Abstract
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by the presence of pathogenic autoantibodies, many of which are directed against nuclear antigens, in particular double-stranded (ds) DNA. Both clinical studies and animal models have shown that anti-dsDNA antibodies contribute to kidney disease, which is present in 50% of lupus patients and is a major cause of mortality. We previously demonstrated that a subset of nephrotoxic anti-dsDNA antibodies also recognizes the pentapeptide consensus sequence D/E W D/E Y S/G (DWEYS) present in the NR2A and NR2B subunits of the N-methyl-d-aspartate receptor (NMDAR). Autoantibodies with this specificity are present in ≈40% of lupus patient sera and are both nephrotoxic and neurotoxic. Elevated titers are present in cerebrospinal fluid of patients with central nervous system manifestations of SLE. Administration of the nonnaturally occurring D form of the DWEYS pentapeptide prevents these antibodies from depositing in glomeruli and from mediating neuronal excitotoxicity. To craft a more useful therapeutic, we used the structural features of the DWEYS peptide to design a unique, selective, and potent small molecule peptidomimetic, FISLE-412, which neutralizes anti-dsDNA/NMDAR lupus autoantibodies and prevents their pathogenic interaction with tissue antigens. This compound, or others derived from it, may provide a unique strategy for the development of lupus therapeutics.
Keywords: autoimmunity, glomerolotoxicity, neurotoxicity, orally absorbable
Although multiple species of autoantibodies can contribute to systemic lupus erythematosus (SLE) pathogenesis, antibodies to dsDNA are diagnostic, correlate with disease activity, and mediate both systemic and local inflammation (1–7). Through direct binding of chromatin or through antigenic cross-reactivity, they deposit in tissue and activate complement and Fc receptors (FcRs) on FcR-bearing cells, thereby initiating inflammatory cascades. Some nephrotoxic anti-DNA antibodies bind to glomeruli even after DNase treatment of the tissue, indicating their ability to bind non-DNA cross-reactive tissue antigens. Alternatively, they bind cell surface molecules and alter cell function (8–11). Finally, DNA containing immune complexes can activate toll-like receptor 9 in dendritic cells and promote an inflammatory milieu.
Previously, we determined that a nephritogenic mouse monoclonal anti-dsDNA antibody, R4A, bound a consensus pentapeptide sequence, D/E W D/E Y S/G (DWEYS), that is present in both the NR2A and NR2B subunits of mouse and human NMDAR (12–15). The R4A autoantibody enhances the glutamate-induced excitatory postsynaptic potentials in a mouse hippocampal slice preparation and causes apoptosis-mediated neuronal death when microinjected in vivo into a mouse brain (16). Mice immunized with a multimerized configuration of DWEYS produce anti-dsDNA/NMDAR antibodies, display glomerular Ig deposition, and exhibit neuronal damage after a breach of the blood–brain barrier (BBB) that allows transit of these antibodies into the CNS (17). In humans, elevated titers of cross-reactive anti-dsDNA/NMDAR antibodies are present in the serum of 40% of lupus patients and their presence in CSF of lupus patients correlates with CNS manifestations of neuropsychiatric lupus (NPSLE), which afflicts up to 80% of lupus patients (15, 18–22). Moreover, human anti-dsDNA/NMDAR antibodies have been eluted from postmortem brain tissue, and such antibodies isolated from lupus patient sera can cause brain damage (e.g., cognitive or behavioral dysfunction) in mice (23, 24). Similarly, gestating mice that harbor anti-dsDNA/NMDAR antibodies in their circulation give birth to offspring with impaired brain development (25) because the BBB does not form until the end of gestation and the fetal brain is exposed in utero to the maternal antibody. Of therapeutic importance, the d-isoform of the DWEYS peptide can protect tissue against antibody binding: In vivo administration of d-DWEYS peptide blocks renal and brain deposition of anti-dsDNA/NMDAR antibodies and ameliorates ongoing disease in mouse lupus models (12, 23, 24). These studies underscore the principle that anti-dsDNA/NMDAR antibodies are pathogenic in lupus and suggest a possible clinical utility for a molecule with properties of the DWEYS peptide. The peptide itself is of limited utility because it is not orally absorbed. Here, we have used the structural features of the DWEYS peptide to design a unique small molecule that neutralizes anti-dsDNA/NMDAR antibodies both in vitro and in vivo.
Results
Creation of a Small Molecule Peptidomimetic, FISLE-412.
