Abstract
We studied two groups of adult macaque monkeys to determine the time course of adult neurogenesis in the dentate gyrus of the hippocampus. In the first group, six adult monkeys (Macaca mulatta) received a single injection of the thymidine analog BrdU (75 mg/kg), which is incorporated into replicating DNA and serves as a marker for new cell birth. Brain tissue was collected 48 h, 2 wk, and 6 wk after BrdU injection to examine the initial stages of neurogenesis. Because mature neurons were not evident at 6 wk, we examined tissue collected over a longer time course in a second study. In this study, eight monkeys (Macaca fascicularis) who were subjects in a separate exercise study received 10 weekly injections of BrdU (75 mg/kg), and brain tissue was collected at 16 and 28 wk from the first injection. Based on the timing of expression of neuronal cell markers (βIII-tubulin, doublecortin, NeuN), the extent of dendritic arborization, and acquisition of mature cell body morphology, we show that granule cell maturation in the dentate gyrus of a nonhuman primate is protracted over a minimum of a 6-mo time period, more than 6 times longer than in rodents. The lengthened time course for new cell maturation in nonhuman primates may be appropriate for preservation of neural plasticity over their longer life span and is relevant to our understanding of antidepressant and other therapies that have been linked to neurogenesis in humans.
Keywords: immunohistochemistry, neuronal maturation, subgranular zone, granule cell layer, neuroprogenitor cell
The generation of new neurons has been shown to occur in the hippocampal dentate gyrus of mammals (1–3). The potential that adult hippocampal neurogenesis can be manipulated has inspired hope for treatments to slow or even repair brain damage from disease or injury. Adult hippocampal neurogenesis is thought to play a role in many brain processes including learning and memory (4–6), cognitive change with age (7), and disorders such as depression (8) and schizophrenia (9). One prominent theory suggests that the special properties (e.g., hyper-plasticity and functional naivety) of the maturing new neurons play an important role in hippocampal function (5, 9). Thus, understanding how new neurons mature in nonhuman primates is an important step for bridging our knowledge of adult neurogenesis in rodent models to a better understanding of this process in humans.
A sequence of events in the maturation of adult born neurons has been established in rodents. Granule cells in the dentate gyrus of the hippocampus are the primary neuron type added. New granule cells divide from progenitor cells in the subgranular zone (SGZ), migrate approximately 2 cell body widths from the SGZ into the granule cell layer (GCL), and then extend axons and dendrites that make the appropriate connections and become functionally integrated into the hippocampal circuit (10). Electrophysiological maturation of new granule cells progresses over the period of 2–7 wk after cell division (10–14). These functional changes are accompanied by changes in expression of the neuron specific cytoskeletal proteins [βIII-tubulin and doublecortin (DCX)] for the first 3 wk, with a subsequent transition to the mature neuronal marker NeuN by 4–6 wk (15–18). Previous studies in primates have not resolved whether new neurons mature through similar transitional stages and over a similar time course (3, 19–24). Here, we report evidence that new granule cells in the dentate gyrus of macaque monkeys take more than 6 times longer to mature than has been demonstrated in rodents. The longer maturation time for new neurons in monkeys suggests that the maturation time may be even longer in humans and, therefore, that direct comparisons of rodent and human neurogenesis must be reconsidered. The present report is part of an ongoing study of the effects of age and exercise on the nonhuman primate brain (25).
Results
Cell Birth and Migration.
