Abstract
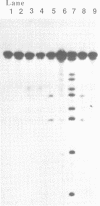
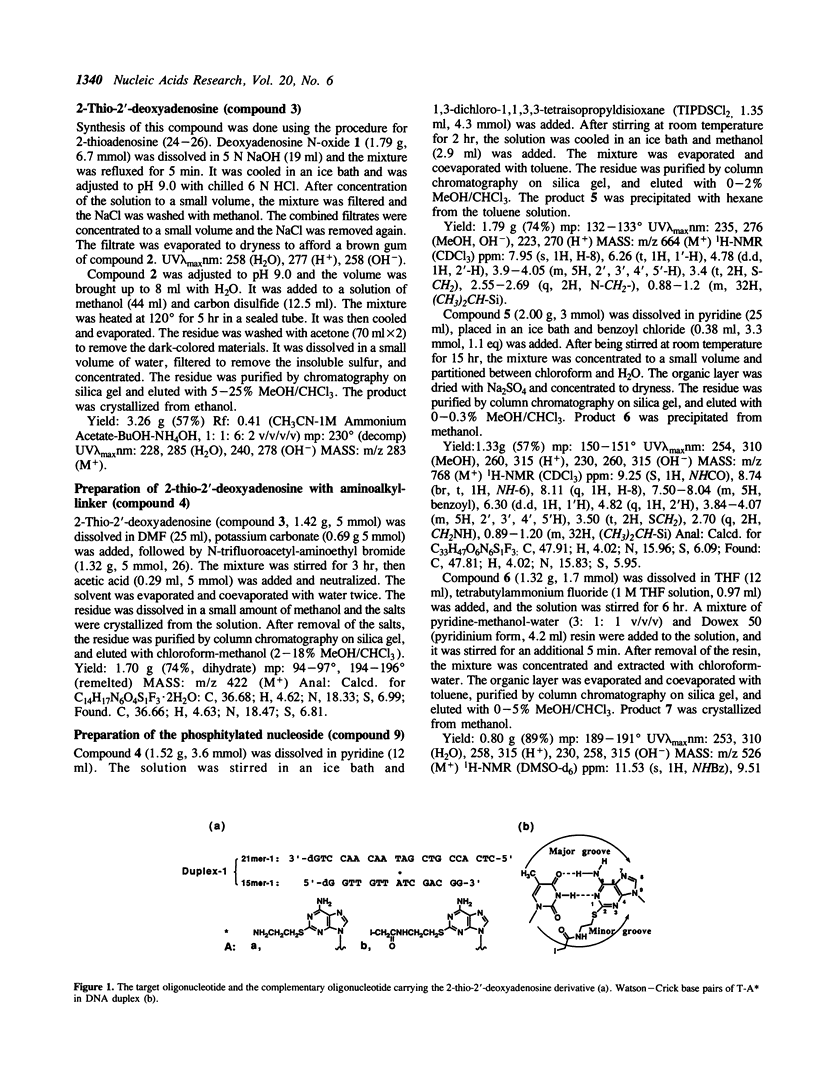
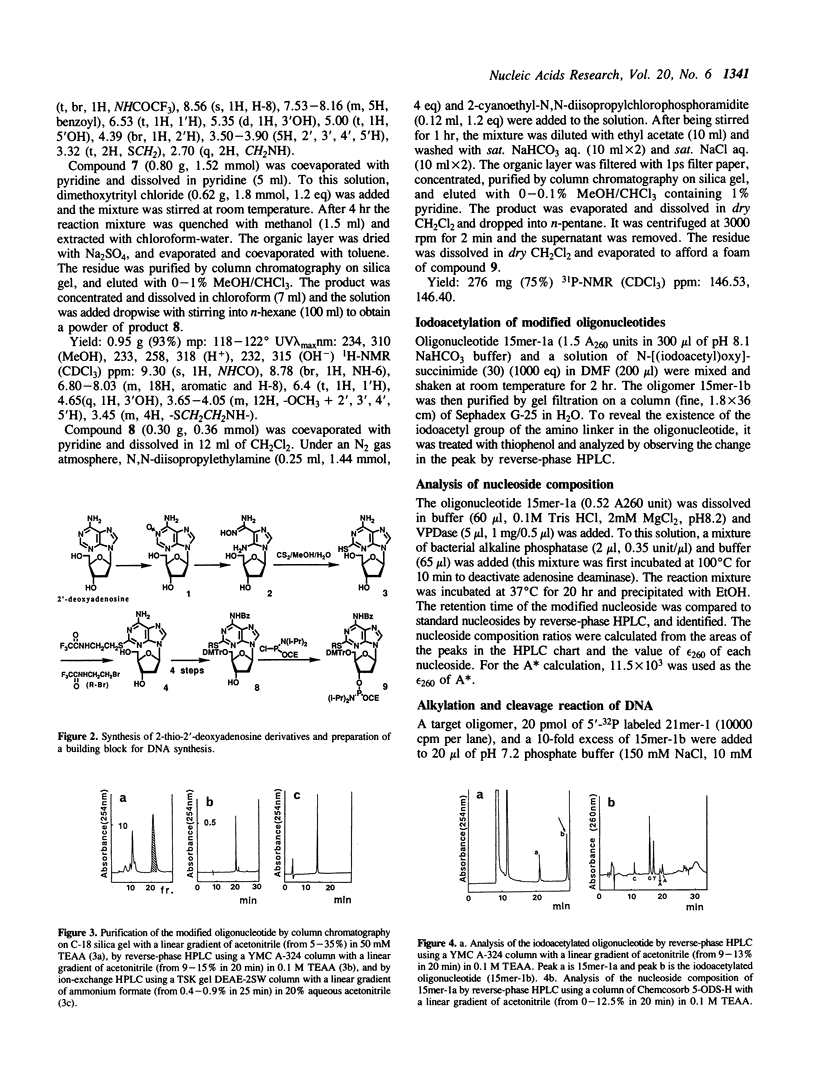
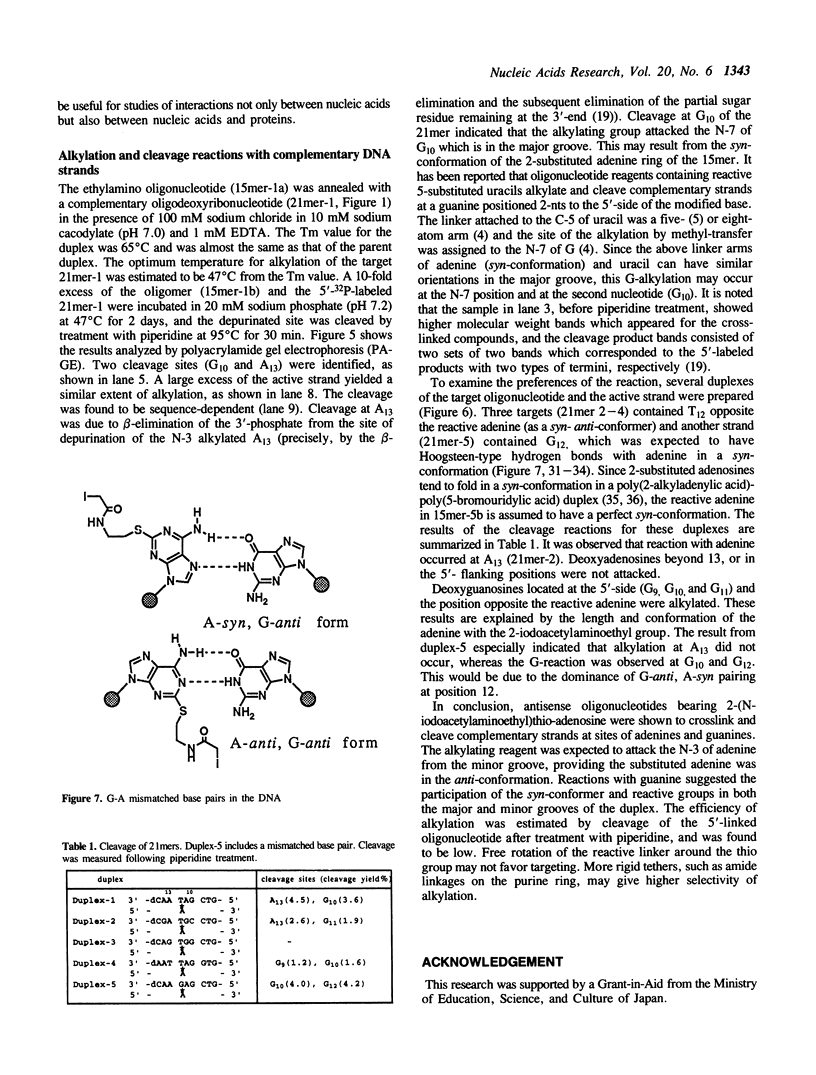
Antisense oligodeoxyribonucleotides (15mers), containing a 2-(N-iodoacetylaminoethyl)thio-adenine, were synthesized and tested for their ability to cleave complementary DNAs (21mers). Cleavage of the target DNAs was done by alkylation followed by treatment with piperidine, and the positions of the alkylated sites were estimated by identification of the cleaved products. By using several combinations of the modified strands and their target DNAs, it was determined that alkylation occurred at adenine or guanine, depending on the torsion angle of the modified nucleoside.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen C. H., Sigman D. S. Nuclease activity of 1,10-phenanthroline-copper: sequence-specific targeting. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7147–7151. doi: 10.1073/pnas.83.19.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu B. C., Orgel L. E. Nonenzymatic sequence-specific cleavage of single-stranded DNA. Proc Natl Acad Sci U S A. 1985 Feb;82(4):963–967. doi: 10.1073/pnas.82.4.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer G. B., Dervan P. B. Sequence-specific cleavage of single-stranded DNA: oligodeoxynucleotide-EDTA X Fe(II). Proc Natl Acad Sci U S A. 1985 Feb;82(4):968–972. doi: 10.1073/pnas.82.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazakerley G. V., Quignard E., Woisard A., Guschlbauer W., van der Marel G. A., van Boom J. H., Jones M., Radman M. Structures of mismatched base pairs in DNA and their recognition by the Escherichia coli