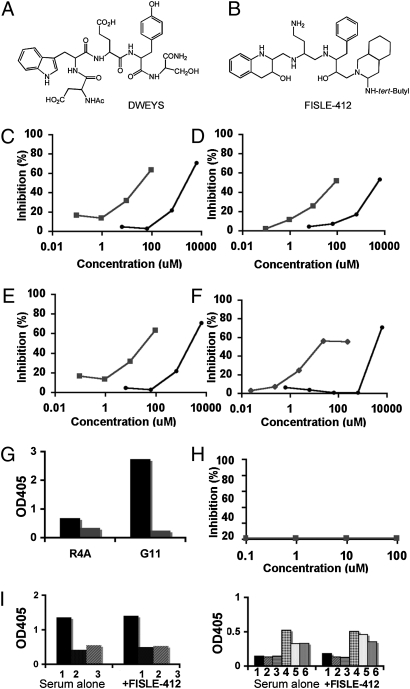
To identify compounds that inhibit anti-dsDNA/NMDAR lupus antibodies from mediating tissue damage, we focused our approach on imitating the molecular topology of the DWEYS structure and designed molecular scaffolds predicted to have the desired DWEYS mimetic properties. Thus, the tryptophan and tyrosine residues were replaced, respectively, by 1,2,3,4-tetrahydroquinolin-3-ol and phenyl moieties mobilized on a polyamine scaffold (Fig. 1 A and B). FISLE-412 is a unique molecule that was synthesized in 12 steps and characterized by mass spectrometry and NMR. We screened this small molecule for its ability to mimic neutralizing activities of the DWEYS peptide by using in vitro, ex vivo, and in vivo assays. First, we asked whether FISLE-412 could block two well-described lupus anti-dsDNA/NMDAR monoclonal antibodies, R4A (derived from a mouse producing anti-dsDNA antibodies) and G11 (derived from a peripheral blood B cell of a lupus patient), from binding to their known antigens in a competitive ELISA. As expected, the DWEYS peptide inhibited in a dose-dependent manner the binding of both R4A and G11, to dsDNA and to the DWEYS peptide itself, at micromolar concentrations (Fig. 1 C–F, black circles). Indeed, as shown in Fig. 1 C–F, FISLE-412 also inhibited the binding of R4A and G11 to these same antigens, but did so with markedly increased potency, at micromolar concentrations (Fig. 1 C–F, green squares). Cross-reactivity of anti-DNA antibodies with cardiolipin has been reported previously. R4A and G11 bind to cardiolipin (Fig. 1G, black bars), and this binding was also inhibited by FISLE-412 (Fig. 1G, green bars). Control experiments underscored the specificity of these interactions in that neither a scrambled pentapeptide nor an altered FISLE scaffold small molecule could inhibit the binding of anti-dsDNA/NMDAR monoclonal antibody to dsDNA or DWEYS, even at high concentrations. FISLE-412 did not inhibit binding of an anti-BSA antibody to BSA (Fig. 1H), nor did it significantly inhibit binding to histone by human or mouse SLE sera (Fig. 1I). Thus, FISLE-412 showed specificity for DNA-reactive antibodies and did not nonspecifically inhibit binding to all SLE autoantigens (Fig. 1I).
Fig. 1.
FISLE-412 is a unique peptidomimetic that is 30-fold more potent than DWEYS at inhibiting SLE anti-dsDNA/NMDAR autoantibodies in vitro. (A and B) Chemical structure of the DWEYS peptide (A) and FISLE-412 (B). (C and D) FISLE-412 inhibits mouse monoclonal anti-dsDNA/NMDAR antibody R4A binding to either DWEYS peptide (C) or dsDNA (D) in a dose-dependent manner. Representative assays (n = 4) show inhibition of R4A binding in the micromolar range (FISLE-412, green) and in the millimolar range (DWEYS peptide, black). (E and F) Identical competitive ELISAs were performed with the human monoclonal anti-dsDNA/NMDAR antibody G11. FISLE-412 blocked G11 binding to both DWEYS peptide (E) and dsDNA (F). Representative assays are shown (n = 4). (G) An ELISA for binding of R4A and G11 antibodies to cardiolipin was performed in the absence (black bars) and presence (green bars) of FISLE-412 (100 μM). As shown, FISLE-412 blocked binding to cardioliopin (green squares). (H) A commercial ELISA for anti-BSA antibodies was performed in the presence and absence of FISLE-412. As shown, there was no inhibition of the anti-BSA antibody (16 ng/mL) binding to its substrate in the presence of FISLE-412 (0.1–100 μM). (I) An ELISA for binding of human (Right, 1–3) and mouse (Left, 1–6) lupus sera to histones was performed in the absence (Left) and presence (Right) of FISLE-412 (250 μM). As shown, FISLE-412 did not inhibit histone binding by lupus sera.