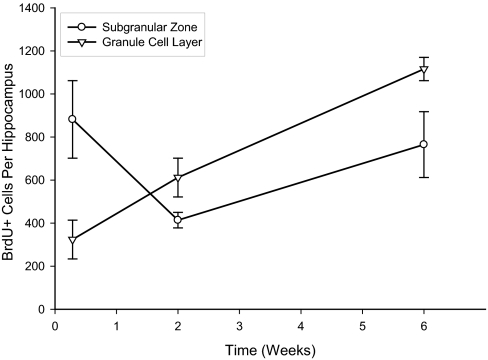
In study 1, we examined the cell birth and migration pattern at 48 h, 2 wk, and 6 wk after single injections of BrdU. Total BrdU+ cell counts are discussed only with regards to the findings of this study (Fig. 1). At 48 h, 75% of BrdU+ cells were located in SGZ and were in pairs within 1 cell body diameter of each other, consistent with recent cell divisions. From 48 h to 2 wk post-BrdU injection, the total number of BrdU+ cells in the SGZ decreased at the same time that BrdU+ cells increased in the GCL (2-way ANOVA, effect of time, P = 0.02; time vs. layer interaction, P = 0.014), indicating cell migration from the SGZ to the GCL during this time frame. The estimated total number of BrdU+ cells in the dentate gyrus continued to increase through 6 wk after a single injection of BrdU (r2 = 0.940, P = 0.001). No other comparisons were statistically significant.
Fig. 1.
Study 1: Total number of BrdU+ cells born after a single BrdU injection (mean ± SEM). There was an inverse relationship between the numbers of new cells in SGZ (○) and GCL (▽) consistent with new cell migration from the SGZ to GCl during that time period (ANOVA, effect of time, P = 0.020; time vs. layer interaction, P = 0.014). There was also an increase in the number of BrdU+ cells over the 6 wk in study 1 (r2 = 0.940, P = 0.001).
Maturation of Adult Born Granule Cells Identified by Immunohistochemistry.
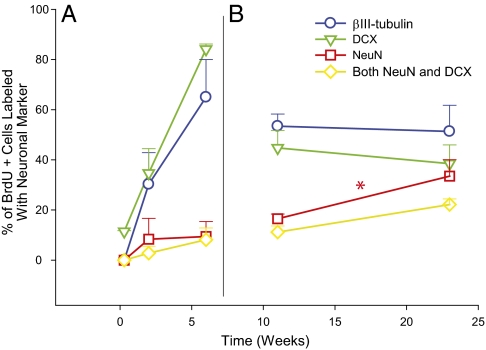
The percentage of adult born cells that were co-labeled with DCX, βIII-tubulin, or NeuN was assessed to study cell maturation during the 4- to 6-wk period when most new granule cells in rodents were fully mature (Table 1). After a single BrdU injection, no cells were immunopositive for the markers βIII-tubulin or NeuN at 48 h, and <10% of the BrdU+ cells were DCX+ (Fig. 2A). Neurons double-labeled for BrdU and the immature markers DCX or βIII-tubulin increased over the period from 48 h to 6 wk. At the end of this period, 84% of BrdU+ cells were DCX+ and 65% were βIII-tubulin+ (Fig. 2A). Only 10% of the BrdU+ cells were NeuN+ and 7% were both NeuN+ and DCX+. The 6-wk time point had the highest percentage of adult born cells labeled with immature markers, indicating that few new granule cells had matured by 6 wk.
Table 1.
