Abstract
Nitric oxide (NO) physiologically regulates numerous cellular responses through S-nitrosylation of protein cysteine residues. We performed antibody-array screening in conjunction with biotin-switch assays to look for S-nitrosylated proteins. Using this combination of techniques, we found that phosphatase with sequence homology to tensin (PTEN) is selectively S-nitrosylated by low concentrations of NO at a specific cysteine residue (Cys-83). S-nitrosylation of PTEN (forming SNO-PTEN) inhibits enzymatic activity and consequently stimulates the downstream Akt cascade, indicating that Cys-83 is a critical site for redox regulation of PTEN function. In ischemic mouse brain, we observed SNO-PTEN in the core and penumbra regions but found SNO-Akt, which is known to inhibit Akt activity, only in the ischemic core. These findings suggest that low concentrations of NO, as found in the penumbra, preferentially S-nitrosylate PTEN, whereas higher concentrations of NO, known to exist in the ischemic core, also S-nitrosylate Akt. In the penumbra, inhibition of PTEN (but not Akt) activity by S-nitrosylation would be expected to contribute to cell survival by means of enhanced Akt signaling. In contrast, in the ischemic core, SNO-Akt formation would inhibit this neuroprotective pathway. In vitro model systems support this notion. Thus, we identify unique sites of PTEN and Akt regulation by means of S-nitrosylation, resulting in an “on–off” pattern of control of Akt signaling.
Keywords: apoptosis, ischemia, oxidation
Nitric oxide (NO) exerts pleiotropic cellular responses on proliferation, apoptosis, neurotransmission, and neurotoxicity in several types of cells by means of protein S-nitrosylation. This modification occurs by means of oxidative reaction between NO and cysteine (Cys) thiol in the presence of an electron acceptor (such as O2 or a transition metal) or through transnitrosylation from S-nitrosothiol to another Cys thiol (1–3). Several methods have been published to detect S-nitrosylated proteins (SNO-Ps) by using antibodies, photolysis, and mercury affinity (4). In particular, the biotin-switch assay is a modified immunoblot developed by Jaffrey and Snyder that has been commonly used to detect endogenous SNO-Ps; this method has greatly advanced the field (5). Subsequently, other methods have been developed to detect SNO-Ps (6), but some of them involve samples treated with high concentrations of NO donor. In the presence of high concentrations of NO, however, it is possible that some Cys residues are artifactually S-nitrosylated.
Antibody arrays have been used to profile protein expression levels with high sensitivity. Each spotted antibody can be validated for its ability to bind proteins in the assay. Samples hybridizing to each antibody on the array can be easily detected. Although a number of proteins have been identified as substrates for S-nitrosylation in the past several years (3–6), we hypothesized that many more candidates modified by physiological levels of NO might still remain to be identified. We therefore tested whether an antibody array might be adapted for identification of additional SNO-Ps and their (patho)physiological functions.
In the present study, we attempted to isolate SNO-Ps in physiological condition by an antibody array. We prepared extracts from cells treated or untreated with NO and specifically labeled with biotin. We found that phosphatase with sequence homology to tensin (PTEN) is preferentially S-nitrosylated by low concentrations of NO. Although other reports have shown that PTEN can undergo S-nitrosylation by high concentrations of NO (7–13), here we found a significance of the (patho)physiological function of S-nitrosylated PTEN (SNO-PTEN) on the Akt pathway using in vivo and in vitro systems. Our results suggest that inhibition of PTEN activity through S-nitrosylation augments Akt signaling, thereby contributing to cell survival in ischemic brains and activation of endothelial NO synthase (eNOS).
Results
Screening for S-Nitrosylated Neural Proteins.
Initially, we developed a unique screening system for isolating previously undescribed SNO-Ps in neuronal systems using an antibody array. Samples were prepared from human neuroblastoma SH-SY5Y cells that had been exposed to calcium ionophore A23187, which activates endogenous neuronal NO synthase (nNOS) and eNOS to produce physiological concentrations of NO. S-nitrosylated Cys residues in cell lysates were converted to their biotinylated form by using the biotin-switch technique (5). These samples, containing biotinylated cysteines, were subjected to antibody array, and fluorescent intensities were detected (Fig. S1). Twenty-five candidates were identified, including known SNO-Ps such as caspase, NOS, and HDAC (Table S1; refs. 14–16). Among the other candidates, we focused on the effect of S-nitrosylation on PTEN activity and its physiological functions. PTEN is an inhibitory regulator of the PI3-kinase/Akt signaling pathway, thereby attenuating cell growth, migration, and survival (17–21).
S-Nitrosylation of PTEN.
To validate S-nitrosylation of PTEN, we examined whether PTEN was significantly S-nitrosylated by a low concentration of the physiological NO donor S-nitrosocysteine (SNOC). SNOC markedly enhanced the level of SNO-PTEN in cell lysates and intact cells. SNO-PTEN was not detected in control biotin-switch assays performed without ascorbate to remove NO, thus preventing replacement of NO by biotin (Fig. 1A). As expected, PTEN was highly sensitive to NO; SNO-PTEN formation was detected in human embryonic kidney (HEK) 293 cells after treatment with 1–10 μM SNOC (Fig. 1 B and C). Furthermore, this modification was also found in cells exposed to various other types of NO donors, including S-nitroso-glutathione (GSNO) and 2-(N,N-diethylamino)-diazenolate-2-oxide (DETA-NONOate; Fig. S2).