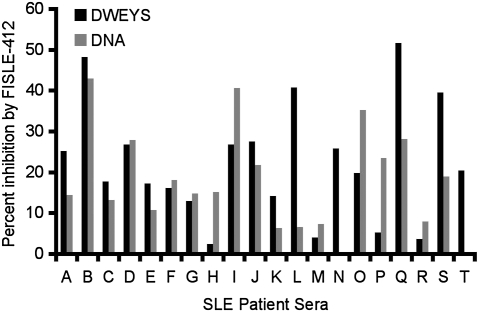
Given its effectiveness in neutralizing mouse or human anti-dsDNA/NMDAR monoclonal antibodies, we next asked whether FISLE-412 would be effective in inhibiting the polyclonal autoreactivity present in human lupus sera. Analogous to the studies of the monoclonal antibodies, we tested whether FISLE-412 inhibited autoantigen binding by SLE sera in an ELISA. FISLE-412 was able to inhibit a significant percent of DWEYS and DNA reactivity in most, but not all, lupus patient sera (Fig. 2), suggesting potential clinical utility in a serologically defined subset of patients.
Fig. 2.
FISLE-412 inhibits lupus autoantibody-mediated antigen recognition by human SLE patient sera. FISLE-412 inhibited human SLE sera binding to DWEYS peptide (black) and dsDNA (gray) substrates in an ELISA. A representative assay (n = 2) shows a range of inhibition of sera binding by FISLE-412 on both antigen substrates.
Effects of FISLE-412 on SLE Autoantibody Binding in Situ.
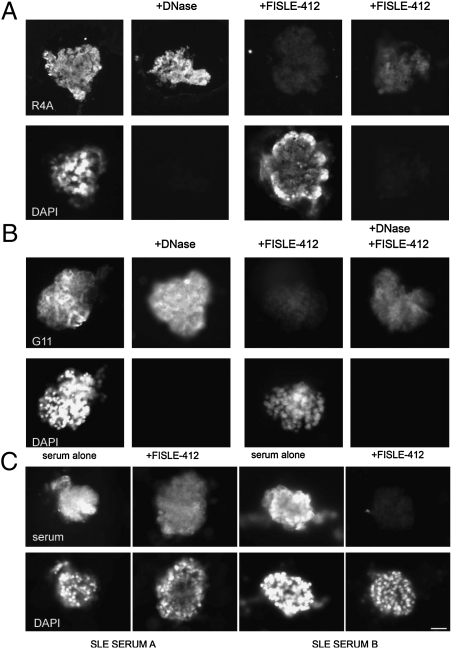
Because FILSE-412 was able to block DNA binding by SLE autoantibodies in vitro, we next assayed the ability of FISLE-412 to diminish or abrogate tissue binding by pathogenic anti-dsDNA, anti-DWEYS cross-reactive antibodies in a more complex tissue environment: the isolated kidney glomerulus. To test whether FISLE-412 could block glomerular binding, we incubated the R4A antibody with mouse glomeruli in the presence or absence of FISLE-412 and assessed binding with fluorescently conjugated secondary antibodies. As demonstrated previously, R4A bound to glomeruli before and after treatment with DNase (Fig. 3A, Left). In marked contrast, R4A pretreated with FISLE-412 showed greatly diminished binding to glomerular antigen (Fig. 3A, Right). Similarly, FISLE-412 mediated the loss of binding to glomerular tissue by the human monoclonal antibody G11 (Fig. 3B).
Fig. 3.