Summary data from study 1 and study 2 describing species, ages, cell counts, and proportion of cells in each animal
| Cell counts per hippocampus in number (%) |
|||||||
| Species | Time point | y | BrdU | βIII-Tubulin+ | DCX+ | NeuN+ | DCX+/NeuN+ |
| Study 1: M. mulatta | 48 h | 6.1 | 252 | 0 (0.0) | 36 (11.1) | 0 (0.0) | 0 (0.0) |
| 6.9 | 414 | 0 (0.0) | 72 (11.8) | 0 (0.0) | 0 (0.0) | ||
| 2 wk | 7.1 | 522 | 108 (17.6) | 108 (25.0) | 0 (0.0) | 0 (0.0) | |
| 5.6 | 702 | 324 (42.9) | 288 (44.4) | 108 (16.7) | 36 (5.6) | ||
| 6 wk | 5.8 | 1,170 | 468 (50.0) | 1,152 (82.1) | 216 (15.4) | 180 (12.8) | |
| 5.7 | 1,062 | 864 (80.0) | 900 (86.2) | 36 (3.4) | 36 (3.4) | ||
| Study 2: M. fascicularis | 11 wk | 10.0 | 2,268 | 1,836 (54.3) | 576 (50.0) | 180 (15.6) | 72 (6.3) |
| 8.3 | 1,872 | 1,152 (41.0) | 360 (38.5) | 108 (11.5) | 72 (7.7) | ||
| 8.8 | 2,754 | 2,844 (64.8) | 684 (61.3) | 216 (19.4) | 180 (16.1) | ||
| 9.4 | 2,214 | 1,584 (53.7) | 432 (29.3) | 288 (19.5) | 216 (14.6) | ||
| 23 wk | 9.0 | 2,646 | 1,188 (28.2) | 180 (16.7) | 252 (23.3) | 180 (16.7) | |
| 10.2 | 2,358 | 1,584 (53.7) | 756 (42.9) | 648 (36.7) | 432 (24.5) | ||
| 9.2 | 1,818 | 1,008 (45.2) | 612 (43.6) | 324 (23.1) | 288 (20.5) | ||
| 8.8 | 2,790 | 2,592 (78.3) | 1,152 (50.8) | 1,152 (50.8) | 612 (27.0) | ||
Counts of BrdU+ cells represent an average from two staining sets in which BrdU was combined with βIII-tubulin and DCX or NeuN and DCX. Total BrdU does not correspond with the denominator used to calculate the proportion of cells expressing each marker in each animal.
Fig. 2.
New neurons in the GCL showed a partial transition from immature to mature neuronal markers throughout the time points of study 1 and 2 (mean ± SEM). (A) In study 1, 84% of BrdU+ cells were labeled with the immature marker DCX at 6 wk, whereas only 10% were labeled with the mature marker NeuN. (B) In study 2, there was a significant increase in the number of NeuN+ cells from 11 to 23 wk (*P = 0.048) and at the 23-wk time point, 54% of BrdU+ neurons still maintained expression of the immature marker βIII-tubulin, whereas only 34% were NeuN+ (βIII-tubulin, ○; DCX, ▽; NeuN, □; both NeuN and DCX, ◇).
In study 2, the percentage of adult born cells labeled with mature marker NeuN increased significantly between 11 and 23 wk (17 vs. 34%, P = 0.048), but almost two-thirds of the BrdU+/NeuN+ cells at 23 wk were also labeled with DCX (Fig. 2B). Also, the percentages of BrdU+ cells with immature markers at these late time points were greater than the percentages of NeuN labeled cells (βIII-tubulin, 53 vs. 51%; DCX, 45 vs. 38%, respectively; Fig. 2B, 4 h). Interestingly, of all cells expressing DCX, irrespective of being BrdU immunoreactive, 33% also expressed NeuN.
Maturation of Granule Cells Measured by Dendritic Branching and Cell Morphology.
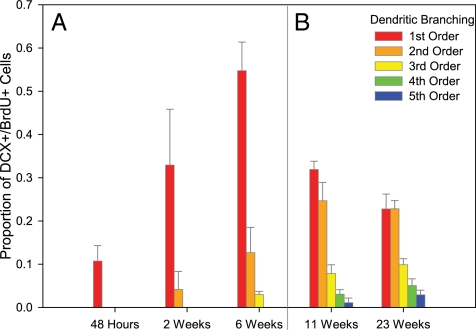
The number of DCX+ dendrites and dendritic branches was also quantified as another measure of cell maturation. In study 1, <5% of the BrdU+ cells had an identifiable DCX+ dendrite on the apical side of the cell 48 h after a single BrdU injection (Figs. 3A and 4A). At 2 and 6 wk, BrdU+/DCX+ cells began to show second and third order branching of a single primary dendrite; however, a majority of new cells had no dendrites or a single unbranched dendrite (55%; Figs. 3A and 4B). The NeuN+/BrdU+ cells observed at 6 wk had fusiform shaped cell bodies (Fig. 4E).