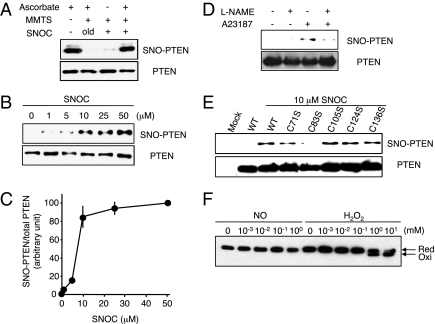
Fig. 1.
S-nitrosylation of PTEN in vitro and in vivo. (A, Upper) Cell lysates from HEK293T cells were incubated with 50 μM SNOC or control solution for 20 min, followed by assay for SNO-PTEN using the biotin-switch assay. Control samples were subjected to decayed (old) SNOC. (Lower) Total PTEN in cell lysates was detected by Western blot. (B, Upper) HEK293T cells were exposed to varying concentrations of SNOC for 20 min, followed by assay for SNO-PTEN. (Lower) Total PTEN. (C) Biotin-switch assay and Western analysis were quantified by densitometry; the relative ratio of SNO-PTEN to total PTEN was calculated for each sample. Values are means ± SEM, n = 3. (D, Upper) HEK cells expressing nNOS were assayed for endogenous SNO-PTEN. nNOS was activated by Ca2+ ionophore A23187 (5 μM) in the presence or absence of NOS inhibitor (NG-Nitro-l-arginine methyl ester, l-NAME, 1 mM). (Lower) Total PTEN. (E, Upper) S-nitrosylation of Cys-83 in PTEN. HEK cell lysates transduced with WT or C-to-S mutant FLAG-PTEN proteins were exposed to 10 μM SNOC or control for 20 min. SNO-PTEN was detected by biotin-switch assay using anti-FLAG antibody. Mutation of a critical cysteine thiol group in the phosphatase domain of PTEN(C83S) prevented S-nitrosylation by SNOC. (Lower) Total PTEN. (F) Effect of NO on oxidation of PTEN. HEK293 cells were incubated with the indicated concentration of SNOC or H2O2 for 20 min. Cell protein extracts were prepared in lysis buffer (pH 6.8) containing 40 mM N-ethylmaleimide and fractionated by nonreducing SDS/PAGE followed by Western analysis with anti-PTEN antibody.
Next, to investigate whether endogenously generated NO also induces SNO-PTEN formation, we used HEK cells stably expressing nNOS. PTEN was S-nitrosylated by endogenous NO in response to A23187 in a NOS-inhibitor–sensitive manner (Fig. 1D and Fig. S3). Mammalian PTEN has five cysteines in its phosphatase domain (17). To determine the target site of S-nitrosylation on PTEN, we mutated each cysteine to serine and assayed for SNO-PTEN formation using the biotin-switch method. HEK cells were transfected with expression vectors encoding either wild-type (WT) FLAG-PTEN or mutant forms of the protein. After 24 h, cells were exposed to SNOC or control conditions and monitored for SNO-PTEN. We found that C83S mutant PTEN produced almost no signal, suggesting that Cys-83 was the predominant S-nitrosylation site (Fig. 1E). Furthermore, we performed a chemical assay on purified recombinant PTEN to detect S-nitrosylation using 2,3-diaminonaphthalene (DAN). DAN stoichiometrically converts to fluorescent 2,3-naphthyltriazole in the presence of NO released from S-nitrosothiol. SNOC-treated PTEN resulted in significant SNO-P formation, whereas the PTEN(C83S) mutant was completely devoid of fluorescent signal (Fig. S3).
PTEN is known to be oxidized by high concentrations of H2O2 (>0.5 mM), which result in disulfide bond formation between Cys-71 and active site Cys-124 (22). Thus, we tested whether NO also induced disulfide bond formation in PTEN. However, even high concentrations of NO did not result in disulfide formation (Fig. 1F), consistent with the notion that S-nitrosylation of PTEN occurred solely at Cys-83. Thus, disparate Cys residues appear to be involved in S-nitrosylation and H2O2-mediated oxidation of PTEN.
SNO-PTEN Inhibits Phosphatase Activity Through C83.