FISLE-412 suppresses pathogenic deposition of SLE autoantibodies to glomeruli ex vivo. (A) A glomeruli binding assay with the anti-dsDNA/NMDAR R4A autoantibody was performed. R4A reactivity was observed in the presence and absence of DNase treatment of glomeruli, underscoring its known cross-reactivity with DNA and non-DNA antigens (see DAPI staining, Lower). In Center Right, R4A alone was incubated with glomeruli. In Far Right, R4A was preincubated with FISLE-412 (250 μM) before incubation with glomeruli. FISLE-412 blocked antigen recognition in the presence and absence of DNase exposure, indicating its ability to inhibit antibody binding in a complex tissue environment. (B) Glomeruli binding assay was performed by using the human monoclonal lupus anti-dsDNA/NMDAR autoantibody G11, as done for R4A. FISLE-412 efficiently blocked the binding of G11 in tissue in the presence and absence of DNase treatment. (C) Human SLE sera (patients A and B from Fig. 1A) bound to glomeruli antigens to varying degrees (Upper Left and Upper Center Left). Preincubation of the serum with FISLE-412 (250 μM) before incubation with glomeruli blocked binding to tissue antigens (Upper Right and Upper Center Right). DAPI staining (Lower) reveals the glomeruli. Fluorescence intensity of secondary antibody recognizing IgG (Upper) is reduced in the presence of FISLE-412 (Upper Right and Upper Center Right vs. Upper Left and Upper Center Left).
Given the success of FISLE-412 in blocking SLE monoclonal antibody binding in tissue ex vivo, and polyclonal binding in an ELISA, we next tested whether it could block the binding of polyclonal autoantibodies present in lupus sera to glomerular tissue. Not surprisingly, sera A and B, which displayed different profiles of inhibition by FISLE-412 in the ELISA (Fig. 2), were inhibited to different degrees in the glomerular binding assay (Fig. 3C). Specifically, serum A was only partially neutralized in the glomerular binding assay; in contrast, serum B was effectively neutralized, despite the fact that FISLE-412 inhibited only ≈50% of dsDNA binding by serum B in an ELISA. These data are consistent with previous data demonstrating a variable amount of cross-reactive antibody in patient sera and suggest that FISLE-412 targets a particularly nephrotoxic subset of anti-dsDNA antibodies and will be effective in inhibiting glomerular IgG deposition in some, but not all, lupus patients.
Inhibition of SLE Autoantibody Neurotoxicity by FISLE-412.
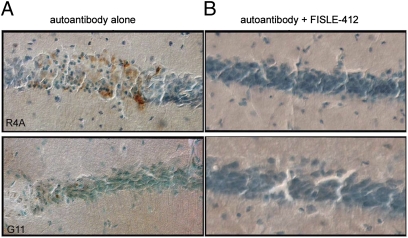
Because both R4A and G11 antibodies cause excitotoxic death of neurons in the mouse hippocampus (16, 26), we next tested whether FISLE-412 would block the neurotoxicity of these antibodies in an in vivo assay. R4A and G11 were independently stereotaxically injected into the dorsal hippocampus of a living mouse in the presence or absence of FISLE-412. As expected, R4A or G11 alone caused neuronal apoptosis, as indicated by positive TUNEL staining (Fig. 4, Upper Left and Lower Left). In contrast, pretreatment of either antibody with FISLE-412 completely abrogated this neurotoxic effect (Fig. 4, Upper Right and Lower Right). In agreement with the competitive ELISA data, the neuroprotective dose of FISLE-412 was ≈2 logs less than the dose of DWEYS peptide needed to achieve similar neuroprotection (23). These data suggest that FISLE-412 will maintain its neutralizing capacity within the complex environment of whole tissues or organs.
Fig. 4.
FISLE-412 blocks neurotoxicity of SLE autoantibodies in vivo. (A and B) The pathogenic lupus autoantibody R4A (A) or G11 (B) was injected intracerebrally into a mouse hippocampus without (Left) or with (Right) preincubation with FISLE-412. Panels show region around the CA1 hippocampal injection site. Injection of either R4A or G11 antibodies alone elicited neurotoxicity, as indicated by TUNEL positive staining (Left). In contrast, neurons were protected when the autoantibodies were preincubated with FISLE-412 (Right). Micrographs were acquired with a 40× lens. Data are representative of n = 6 animals (G11) and n = 5 animals (R4A).