mismatch repair system. EMBO J. 1986 Dec 20;5(13):3697–3703. doi: 10.1002/j.1460-2075.1986.tb04702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazakerley G. V., Sowers L. C., Eritja R., Kaplan B. E., Goodman M. F. NMR studies on an oligodeoxynucleotide containing 2-aminopurine opposite adenine. Biochemistry. 1987 Sep 8;26(18):5641–5646. doi: 10.1021/bi00392a009. [DOI] [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Chassignol M., Thuong N. T., Helene C. Sequence-targeted cleavage of single- and double-stranded DNA by oligothymidylates covalently linked to 1,10-phenanthroline. J Biol Chem. 1989 Apr 5;264(10):5891–5898. [PubMed] [Google Scholar]
- Frolova E. I., Ivanova E. M., Zarytova V. F., Abramova T. V., Vlassov V. V. Porphyrin-linked oligonucleotides. Synthesis and sequence-specific modification of ssDNA. FEBS Lett. 1990 Aug 20;269(1):101–104. doi: 10.1016/0014-5793(90)81129-c. [DOI] [PubMed] [Google Scholar]
- Iverson B. L., Dervan P. B. Nonenzymatic sequence-specific methyl transfer to single-stranded DNA. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4615–4619. doi: 10.1073/pnas.85.13.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikugawa K., Suehiro H., Ichino M. Platelet aggregation inhibitors. 6. 2-Thioadenosine derivatives. J Med Chem. 1973 Dec;16(12):1381–1388. doi: 10.1021/jm00270a014. [DOI] [PubMed] [Google Scholar]
- Kikugawa K., Suehiro H., Yanase R., Aoki A. Platelet aggregation inhibitors. IX. Chemical transformation of adenosine into 2-thioadenosine derivatives. Chem Pharm Bull (Tokyo) 1977 Aug;25(8):1959–1969. doi: 10.1248/cpb.25.1959. [DOI] [PubMed] [Google Scholar]
