DOI] [PubMed] [Google Scholar]
- 60.Hewlett G. Strategies for optimising serum-free media. Cytotechnology. 1991;5:3–14. doi: 10.1007/BF00365530. [DOI] [PubMed] [Google Scholar]
- 61.Sato GH, Sato JD, Okamoto T, McKeehan WL, Barnes DW. Tissue culture: the unlimited potential. In Vitro Cell Dev Biol Anim. 2010;46:590–594. doi: 10.1007/s11626-010-9315-1. [DOI] [PubMed] [Google Scholar]
- 62.Sato GH. The role of serum in cell culture. In: Litwack G, editor. Biochemical Actions of Hormones. New York: Academic Press; 1975. pp. 391–396. [Google Scholar]
- 63.Lawrenz B, Schiller H, Willbold E, Ruediger M, Muhs A, et al. Highly sensitive biosafety model for stem-cell-derived grafts. Cytotherapy. 2004;6:212–222. doi: 10.1080/14653240410006031. [DOI] [PubMed] [Google Scholar]
- 64.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends in Biochemical Sciences. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 67.Watanabe S, Umehara H, Murayama K, Okabe M, Kimura T, et al. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. 2006;25:2697–2707. doi: 10.1038/sj.onc.1209307. [DOI] [PubMed] [Google Scholar]
- 68.Paling NR, Wheadon H, Bone HK, Welham MJ. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J Biol Chem. 2004;279:48063–48070. doi: 10.1074/jbc.M406467200. [DOI] [PubMed] [Google Scholar]
- 69.Itasaki N, Hoppler S. Crosstalk between Wnt and bone morphogenic protein signaling: a turbulent relationship. Dev Dyn. 2010;239:16–33. doi: 10.1002/dvdy.22009. [DOI] [PubMed] [Google Scholar]
- 70.Zhang W, Zhao Y, Tong C, Wang G, Wang B, et al. Hedgehog-regulated Costal2-kinase complexes control phosphorylation and proteolytic processing of Cubitus interruptus. Dev Cell. 2005;8:267–278. doi: 10.1016/j.devcel.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Riobo NA, Lu K, Ai X, Haines GM, Emerson CP., Jr Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc Natl Acad Sci U S A. 2006;103:4505–4510. doi: 10.1073/pnas.0504337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim WY, Wang X, Wu Y, Doble BW, Patel S, et al. GSK-3 is a master regulator of neural progenitor homeostasis. Nat Neurosci. 2009;12:1390–1397. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. Embo J. 1999;18:4261–4269. doi: 10.1093/emboj/18.15.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chowdhury F, Li Y, Poh Y-C, Yokohama-Tamaki T, Wang N, et al. Soft Substrates Promote Homogeneous Self-Renewal of Embryonic Stem Cells via Downregulating Cell-Matrix Tractions. PLoS ONE. 2010;5:e15655. doi: 10.1371/journal.pone.0015655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 77.Nakagawa M, Takizawa N, Narita M, Ichisaka T, Yamanaka S. Promotion of direct reprogramming by transformation-deficient Myc. Proc Natl Acad Sci U S A. 2010;107:14152–14157. doi: 10.1073/pnas.1009374107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 79.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder J. Derivation of Completely Cell Culture-Derived Mice from Early-Passage Embryonic Stem Cells. PNAS. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Viswanathan S, Benatar T, Mileikovsky M, Lauffenburger DA, Nagy A, et al. Supplementation-dependent differences in the rates of embryonic stem cell self-renewal, differentiation, and apoptosis. Biotechnol Bioeng. 2003;84:505–517. doi: 10.1002/bit.10799. [DOI] [PubMed] [Google Scholar]
- 81.Tompers DM, Labosky PA. Electroporation of Murine Embryonic Stem Cells: A Step-by-Step Guide. Stem Cells. 2004;22:243–249. doi: 10.1634/stemcells.22-3-243. [DOI] [PubMed] [Google Scholar]
- 82.Tanaka TS, Lopez de Silanes I, Sharova LV, Akutsu H, Yoshikawa T, et al. Esg1, expressed exclusively in preimplantation embryos, germline, and embryonic stem cells, is a putative RNA-binding protein with broad RNA targets. Dev Growth Differ. 2006;48:381–390. doi: 10.1111/j.1440-169X.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 83.Tanaka TS, Davey RE, Lan Q, Zandstra PW, Stanford WL. Development of a gene trap vector with a highly-sensitive fluorescent protein reporter system aiming for the real-time single cell expression profiling. Genesis. 2008;46:347–356. doi: 10.1002/dvg.20404. [DOI] [PubMed] [Google Scholar]