Fig. 3.
New DCX+ neurons showed a pattern of consistent increase in the number of apical dendritic branches over the length of the study (mean ± SEM). (A) In study 1, at 48 h, only 10% of DCX+ cells had a dendritic process and at 6 wk, 55% of cells still had a single unbranched dendrite. (B) In study 2, at the 11- and 23-wk time points, there were increasing numbers of dendrites with 4–5 branches (first order, red; second order, orange; third order, yellow; fourth order, green; fifth order, blue).
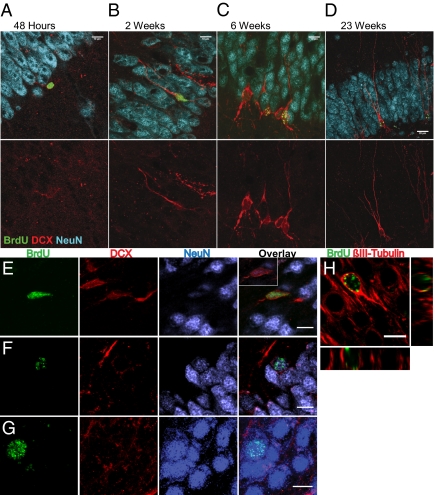
Fig. 4.
Confocal micrographs demonstrating the sequence of neuron maturation. (A–D) Micrographs represent the most mature cells observed at each time point (Upper, an overlay of BrdU and DCX; Lower, the same image showing DCX only). Study 1 (A–C). (A) At 48 h, the most mature neurons were weakly DCX positive with a single apical dendrite (scale bar: 10 μm). (B) At 2 wk, the most mature cells had fusiform nuclei and dendrites reaching the edge of the GCL (scale bar: 10 μm). (C) At 6 wk, the most mature neurons had dendrites with 2 or 3 branches (scale bar: 10 μm). (D) In Study 2 at 23 wk, the most mature DCX+ neurons still had fusiform shapes with basilar dendrites (scale bar: 20 μm; BrdU, green; DCX, red; NeuN, blue). (E–G) NeuN+/BrdU+ cells showed immature morphology until the 23-wk time point. (E) In study 1 at 6 wk, nuclei and cell bodies of NeuN+ cells were fusiform in shape. However, in study 2 at the 23-wk time point, some NeuN+ cells had round nuclei comparable to mature granule cells and were either weakly labeled (F) or not labeled with DCX (G; scale bar: 10 μm; BrdU, green; DCX, red; NeuN, blue). (H) Confocal micrograph of βIII-tubulin+/BrdU+ cell with oblique views (scale bar: 10 μm).
In study 2, fourth and fifth order branching was observed at the 11- and 23-wk time points (Fig. 3B) as well as a greater number of dendrites emanating from the soma (Fig. 4D), yet over 20% of cells still had only a single unbranched dendrite. At 23 wk, DCX+ neurons with the highest branching order still maintained an immature-looking, fusiform cell body and basilar dendrites, whereas the few BrdU+/NeuN+ cells that displayed round cell bodies typical of mature granule cells were either weakly DCX+ (Fig. 4F) or DCX− (Fig. 4G).
Discussion
In the present study, we extend observations from earlier studies in the monkey of adult born granule cells in the hippocampus from 3 to 5 wk (19–22) and 10–14 wk (3, 23, 24) to 28 wk after the first injection of BrdU. We found that at 6 wk in monkeys, only 10% of BrdU+ cells were NeuN+, whereas 84% were DCX+ in comparison with similar percentages at 1 wk in rats (16–18). In contrast, in rats, virtually all new neurons are NeuN+ and no neurons express immature markers by 4 wk (16–18). This period is only slightly longer in mice (15). However, even in the monkeys that had the longest post-BrdU survival (23 wk from the midpoint of weekly injections 18–28 wk prior), only 34% of BrdU+ cells were NeuN+, indicating that at the end of our study, a majority of new neurons had not yet fully matured when measured by immunomarkers. Both the period when new cells reach peak percentage of DCX labeling and the period when new cells approach maturation, as measured by NeuN labeling, are more than 6 times longer in the monkey than in the rat.