Discussion
Using the DWEYS peptide as a model, we have designed a unique small molecule that neutralizes autoantibody binding to target antigen in several validated assays of lupus autoantibody activity, including in vitro, ex vivo, and in vivo assays. Additional validation of our conceptual approach to use features of the DWEYS peptide to target a subset of lupus autoantibodies has been provided by independent research groups who have recently published four studies that used reagents coupled to the DWEYS peptide to successfully target autoreactive B cells in lupus. Although initial studies both prevented disease and ameliorated ongoing disease in lupus-prone mice, the immunogenicity of the peptide-antibody preparation limited the potential utility of these reagents in the clinic. In the first study, the peptide was coupled to an antibody to FcRIIb, the inhibitory Fc receptor expressed on many cell types and the only Fc receptor expressed on B cells (9, 27–32). In the second study, DWEYS peptide was coupled to an anti-CD35 (complement receptor 1) antibody; this reagent caused a selective decrease ex vivo in anti-DNA antibody secreting B cells from peripheral blood of lupus patients (33). More recently, both the DWEYS peptide and an epitope binding to CD22, an inhibitory receptor of recent interest in silencing B cells active in autoimmunity, were coupled to IgG (34, 35). This reagent decreased titers of anti-dsDNA antibodies and improved renal survival in MRL/lpr mice. An additional study described the effects of incorporating the DWEYS peptide and diphtheria toxin A into a pseudovirus; this reagent reduced anti-dsDNA antibodies and improved survival in NZB/W mice (36). These studies showed that it may not be necessary to target all anti-dsDNA antibodies to achieve a therapeutic effect. Each of these groundbreaking studies, however, used a complicated biological reagent that was difficult to manufacture and store, and cannot be administered orally. Moreover, although each reagent used was therapeutic in vivo, each was also immunogenic. Our present creation of a unique small molecule that neutralizes antigen binding and the tissue-destructive activity of dsDNA/DWEYS-reactive autoantibodies circumvents these potential limitations. FISLE-412, therefore, represents a significant therapeutic advance: It combines potent anti-dsDNA/NMDAR neutralization with increased molecular stability and oral availability in a compound that is not expected to elicit neutralizing antibodies. Given that current lupus therapies are inadequate and often introduce the additional risk of immunosuppression, our data provide hope for the development of more specific, less toxic therapy for lupus and provide a model for the development of customized therapeutics for a highly pathogenic subset of lupus antibodies, as well as other antibody-mediated symptoms or diseases.
Materials and Methods
Chemical Synthesis.
FISLE-412 was synthesized and characterized by the Laboratory of Medicinal Chemistry at the institute. MS-ES: m/z = 635 [M+H]+. 1H NMR (500 MHz, acetone-d6): δ7.4–7.2 (5H, multiplet, phenyl CH), δ 7.0–6.9 (2H, multiplet, isoquinoline CH), δ6.65–6.55 (2H, multiplet, isoquinoline CH), δ4.6 (1H, broad, isoquinoline NH), δ4.05–3.9 (2H, multiplet, CH-OH), δ3.9–3.3 (multiplet, CHx-NHx methine and methylene), δ3.15–2.75 (5H, isoquinoline piperidine CHx), δ2.55–2.45 (1H, multiplet, piperidine CH), δ2.35–2.25 (2H, multiplet, piperidine CH2), δ1.65–1.55 (broad, OH, NH, D2O exchangeable), δ1.45–1.25 (multiplet, cyclohexane CH2 and t-Butyl CH3). 13C NMR (125 MHz, acetone-d6): δ145 (C1, isoquinoline) δ139 (C1, benzene), δ131–128 (CH, benzene), δ121–115 (CH, isoquinoline), δ67.5–67.0 (CH-OH), δ65.5 (CH, isoquinoline), δ59.5 (CH, benzyl), δ56.5–56.0 (CH and CH2, piperidine), δ50–43 (CH2-NH), δ37.0 (CH2-NH2), δ31.0–29.5 (CH2, piperidine and CH3, t-Butyl), δ28.0–26.0 (CH2, cyclohexane).
ELISAs.