- Knorre D. G., Vlassov V. V. Complementary-addressed (sequence-specific) modification of nucleic acids. Prog Nucleic Acid Res Mol Biol. 1985;32:291–320. doi: 10.1016/s0079-6603(08)60352-9. [DOI] [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Doan T., Perrouault L., Helene C., Chassignol M., Thuong N. T. Targeted cleavage of polynucleotides by complementary oligonucleotides covalently linked to iron-porphyrins. Biochemistry. 1986 Nov 4;25(22):6736–6739. doi: 10.1021/bi00370a002. [DOI] [PubMed] [Google Scholar]
- Li Y., Zon G., Wilson W. D. NMR and molecular modeling evidence for a G.A mismatch base pair in a purine-rich DNA duplex. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):26–30. doi: 10.1073/pnas.88.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. H., Beale J. M., Hurley L. H. Structure of the (+)-CC-1065-DNA adduct: critical role of ordered water molecules and implications for involvement of phosphate catalysis in the covalent reaction. Biochemistry. 1991 Apr 16;30(15):3597–3602. doi: 10.1021/bi00229a002. [DOI] [PubMed] [Google Scholar]
- Lin C. H., Hurley L. H. Determination of the major tautomeric form of the covalently modified adenine in the (+)-CC-1065-DNA adduct by 1H and 15N NMR studies. Biochemistry. 1990 Oct 16;29(41):9503–9507. doi: 10.1021/bi00493a002. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P. DNA mismatch correction. Annu Rev Biochem. 1987;56:435–466. doi: 10.1146/annurev.bi.56.070187.002251. [DOI] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Murata T., Iwai S., Ohtsuka E. Synthesis and characterization of a substrate for T4 endonuclease V containing a phosphorodithioate linkage at the thymine dimer site. Nucleic Acids Res. 1990 Dec 25;18(24):7279–7286. doi: 10.1093/nar/18.24.