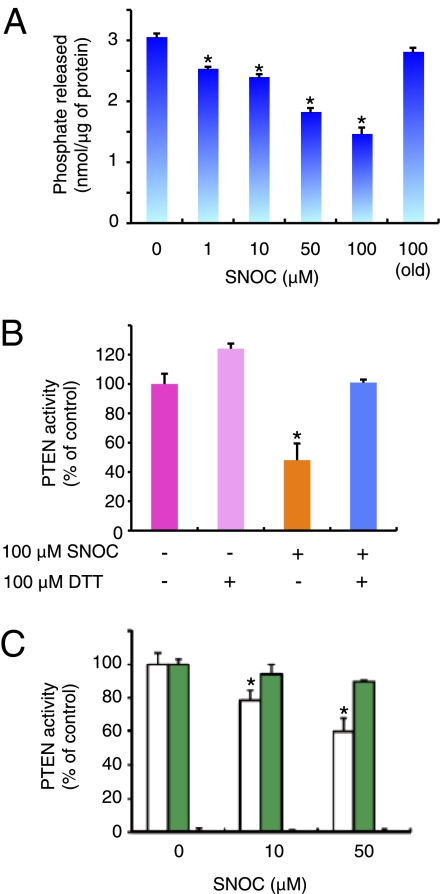
To determine whether S-nitrosylation affected PTEN activity, we initially monitored recombinant PTEN enzyme activity. Phosphatase activity was evaluated with a standard assay by measuring phosphate released from phosphatidylinositol-3,4,5-trisphosphate [PI(3,4,5)P3], a physiological substrate (23). Exposure of recombinant PTEN to SNOC significantly decreased the level of phosphate in a dose-dependent manner (Fig. 2A). This decline was reversed to basal levels after incubation with the chemical-reducing agent DTT, indicating that S-nitrosylation in PTEN was reversible (Fig. 2B). Next, we assessed the enzymatic activity of WT recombinant PTEN and Cys mutants. Cys-124 is essential for PTEN activity; thus, even in the absence of SNOC exposure, the C124S mutant completely lost its enzyme activity (Fig. 2C). In contrast, the C83S mutant maintained its enzymatic activity even after exposure to high concentrations of SNOC (Fig. 2C). Therefore, we made the observation that not only is Cys-83 the principal target site for PTEN oxidation by S-nitrosylation, but that this modification influences enzymatic activity by a mechanism distinct from that of oxidation by H2O2.
Fig. 2.
S-nitrosylation of PTEN regulates its phosphatase activity. (A and B) Effect of S-nitrosylation on PTEN phosphatase activity. (A) In vitro expressed GST-fused PTEN was incubated with the indicated concentrations of SNOC and evaluated by phosphatase assay. (B) Recombinant PTEN and SNO-PTEN were assayed for lipid phosphatase activity against PI(3,4,5)P3 with or without DTT. Release of phosphate was detected colorimetrically with Biomol green reagent. Values are means ± SEM, n = 5; *P < 0.01 by ANOVA. (C) GST-fused WT-PTEN (white), PTEN(C83S) (green), and dominant-negative PTEN(C124S) (black) were expressed and purified from bacteria, exposed to SNOC, and assayed for phosphatase activity. Values, expressed as percentage of WT in the absence of SNOC, are means ± SEM, n = 5; *P < 0.01 by ANOVA.
Low Concentrations of NO Inhibit PTEN to Increase Akt Activity.
Because PTEN phosphatase activity negatively regulates the PI3-kinase/Akt signaling cascade and acts upstream of Akt (17–19), we speculated that PTEN inhibition induced by S-nitrosylation might activate the Akt pathway. Therefore, we first investigated how various concentrations of SNOC affected Akt activity and its downstream cascade. We found that a relatively low concentration of SNOC (10 μM) markedly increased the level of phosphorylated Akt (pAkt; at Thr-308 and Ser-473) in a time-dependent fashion (Fig. 3 A and B). In contrast, high SNOC concentrations (e.g., 250 μM) did not result in increased pAkt (Fig. 3A), even though SNO-PTEN formation was still evident at >50 μM SNOC (Fig. 1B). Thus, under these conditions the increase in pAkt levels appeared only after exposure to low concentrations (10 μM) of SNOC and not after high concentrations (250 μM). We next monitored Akt activity in cells in response to various concentrations of SNOC. Low concentrations of SNOC (≤10 μM) enhanced, whereas high concentrations (≥250 μM) attenuated, substrate phosphorylation by Akt (peNOS at Ser-1177; Fig. 3 C and D).
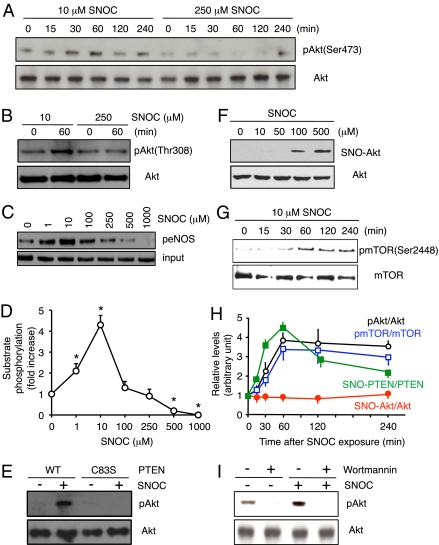
Fig. 3.