The anti-dsDNA/DWEYS inhibition ELISAs were performed essentially as described (15, 24). Antigens were adsorbed overnight at 37 °C onto Costar plates (catalog no. 3690, Costar), either with calf thymus DNA (100 μg/mL) in NaHCO3 (0.1 M, pH 8.6) or d-DWEYS (20 μg/mL) in PBS. The inhibition ELISA was performed as described above on R4A or G11 monoclonal antibodies (20 μg/mL) (mouse and human, respectively), or a panel of deidentified human lupus sera (diluted 1:100), purchased from RDL. All were preincubated with dilutions of DWEYS peptide or FISLE-412 at varying concentrations for 1 h at 37 °C and then transferred to the 96-well plate for 1 h at 37 °C. The plates were washed and goat anti-mouse or anti-human IgG antibody was added for 1 h at 37 °C, essentially as described (12, 15, 24). The percent inhibition was calculated based on the OD at 405 nm: [(OD of test antibody, R4A, G11, or serum, minus OD of test antibody and FISLE-412 or DWEYS)/OD of test antibody alone] × 100. The d-DWEYS peptide was synthesized and purified by James I. Elliott at Yale University. An anti-cardiolipin ELISA was performed as follows: Cardiolipin (Sigma catalog no. C-0563, 75 μg/mL in 100% ethanol) was adsorbed overnight at room temperature onto Immulon IIHB plates (catalog no. 3455, Thermo Scientific). Plates were blocked with 3% BSA/PBS for 1 h at 37 °C. The inhibition ELISA was performed on R4A or G11 monoclonal antibodies (20 μg/mL), (mouse and human, respectively), as described above for the anti-dsDNA inhibition ELISA using FISLE-412 at 100 μM. An anti-BSA antibody ELISA (Cygnus Technologies, catalog no. F030) was performed according to the manufacturer's recommendations in the absence and presence of FISLE-412. The anti-BSA antibody (final concentration of 16 ng/mL) was preincubated with FISLE-412 at varying concentrations, as shown in Fig. 1H, for 1 h at 37 °C and then incubated on a substrate coated 96-well plate for 90 min at room temperature. The plate was developed according the manufacturer's protocol. An anti-histone ELISA was performed as follows: Calf thymus histones (H1, H2A, H2B, H3, and H4, Roche catalog no. 10223565001, at 10 μg/mL in PBS) were adsorbed overnight at 4 °C onto Costar plates (catalog no. 9018, Costar). An inhibition ELISA was performed as described above in the presence and absence of FISLE-412 at 250 μM. Human SLE sera (1–3, obtained for research, deidentified) were diluted 1:200, mouse sera were diluted 1:50 from C57BL/6 sle, 1, 2, and 3 mice (serum 1–3), and 3 from NZB/W mice (serum 4–6).
Neurotoxicity.
These procedures were in compliance with the Animal Welfare Act, the Public Health Service Policy on Humane Care and Use of Laboratory Animals, the US Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training, the National Institutes of Health Guide for the Use and Care of Laboratory Animals, and the Research Animal Resource Center Users Guide. Adult male C57BL/6 mice (30−34 g) were anesthetized with 2.5% Avertin (i.p.) and placed in a stereotaxic frame, and injected stereotaxically (2 μL) into the hippocampus, as described (15, 24, 26). Mice were euthanized 48 h later, and their brains were fixed and removed. Hippocampal sections (40 μm) were cryoprotected and subjected to TACS-Tdt Apoptosis Detection (R&D Systems no. TA4625 and counterstained with methylene green, as described (25). TUNEL-stained tissue was visualized with an upright microscope (Axio-Imager; Zeiss). Images were captured digitally (12-bit camera, 25 MHz, 1388 × 1040 resolution), processed (AxioVision 4.7, Zeiss), imported to Photoshop-CS4 as 8-bit TIFF files, and scale-adjusted equally for all frames.
Glomerular Binding Assay.
Murine glomeruli were isolated, added to glass slides, and the glomeruli binding assay was performed essentially as described (26). Monoclonal antibodies R4A (mouse) or G11 (human) were applied at 20 μg/mL or human lupus sera at 1:100 for 1 h at room temperature and visualized with anti-mouse or anti-human IgG antibodies conjugated to FITC or TRITC (Jackson ImmunoResearch Laboratories). For examination of antibody binding in the absence of DNA, some glomeruli were treated with DNase (100 μg/mL, 45 min at 37 °C) before antibody incubation. To visualize the presence or absence of DNA, DAPI (Invitrogen) was applied to glomeruli for 1 min at room temperature (1 μg/mL) before secondary antibody application. Slides were mounted by using Vectashield. For inhibition of antibody or sera binding, R4A, G11, or human lupus sera were preincubated with FISLE-412 (250 μM) for 1 h at 37 °C, before incubation with glomeruli, as done for ELISA inhibition assays. Slides were washed with PBS and mounted with coverslips by using Vectashield Mounting Medium (catalog no. H-1000; Vector Laboratories). Images were acquired at room temperature on an upright microscope (Axioplan 2; Zeiss) with a Zeiss Plan-Neofluar 40× lens using the OpenLab version 4.04 Software (Improvision). For each antibody or experimental condition, images were acquired with a Hamamatsu ORCA-ER Digital Camera (model no. C4742-80), at the same exposure for all conditions, imported into Adobe Photoshop-CS3 and then adjusted for contrast and brightness identically in all frames. Images were enlarged 2× for the figures.