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrouault L., Asseline U., Rivalle C., Thuong N. T., Bisagni E., Giovannangeli C., Le Doan T., Hélène C. Sequence-specific artificial photo-induced endonucleases based on triple helix-forming oligonucleotides. Nature. 1990 Mar 22;344(6264):358–360. doi: 10.1038/344358a0. [DOI] [PubMed] [Google Scholar]
- Saneyoshi M., Nishimura S., Okabe M., Fukuoka F. Synthetic nucleosides and nucleotides. XIV. Facile synthesis and antitumor activities of 6-mercaptopurine 2'-deoxyriboside and related compounds. J Pharmacobiodyn. 1980 Feb;3(2):105–110. doi: 10.1248/bpb1978.3.105. [DOI] [PubMed] [Google Scholar]
- Schmidt F. J., Bock R. M., Hecht S. M. Chemical modifications of transfer rna species. Heavy atom derivatization of aminoacyl tRNA. Biochem Biophys Res Commun. 1972 Jul 25;48(2):451–456. doi: 10.1016/s0006-291x(72)80072-x. [DOI] [PubMed] [Google Scholar]
- Schwartz W. E., Smith P. K., Royer G. P. N-(beta-Iodoethyl)trifluoroacetamide: a new reagent for the aminoethylation of thio groups in proteins. Anal Biochem. 1980 Jul 15;106(1):43–48. doi: 10.1016/0003-2697(80)90116-5. [DOI] [PubMed] [Google Scholar]
- Vlassov V. V., Gaidamakov S. A., Gorn V. V., Grachev S. A. Sequence-specific chemical modification of a 365-nucleotide-long DNA fragment with an alkylating oligonucleotide derivative. FEBS Lett. 1985 Mar 25;182(2):415–418. doi: 10.1016/0014-5793(85)80345-8. [DOI] [PubMed] [Google Scholar]
- Webb T. R., Matteucci M. D. Hybridization triggered cross-linking of deoxyoligonucleotides. Nucleic Acids Res. 1986 Oct 10;14(19):7661–7674. doi: 10.1093/nar/14.19.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]