S-nitrosylation of PTEN by low concentrations of SNOC activates the Akt pathway. (A and B) HEK293T cells were exposed to 10 or 250 μM SNOC for the indicated period. pAkt (Upper) and total Akt (Lower) were detected by Western analysis with anti-pAkt(Ser-473), anti-pAkt(Thr-308), and anti-Akt antibodies. (C and D) Akt kinase activity was enhanced by low and suppressed by high concentrations of SNOC. Kinase activity was monitored in F2 cells 30 min after exposure to SNOC by assessing phosphorylation of substrate protein eNOS using peNOS(Ser-1177) antibody. Values are means ± SEM, n = 4; *P < 0.01 by ANOVA. (E) WT and PTEN(C83S) mutant-expressing HEK cells were treated with 10 μM SNOC for 1 h, and phosphorylation of Akt (Thr-308) was assessed by Western blot. (F) Low concentrations of SNOC did not produce SNO-Akt. (Upper) F2 cells were incubated with the indicated concentration of SNOC and SNO-Akt detected by biotin-switch assay. (Lower) Total Akt. (G) SNOC stimulates phosphorylation of the Akt substrate mTOR. HEK293T cells were treated with 10 μM SNOC for the indicated periods of time. pmTOR (at Ser-2448) and Akt were detected by Western analysis. (H) Ratio of SNO-PTEN/PTEN, SNO-Akt/Akt, pAkt/Akt, and pmTOR/mTOR levels after exposure to 10 μM SNOC. Relative intensity was quantified by using NIH Image software. Values are mean ± SEM, n = 4–7. (I) HEK293T cells were pretreated with 10 nM wortmannin for 10 min and then stimulated with 10 μM SNOC for 2 h. pAkt and total Akt were monitored as in A.
Next, we expressed PTEN(C83S), the nonnitrosylatable mutant of PTEN, in cells to further confirm that attenuation of PTEN phosphatase activity by NO is involved in Akt activation. After transfection with PTEN(C83S), low concentrations of SNOC no longer enhanced pAkt levels, consistent with the notion that inhibition of WT PTEN enzymatic activity that we observed after S-nitrosylation leads to stimulation of a downstream cascade involving Akt (Fig. 3E). In contrast, we observed that after exposure to high concentrations of an NO donor (≥100 μM), not only PTEN but also Akt was S-nitrosylated, and thus Akt was directly inhibited (Fig. 3F), which is consistent with prior reports (24–26).
We then determined whether SNO-PTEN was formed after exposure to endogenous NO in nNOS- and eNOS-expressing cells. Treatment with A23187 to increase Ca2+, and thus stimulate NOS, resulted in formation of SNO-PTEN, but not SNO-Akt, in both cell types. As a measure of NO generated, the amount of nitrite produced by nNOS or eNOS cells was approximately the same as that generated by 10–30 μM SNOC (Fig. S4). These findings showed that S-nitrosylation of PTEN is independent of NOS isoform and that it occurred in the presence of low (physiological) levels of NO (Fig. S4). In contrast, exposing primary cortical neurons to neurotoxic concentrations of NMDA (≥200 μM), which is known to generate high levels of NO, resulted in the formation of both SNO-PTEN and SNO-Akt (Fig. S5). From these results, we conclude that S-nitrosylation of PTEN occurred with physiological levels of NO derived from eNOS or nNOS, while Akt was also nitrosylated in the presence of pathological levels of NO generated by neurotoxic concentrations of NMDA. Moreover, our findings suggest that low (≤10 μM) concentrations of SNOC, although nitrosylating PTEN, could not S-nitrosylate Akt; this modification led to PTEN inhibition and consequently Akt phosphorylation/activation. Thus, the sensitivity of PTEN to NO was higher than that of Akt under physiological conditions in our experiments.
Formation of SNO-PTEN Results in Phosphorylation of Akt Substrates.
We next asked whether activation of Akt after exposure of cells to low concentrations of SNOC led to phosphorylation of Akt substrates. Akt activity was assessed by measuring the level of phosphorylated mTOR (pmTOR; at Ser-2448), an Akt substrate (27). Levels of pmTOR were markedly increased after exposure to 10 μM SNOC, after a time course that paralleled Akt phosphorylation (Fig. 3 G and H). These functional observations were also consistent with the notion that low NO concentrations (≤10 μM) induced formation of SNO-PTEN, but not SNO-Akt, and consequently activated the Akt signaling cascade. To further substantiate our conclusion that activation of Akt after exposure to low concentrations of NO is dependent on S-nitrosylation and resultant inhibition of PTEN, we investigated the effect of wortmannin, a PI3-kinase inhibitor, on NO-induced Akt activation. Treatment with wortmannin resulted in significant attenuation of pAkt formation in response to low concentration of SNOC (Fig. 3I). This observation is consistent with the notion that NO stimulates Akt signaling through formation of SNO-PTEN with resultant inhibition of PTEN activity because this increase in pAkt would occur by means of increased PI3-kinase activity.
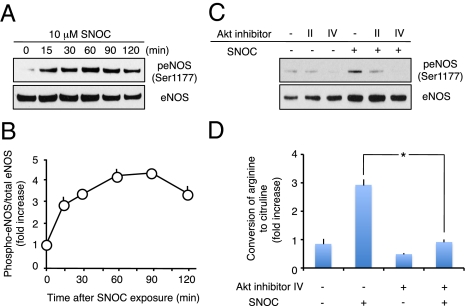
Additionally, it had been shown that eNOS is activated by Akt-dependent phosphorylation (28, 29). We therefore asked whether Akt activation after SNO-PTEN formation led to eNOS phosphorylation/activation in endothelial cells. We found that the level of phosphorylated eNOS (peNOS) protein rapidly increased and was sustained for up to 2 h after exposure to 10 μM SNOC in the mouse F2 endothelial cell line (Fig. 4 A and B). This SNOC-induced eNOS phosphorylation (at Ser-1177) was abrogated by Akt inhibitors (Fig. 4C; refs. 30 and 31). Next, we monitored eNOS activity by measuring the conversion of [3H]arginine to [3H]citrulline (32). We found that SNOC significantly augmented the formation of citrulline from arginine in an Akt inhibitor-sensitive manner (Fig. 4D). These findings are consistent with the notion that enhanced Akt activity, resulting from PTEN nitrosylation, leads to eNOS phosphorylation and activation.