- 84.Ying QL, Smith AG. Defined conditions for neural commitment and differentiation. Methods Enzymol. 2003;365:327–341. doi: 10.1016/s0076-6879(03)65023-8. [DOI] [PubMed] [Google Scholar]
- 85.Tanaka TS, Nishiumi F, Komiya T, Ikenishi K. Characterization of the 38 kDa protein lacking in gastrula-arrested mutant Xenopus embryos. Int J Dev Biol. 2010;54:1347–1353. doi: 10.1387/ijdb.092862tt. [DOI] [PubMed] [Google Scholar]
- 86.Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 87.Tanaka TS, Kunath T, Kimber WL, Jaradat SA, Stagg CA, et al. Gene expression profiling of embryo-derived stem cells reveals candidate genes associated with pluripotency and lineage specificity. Genome Res. 2002;12:1921–1928. doi: 10.1101/gr.670002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9:625–635. doi: 10.1038/ncb1589. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Anatomical images of NOD-SCID mice transplanted with mouse embryonic stem cells. (A-D, F and G): Teratomas developed from mouse embryonic stem cells (mESCs) and embryonic carcinomas (F9 in D) cultured under the conditions indicated are shown. Also, the number of weeks (wks) needed for the experimental animals to reach their end points are shown. Bars, 1cm. CDSF+FBS, CDSF culture supplemented with fetal bovine serum; CDSF-Standard, CDSF conditions followed by standard conditions; CDSF (F9), embryonic carcinoma cells F9 maintained under CDSF conditions; CDSF+Bmp4, CDSF culture supplemented with Bmp4; CDSF+iGsk3β, CDSF culture supplemented with the Gsk3β inhibitor. (E): This animal was sacrificed one week after mESCs cultured under CDSF conditions followed by standard conditions were transplanted. The rectangle indicates the area shown in the inset. Bar, 1cm. The inset shows an enlarged image of a tiny mass of the mESCs depicted by dashed lines with a scale bar of 0.1 cm. See supporting information Fig. S2 for detail.
(TIF)
Mouse embryonic stem cells exhibited initial differentiation as early as one week after transplantation when cultured under CDSF-Standard conditions. Mouse ESCs were cultured in CDSF for three passages followed by transfer to standard conditions for two passages prior to transplantation. (A): An epithelialized cellular mass has aggregates formed among the collagen fibers (stained pale pink) used to transplant the mESCs. Rectangles indicate the areas shown in B, C and D. Bar, 500 µm. (B-D): Two types of cells are prominent, one of which is reminiscent of keratin pearls (B and D), and the other that resembles cartilage (C and D). Bars, 20 µm.
(TIF)
Identification of factors that support the tumor-like growth of mouse embryonic stem cells maintained under CDSF conditions. (A-E): Phase contrast (top) and fluorescence (bottom) images of mESCs under CDSF conditions supplemented with each factor indicated above are shown. Bars, 20 µm. CDSF+RA, CDSF with retinoic acid; CDSF+RL, CDSF with retinol; CDSF+RL+RBP, CDSF with retinol and retinol binding protein; CDSF+Bmp4, CDSF with Bmp4; CDSF+iGsk3β, CDSF with the Gsk3β inhibitor.
(TIF)
The incidence of teratoma formation was compared among different CDSF conditions. The sizes of the teratomas formed (orange bar, left axis) and the number of days required for the experimental NOD-SCID mice to reach their end points (blue stars, right axis) were compared among mESCs cultured under the conditions indicated. Parentheses indicate the number of biological replicates (i.e., mESCs prepared at different passages) per culture condition. Standard errors of the means are indicated by bars. Two transplantations for ESF7 showed no sign of teratoma formation when paired with the standard conditions. Standard, Standard conditions; CDSF, CDSF conditions; BL, other established CDSF conditions supplemented with N2, B27, Bmp4 and LIF; 2i, other established CDSF conditions supplemented with N2, B27, and pharmacological inhibitors of Erk and Gsk3β.
(TIF)
Expression levels of markers were compared among different culture conditions. Abundance of each transcript indicated above was examined in mESCs cultured under each condition on the right by 19, 21 and 23 cycles of PCR (indicated by triangles). Ef1α was used as a reference. White arrowheads indicate the PCR product of c-Myc.
(TIF)
Effects of serum on the tumorigenicity of mouse embryonic stem cells.
(DOCX)