Acknowledgments
These studies were supported by National Institutes of Health Grants R01AR057084 and P01AI73693. We thank Tom Coleman for critical reading, Dr. Nanette Wachter-Jurcsak (Hofstra University) and Ahmad Titi (Hunter College) for running the NMR spectra, and Roseann Berlin for expert technical assistance.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–306. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- 2.Waters ST, et al. Breaking tolerance to double stranded DNA, nucleosome, and other nuclear antigens is not required for the pathogenesis of lupus glomerulonephritis. J Exp Med. 2004;199:255–264. doi: 10.1084/jem.20031519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ebling F, Hahn BH. Restricted subpopulations of DNA antibodies in kidneys of mice with systemic lupus. Comparison of antibodies in serum and renal eluates. Arthritis Rheum. 1980;23:392–403. doi: 10.1002/art.1780230402. [DOI] [PubMed] [Google Scholar]
- 4.Winfield JB, Faiferman I, Koffler D. Avidity of anti-DNA antibodies in serum and IgG glomerular eluates from patients with systemic lupus erythematosus. Association of high avidity antinative DNA antibody with glomerulonephritis. J Clin Invest. 1977;59:90–96. doi: 10.1172/JCI108626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson L, Lightfoot RW., Jr Correlation of DNA-anti-DNA association rates with clinical activity in systemic lupus erythematosus (SLE) J Immunol. 1981;126:16–19. [PubMed] [Google Scholar]
- 6.Davis P, Cumming RH, Verrier-Jones J. Relationship between anti-DNA antibodies complement consumption and circulating immune complexes in systemic lupus erythematosus. Clin Exp Immunol. 1977;28:226–232. [PMC free article] [PubMed] [Google Scholar]
- 7.Bertsias GK, Salmon JE, Boumpas DT. Therapeutic opportunities in systemic lupus erythematosus: State of the art and prospects for the new decade. Ann Rheum Dis. 2010;69:1603–1611. doi: 10.1136/ard.2010.135186. [DOI] [PubMed] [Google Scholar]
- 8.Avalos AM, Busconi L, Marshak-Rothstein A. Regulation of autoreactive B cell responses to endogenous TLR ligands. Autoimmunity. 2010;43:76–83. doi: 10.3109/08916930903374618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- 10.Park SY, et al. Resistance of Fc receptor- deficient mice to fatal glomerulonephritis. J Clin Invest. 1998;102:1229–1238. doi: 10.1172/JCI3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ter Borg EJ, Horst G, Hummel EJ, Limburg PC, Kallenberg CG. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum. 1990;33:634–643. doi: 10.1002/art.1780330505. [DOI] [PubMed] [Google Scholar]
- 12.Gaynor B, et al. Peptide inhibition of glomerular deposition of an anti-DNA antibody. Proc Natl Acad Sci USA. 1997;94:1955–1960. doi: 10.1073/pnas.94.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz JB, Limpanasithikul W, Diamond B. Mutational analysis of an autoantibody: Differential binding and pathogenicity. J Exp Med. 1994;180:925–932. doi: 10.1084/jem.180.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shefner R, Kleiner G, Turken A, Papazian L, Diamond B. A novel class of anti-DNA antibodies identified in BALB/c mice. J Exp Med. 1991;173:287–296. doi: 10.1084/jem.173.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeGiorgio LA, et al. A subset of lupus anti-DNA antibodies cross-reacts with the NR2 glutamate receptor in systemic lupus erythematosus. Nat Med. 2001;7:1189–1193. doi: 10.1038/nm1101-1189. [DOI] [PubMed] [Google Scholar]
- 16.Faust TW, et al. Neurotoxic lupus autoantibodies alter brain function through two distinct mechanisms. Proc Natl Acad Sci USA. 2010;107:18569–18574. doi: 10.1073/pnas.1006980107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowal C, et al. Cognition and immunity; antibody impairs memory. Immunity. 2004;21:179–188. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Aranow C, Diamond B, Mackay M. Glutamate receptor biology and its clinical significance in neuropsychiatric systemic lupus erythematosus. Rheum Dis Clin North Am. 2010;36:187–201. doi: 10.1016/j.rdc.2009.12.007. x–xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fragoso-Loyo H, et al. Serum and cerebrospinal fluid autoantibodies in patients with neuropsychiatric lupus erythematosus. Implications for diagnosis and pathogenesis. PLoS ONE. 2008;3:e3347. doi: 10.1371/journal.pone.0003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fragoso-Loyo H, et al. Inflammatory profile in the cerebrospinal fluid of patients with central neuropsychiatric lupus, with and without associated factors. Rheumatology (Oxford) 2009;48:1615–1616. doi: 10.1093/rheumatology/kep297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Husebye ES, et al. Autoantibodies to a NR2A peptide of the glutamate/NMDA receptor in sera of patients with systemic lupus erythematosus. Ann Rheum Dis. 2005;64:1210–1213. doi: 10.1136/ard.2004.029280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshio T, Onda K, Nara H, Minota S. Association of IgG anti-NR2 glutamate receptor antibodies in cerebrospinal fluid with neuropsychiatric systemic lupus erythematosus. Arthritis Rheum. 2006;54:675–678. doi: 10.1002/art.21547. [DOI] [PubMed] [Google Scholar]
- 23.Huerta PT, Kowal C, DeGiorgio LA, Volpe BT, Diamond B. Immunity and behavior: Antibodies alter emotion. Proc Natl Acad Sci USA. 2006;103:678–683. doi: 10.1073/pnas.0510055103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kowal C, et al. Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci USA. 2006;103:19854–19859. doi: 10.1073/pnas.0608397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JY, et al. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat Med. 2009;15:91–96. doi: 10.1038/nm.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, et al. Polyreactive autoantibodies in systemic lupus erythematosus have pathogenic potential. J Autoimmun. 2009;33:270–274. doi: 10.1016/j.jaut.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackay M, et al. Selective dysregulation of the FcgammaIIB receptor on memory B cells in SLE. J Exp Med. 2006;203:2157–2164. doi: 10.1084/jem.20051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGaha TL, Karlsson MC, Ravetch JV. FcgammaRIIB deficiency leads to autoimmunity and a defective response to apoptosis in Mrl-MpJ mice. J Immunol. 2008;180:5670–5679. doi: 10.4049/jimmunol.180.8.5670. [DOI] [PubMed] [Google Scholar]
- 29.Dhodapkar KM, et al. Selective blockade of the inhibitory Fcgamma receptor (FcgammaRIIB) in human dendritic cells and monocytes induces a type I interferon response program. J Exp Med. 2007;204:1359–1369. doi: 10.1084/jem.20062545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bolland S, Yim YS, Tus K, Wakeland EK, Ravetch JV. Genetic modifiers of systemic lupus erythematosus in FcgammaRIIB(-/-) mice. J Exp Med. 2002;195:1167–1174. doi: 10.1084/jem.20020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGaha TL, Sorrentino B, Ravetch JV. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science. 2005;307:590–593. doi: 10.1126/science.1105160. [DOI] [PubMed] [Google Scholar]
- 32.Tchorbanov AI, et al. Selective silencing of DNA-specific B lymphocytes delays lupus activity in MRL/lpr mice. Eur J Immunol. 2007;37:3587–3596. doi: 10.1002/eji.200737143. [DOI] [PubMed] [Google Scholar]
- 33.Voynova E, et al. An antibody-based construct carrying DNA-mimotope and targeting CR1(CD35) selectively suppresses human autoreactive B-lymphocytes. Immunol Lett. 2008;116:168–173. doi: 10.1016/j.imlet.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 34.Mihaylova N, et al. Simultaneous engagement of FcgammaIIb and CD22 inhibitory receptors silences targeted B cells and suppresses autoimmune disease activity. Mol Immunol. 2009;47:123–130. doi: 10.1016/j.molimm.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 35.Nikolova KA, et al. Selective silencing of autoreactive B lymphocytes-Following the Nature's way. Autoimmun Rev. 2010;9:775–779. doi: 10.1016/j.autrev.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 36.Tan H, Yang X, Cui S, Yang Z. A novel pseudovirus encoding Diphtheriatoxin A fragment eliminates autoantibody-producing B cells and inhibits lupus in the BWF1 mouse model. Immunol Lett. 2009;126:43–47. doi: 10.1016/j.imlet.2009.07.011. and retraction (2011) 136:114. [DOI] [PubMed] [Google Scholar]