Fig. 4.
S-nitrosylation of PTEN promotes eNOS phosphorylation by means of Akt activation. (A) Human endothelial F2 cells were exposed to 10 μM SNOC for the indicated periods of time. peNOS (at Ser-1177; Upper) and total eNOS (Lower) were detected by Western analysis. (B) Ratio of increased peNOS levels. Intensity level was quantified from blots by using NIH Image software. Values are means ± SEM, n = 3; *P < 0.01 by ANOVA. (C) Akt inhibitors block phosphorylation of eNOS stimulated by exposure to SNOC. Cultures were treated with 10 μM Akt inhibitor II or IV (or control) for 30 min and then incubated with 10 μM SNOC or control solution. The levels of peNOS and eNOS were examined 30 min later by Western analysis. (D) eNOS activity in protein homogenates was measured with a citrulline assay. Values are means ± SEM, n = 5; *P < 0.01 by ANOVA.
SNO-PTEN Inhibits Neuronal Apoptosis.
To study the effect of SNO-PTEN on apoptotic cell death, we initially used an in vitro model system. For this purpose, we exposed human neural SH-SY5Y cells to staurosporine while overexpressing either WT or C83S mutant PTEN (Fig. S6). After treating the cultures with a low concentration of SNOC to activate the Akt pathway by means of SNO-PTEN formation, we found that apoptosis was significantly attenuated in WT, but not NO-insensitive C83S-PTEN mutant-expressing cells. This finding is in accord with the fact that Akt is known to be important in cell survival signaling and that it ameliorates apoptosis (33, 34).
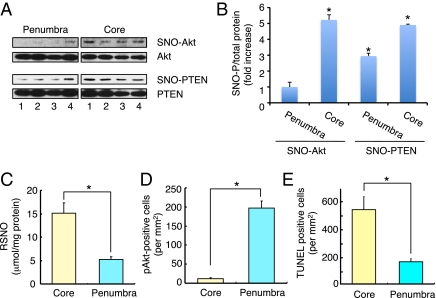
Next, we studied the effect of SNO-PTEN formation in vivo in a rodent model of cerebral ischemia (stroke). In ischemic brain, the generation of NO contributes to neuronal cell death, and NO production is mediated, at least in part, by excessive stimulation of NMDA-type glutamate receptors (35, 36). Within 24 h of focal cerebral ischemia induced by middle cerebral artery occlusion (MCAO), cell death occurs by necrotic and apoptotic mechanisms (37). We surmised that the difference observed between the ischemic core (subcortex, including striatum) and penumbra (cortex) might be partly dependent on Akt/PTEN inactivation through S-nitrosylation. Although SNO-PTEN was observed in both the ischemic core and penumbral regions, SNO-Akt formation was detected only in the ischemic core and not in the penumbra (Fig. 5A). To compare semiquantitatively SNO-P levels in the core and penumbral regions of ischemic brains, we analyzed our blots by densitometry to calculate the ratio of SNO-PTEN or -Akt (determined by biotin-switch assay) to total PTEN or Akt (by Western blot; Fig. 5B). We found that the ratios of both SNO-PTEN/total PTEN and SNO-Akt/total Akt were significantly enhanced in both the ischemic core and penumbra, but only SNO-PTEN/PTEN was elevated in the penumbra. In line with these data, we also found that the levels of nitrosothiol in the core were higher than in the penumbra, as estimated by the Saville reaction on tissue samples (Fig. 5C). Additionally, we precipitated and then separated on SDS/PAGE biotinylated proteins (representing proteins that had been S-nitrosylated and subsequently labeled by the biotin-switch method) followed by silver staining to estimate the number and intensity of total SNO-Ps in the ischemic core and penumbra. The number of SNO-Ps in the ischemic core was distinctly increased compared with the penumbral region (Fig. S7). These results reveal that SNO-P formation in vivo depends upon the concentration of NO in the ischemic areas of the brains.
Fig. 5.
S-nitrosylation of PTEN promotes neuroprotection through Akt activation in vitro and in vivo. (A) S-nitrosylation of PTEN and Akt after cerebral ischemia in mice. Brain tissue from infarcted hemispheres was harvested 24 h after a 2-h MCAO and subjected to biotin-switch assay to detect in vivo S-nitrosylation of PTEN and Akt. (B) Ratio of increased SNO-P to total protein. Blots from biotin-switch assays and Western analyses were quantified by densitometry, and the relative ratio of SNO-PTEN to total PTEN or SNO-Akt to total Akt in both hemispheres was calculated. Values are means ± SEM, n = 4; *P < 0.01 by ANOVA. (C) NO levels, as reflected by total nitrosothiol (RSNO) in ischemic brain tissue, were measured by the Saville reaction. A significant increase in RSNO was found in both the ischemic core and penumbra. Values are means ± SEM, n = 7; *P < 0.05 by ANOVA. (D) Number of pAkt-positive cells in the core and penumbra of ischemic brain. Values are means ± SEM, n = 4; *P < 0.05 by ANOVA. (E) Number of TUNEL-positive apoptotic neurons in the core and penumbra of ischemic brain. Values are means ± SEM, n = 4; *P < 0.05 by ANOVA.
Our finding of enhanced levels of SNO-PTEN, but not SNO-Akt, in the penumbra led us to look for phosphorylation/activation of Akt in this region to determine whether it was indeed phosphorylated after ischemia, as would be predicted. In fact, we found that the predominant location of pAkt-positive cells was in the penumbra and not in the core. Moreover, we found that the pAkt-positive cells mostly coincided with PTEN signal in the penumbral region. In addition under our conditions, we detected fewer TUNEL-positive cells, representing apoptotic neurons, in the penumbra region than in the core region (Fig. 5 D and E and Fig. S8). These findings are consistent with the notion that in the face of low concentrations of NO in the ischemic penumbra, neuroprotection may be enhanced by Akt activation through SNO-PTEN. Hence, the ischemic model provides in vivo support for the dependence of the positive and negative regulatory system of Akt signaling on NO concentration.
Discussion
Protein Cys thiols undergo a range of reactive nitrogen species (RNS)-dependent or reactive oxygen species (ROS)-dependent electrophilic and oxidative modifications. Through these reactions, nitrosative and oxidative stress affect the physiological function of proteins (38–41). Reversible modifications are associated with homeostatic maintenance by means of cellular redox state, but excessive amounts of RNS/ROS can elicit irreversible protein dysfunction. Here, we show that PTEN is highly sensitive to relative low concentrations of NO and that its enzymatic activity is inhibited by the resulting S-nitrosylation of Cys-83. In comparison, high H2O2 concentrations result in the oxidation of Cys residues on PTEN and formation of a disulfide bond between Cys-78 and -124. Heretofore, it has not been reported that distinct Cys residues react with NO and H2O2. Clues for the underlying mechanisms for these disparate reactions may lie in the 3D structure of PTEN. The atomic structure of PTEN reveals that Cys-83 is located between the pα2 and pβ4 regions (42). Asp-77, located proximal to the pα2 region, and Glu-114, located distal to the pα3 region, are both situated in the vicinity of Cys-83 in the 3D structure, showing that Cys-83 is surrounded by a motif favoring nitrosylation (43).
In the present study, we demonstrate that Cys-83 is a direct target of NO, indicating that the modification site and mode of oxidation caused by NO completely differs from H2O2. In contrast to Cys-83, Cys-124 is located in the enzymatic active site of PTEN and forms a disulfide bond with Cys-71 after exposure to high concentrations of H2O2 (22).
Because S-nitrosylation of PTEN inhibits its enzymatic activity, we also found that low concentrations of NO result in less dephosphorylation on Akt and thus increased Akt activity. In contrast to S-nitrosylation of PTEN, SNO-Akt formation results in inhibition of Akt activity (24–26). However, in the present study we show that higher concentrations of NO are necessary to S-nitrosylate Akt than PTEN. Thus, in the presence of low (physiological) concentrations of NO, SNO-PTEN formation would enhance Akt signaling activity, whereas high (pathological) levels of NO would S-nitrosylate Akt to inhibit its function directly or might act on an unknown upstream target to attenuate Akt phosphorylation. Transnitrosylation from one SNO-P to another has recently been demonstrated for several proteins (1–3, 44), so it is possible that under some circumstances SNO-Akt could transfer NO to PTEN because PTEN is a better NO acceptor than Akt. However, for the same reason, it is unlikely that the converse is true—i.e., that Akt activity is attenuated by transnitrosylation derived from SNO-PTEN at physiological concentrations of NO.
Additionally, we explored possible pathophysiological roles of S-nitrosylation of PTEN and Akt in vitro and in vivo. Interestingly, we found that SNO-PTEN is detected in both the core and penumbral regions of a stroke, whereas SNO-Akt is only found in the core region. Although it has been reported that PTEN can react with NO (7–13), the pathophysiological consequences of this reaction have not yet been fully elucidated. Recently, Pei et al. (11) reported that formation of SNO-PTEN occurred during brain ischemia, but the effect of S-nitrosylation on Akt signaling was not determined. Based on our findings, we speculate that a possible contributing factor to rescue of the penumbral region in the ischemic brain is that the lower levels of NO found in the penumbra result in S-nitrosylation of PTEN rather than Akt. Thus, from our findings, the penumbra, where SNO-PTEN is present in the absence of SNO-Akt, would be expected to have increased Akt neuroprotective signaling activity compared with the core of the infarct where Akt is also S-nitrosylated because of higher concentrations of NO.
In summary, although there have been other reports that oxidation/S-nitrosylation suppresses PTEN activity (22, 45), our results demonstrate the unique finding that high concentrations of NO affect not only PTEN but also downstream Akt, which thus inhibits Akt signaling and hinders cell survival in the ischemic core. Our work shows the need to characterize the sensitivity to NO of several proteins in a signaling cascade in order to determine the net effect of S-nitrosylation events (46).
In conclusion, our findings provide mechanistic insight into S-nitrosylation of PTEN, showing that PTEN is negatively regulated by lower NO concentrations than Akt and suggesting that NO could be the physiological oxidative modulator of PTEN rather than high concentrations of H2O2. Moreover, the fact that high concentrations of NO directly inhibit Akt activity through S-nitrosylation suggests the presence of a previously undescribed on–off regulatory system for Akt signaling depending on NO concentration, with low concentrations of NO activating Akt through formation of SNO-PTEN, whereas high concentrations of NO directly deactivate Akt signaling.
Materials and Methods
Materials, biotin-switch assay, screen to detect SNO-P, fluorometric detection of S-nitrosothiols, PTEN activity, assay for eNOS activity, PTEN oxidation, transient focal cerebral ischemia, colorimetric detection of NO2, accumulation in brain tissue, double-immunostaining, TUNEL staining, cell counting, and cell death assay are described in SI Materials and Methods.
Chemicals and Antibodies.
N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio)propionamide was purchased from Pierce Chemical. Akt inhibitors were from Calbiochem. Anti-PTEN, anti-phospho Akt(Thr-308), anti-phospho Akt (Ser-473), anti-Akt, anti-phospho mTOR(Ser-2448), anti-mTOR, antiphospho eNOS(Ser-1177), and anti-eNOS antibodies were obtained from Cell Signaling Technology. All other reagents were from Sigma-Aldrich.
Supplementary Material
Acknowledgments
We thank Dr. Akio Matsumoto for advice; and Akane Katoh, Kana Hyakkoku, and Junya Hamanaka for excellent technical assistance. This work was supported in part by the Japan Ministry of Education, Culture, Sports and Technology (MEXT) Grants-in-Aid for Scientific Research on Innovative Areas 20117011, Scientific Research B 22310039, and Challenging Exploratory Research 21659013; the Urakami Foundation (T.U.); and National Institutes of Health Grants P01 HD29587, P01 ES016738, P30 NS057096, R01 EY09024, and R01 EY05477 (to S.A.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1103503108/-/DCSupplemental.
References
- 1.Foster MW, Hess DT, Stamler JS. Protein S-nitrosylation in health and disease: A current perspective. Trends Mol Med. 2009;15:391–404. doi: 10.1016/j.molmed.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu L, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 3.Foster MW, Forrester MT, Stamler JS. A protein microarray-based analysis of S-nitrosylation. Proc Natl Acad Sci USA. 2009;106:18948–18953. doi: 10.1073/pnas.0900729106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doulias PT, et al. Structural profiling of endogenous S-nitrosocysteine residues reveals unique features that accommodate diverse mechanisms for protein S-nitrosylation. Proc Natl Acad Sci USA. 2010;107:16958–16963. doi: 10.1073/pnas.1008036107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein S-nitrosylation: A physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3:193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 6.Hao G, Derakhshan B, Shi L, Campagne F, Gross SS. SNOSID, a proteomic method for identification of cysteine S-nitrosylation sites in complex protein mixtures. Proc Natl Acad Sci USA. 2006;103:1012–1017. doi: 10.1073/pnas.0508412103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu CX, Li S, Whorton AR. Redox regulation of PTEN by S-nitrosothiols. Mol Pharmacol. 2005;68:847–854. doi: 10.1124/mol.104.010504. [DOI] [PubMed] [Google Scholar]
- 8.Lim S, Clément MV. Phosphorylation of the survival kinase Akt by superoxide is dependent on an ascorbate-reversible oxidation of PTEN. Free Radic Biol Med. 2007;42:1178–1192. doi: 10.1016/j.freeradbiomed.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Carver DJ, Gaston B, Deronde K, Palmer LA. Akt-mediated activation of HIF-1 in pulmonary vascular endothelial cells by S-nitrosoglutathione. Am J Respir Cell Mol Biol. 2007;37:255–263. doi: 10.1165/rcmb.2006-0289SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Floyd RA, et al. Nitric oxide and cancer development. J Toxicol Pathol. 2007;20:77–92. [Google Scholar]
- 11.Pei DS, Sun YF, Song YJ. S-nitrosylation of PTEN Invovled in ischemic brain injury in rat hippocampal CA1 region. Neurochem Res. 2009;34:1507–1512. doi: 10.1007/s11064-009-9938-3. [DOI] [PubMed] [Google Scholar]
- 12.Sun J, Murphy E. Protein S-nitrosylation and cardioprotection. Circ Res. 2010;106:285–296. doi: 10.1161/CIRCRESAHA.109.209452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwak YD, et al. NO signaling and S-nitrosylation regulate PTEN inhibition in neurodegeneration. Mol Neurodegener. 2010;5:49. doi: 10.1186/1750-1326-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mannick JB, et al. Fas-induced caspase denitrosylation. Science. 1999;284:651–654. doi: 10.1126/science.284.5414.651. [DOI] [PubMed] [Google Scholar]
- 15.Ravi K, Brennan LA, Levic S, Ross PA, Black SM. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci USA. 2004;101:2619–2624. doi: 10.1073/pnas.0300464101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nott A, Watson PM, Robinson JD, Crepaldi L, Riccio A. S-Nitrosylation of histone deacetylase 2 induces chromatin remodelling in neurons. Nature. 2008;455:411–415. doi: 10.1038/nature07238. [DOI] [PubMed] [Google Scholar]
- 17.Li J, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 18.Tamura M, et al. Inhibition of cell migration, spreading, and focal adhesions by tumor suppressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 19.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: Of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 20.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 21.Stambolic V, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 22.Lee SR, et al. Reversible inactivation of the tumor suppressor PTEN by H2O2. J Biol Chem. 2002;277:20336–20342. doi: 10.1074/jbc.M111899200. [DOI] [PubMed] [Google Scholar]
- 23.Okumura K, et al. PCAF modulates PTEN activity. J Biol Chem. 2006;281:26562–26568. doi: 10.1074/jbc.M605391200. [DOI] [PubMed] [Google Scholar]
- 24.Yasukawa T, et al. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem. 2005;280:7511–7518. doi: 10.1074/jbc.M411871200. [DOI] [PubMed] [Google Scholar]
- 25.Wu M, et al. Aging-associated dysfunction of Akt/protein kinase B: S-nitrosylation and acetaminophen intervention. PLoS ONE. 2009;4:e6430. doi: 10.1371/journal.pone.0006430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carvalho-Filho MA, et al. Aspirin attenuates insulin resistance in muscle of diet-induced obese rats by inhibiting inducible nitric oxide synthase production and S-nitrosylation of IRbeta/IRS-1 and Akt. Diabetologia. 2009;52:2425–2434. doi: 10.1007/s00125-009-1498-1. [DOI] [PubMed] [Google Scholar]
- 27.Navé BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR. Mammalian target of rapamycin is a direct target for protein kinase B: Identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J. 1999;344:427–431. [PMC free article] [PubMed] [Google Scholar]
- 28.Fulton D, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimmeler S, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 30.Kau TR, et al. A chemical genetic screen identifies inhibitors of regulated nuclear export of a Forkhead transcription factor in PTEN-deficient tumor cells. Cancer Cell. 2003;4:463–476. doi: 10.1016/s1535-6108(03)00303-9. [DOI] [PubMed] [Google Scholar]
- 31.Gills JJ, et al. Phosphatidylinositol ether lipid analogues that inhibit AKT also independently activate the stress kinase, p38alpha, through MKK3/6-independent and -dependent mechanisms. J Biol Chem. 2007;282:27020–27029. doi: 10.1074/jbc.M701108200. [DOI] [PubMed] [Google Scholar]
- 32.Knowles RG, Palacios M, Palmer RM, Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: A transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci USA. 1989;86:5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudek H, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy SG, et al. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- 35.Lei SZ, et al. Effect of nitric oxide production on the redox modulatory site of the NMDA receptor-channel complex. Neuron. 1992;8:1087–1099. doi: 10.1016/0896-6273(92)90130-6. [DOI] [PubMed] [Google Scholar]
- 36.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- 37.Wei L, Ying DJ, Cui L, Langsdorf J, Yu SP. Necrosis, apoptosis and hybrid death in the cortex and thalamus after barrel cortex ischemia in rats. Brain Res. 2004;1022:54–61. doi: 10.1016/j.brainres.2004.06.080. [DOI] [PubMed] [Google Scholar]
- 38.Yao D, et al. Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci USA. 2004;101:10810–10814. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uehara T, et al. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 40.Fang J, Nakamura T, Cho DH, Gu Z, Lipton SA. S-nitrosylation of peroxiredoxin 2 promotes oxidative stress-induced neuronal cell death in Parkinson's disease. Proc Natl Acad Sci USA. 2007;104:18742–18747. doi: 10.1073/pnas.0705904104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho DH, et al. S-nitrosylation of Drp1 mediates β-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JO, et al. Crystal structure of the PTEN tumor suppressor: Implications for its phosphoinositide phosphatase activity and membrane association. Cell. 1999;99:323–334. doi: 10.1016/s0092-8674(00)81663-3. [DOI] [PubMed] [Google Scholar]
- 43.Stamler JS, Toone EJ, Lipton SA, Sucher NJ. (S)NO signals: Translocation, regulation, and a consensus motif. Neuron. 1997;18:691–696. doi: 10.1016/s0896-6273(00)80310-4. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura T, et al. Transnitrosylation of XIAP regulates caspase-dependent neuronal cell death. Mol Cell. 2010;39:184–195. doi: 10.1016/j.molcel.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delgado-Esteban M, Martin-Zanca D, Andres-Martin L, Almeida A, Bolaños JP. Inhibition of PTEN by peroxynitrite activates the phosphoinositide-3-kinase/Akt neuroprotective signaling pathway. J Neurochem. 2007;102:194–205. doi: 10.1111/j.1471-4159.2007.04450.x. [DOI] [PubMed] [Google Scholar]
- 46.Lipton SA, et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.