The structural maturation of granule cell dendrites in monkeys is also prolonged. At 48 h, only 11% of DCX+ cells were found to display a process on the apical side of the cell and by 2 wk, dendrites were just beginning to reach the molecular layer. It was not until 11 wk that dendritic branching of fourth order and higher appeared in monkeys. In contrast, dendritic processes in mouse dentate gyrus have already reached the molecular layer at 3 d post-BrdU injection, and by 2 wk, fourth and fifth order branching of dendrites were observed (14).
There are reasons to believe that there would be fundamental differences in the time course of neuronal maturation in rodents vs. primates. For example, the total length of the embryonic neurogenic period in nonhuman primates and humans is protracted compared with that of rodents (humans, 100 d; monkeys, 60 d; mice, 6 d) (26–29). Moreover, the cell cycle of neuroprogenitor cells is 2–5 times longer in nonhuman primates than it is in rodents during embryonic neurogenesis (monkeys, 22–54 h; mice 8–17 h) (30, 31). It makes sense that differences between rodents and primates in embryonic neurogenesis would be recapitulated in adult neurogenesis (13). In fact, in our study, new cells continued to increase through 6 wk, whereas in rats, new cells increase until 7 d post-BrdU administration (16, 18, 32). This increase has been attributed to continued division of labeled precursor cells approximately every 14 h in mice (33) and 24 h in rats until BrdU is diluted from the DNA by the normal base thymidine (32–35). The longer period of increase in the number of new cells in monkeys means that the time it takes to dilute the BrdU label from these cells is longer, suggesting that precursor cells divide at a slower rate.
A third piece of evidence for a significantly longer time course of maturation in primates is the period of transition from immature to mature neuronal markers. In this study, we found that monkeys enter this transition period at ∼11 wk. At the 23-wk time point, 40–50% of new cells still expressed immature markers while NeuN+ cells continued to increase. A substantial proportion of new DCX+ granule cells at this time point co-labeled with NeuN (22%, Fig. 2B), but this was less than the 33% of BrdU-/DCX+ cells that labeled with NeuN in the same tissue. This finding suggests that post-BrdU injection survivals extending beyond the 28 wk that we used in this study might increase the percentages of transitional (BrdU+/DCX+/NeuN+) granule cells. Some of the new cells at the longest time point likely resulted from multiple cell divisions of labeled precursor cells and, because of the delay in onset of immature neuronal marker expression (∼6 wk for some cells in our study), it is probable that most immature-appearing NeuN expressing cells were born closer to the BrdU injection made 18 wk before the monkeys were euthanized and the more mature cells were born closer to the injection made 28 wk before death. Nevertheless, in rats, the period of transition in which DCX and NeuN co-label is from 10 to 21 d (16), indicating a much shorter period of development than in the monkey.
Our discovery of a prolonged maturation time for new granule cells in the monkey hippocampus was the result of analysis of two studies using different BrdU administration protocols and different monkey species of a single sex in which monkeys in one study were subjects in a separate exercise experiment. Although it is possible that differences in BrdU administration, sex, monkey species, and experimental protocol had subtle effects on maturation, they cannot alter the conclusion that at 6 wk after single BrdU injections in M. mulatta monkeys, 84% of BrdU+ cells express immature markers and have immature morphology when rodent granule cells have all matured. The brains of the two species of macaque used in this study are very similar, and both species are used interchangeably in anatomical, behavioral, and physiological studies, so continued expression of immature markers and morphology with a progression toward maturity out to 28 wk suggests that the findings in M. fascicularis are consistent with those in M. mulatta. Although exercise does affect the generation of new neurons in the hippocampus (36–38), we based our analysis on the proportion of new cells expressing markers of maturation to avoid the confounding variables associated with measures of cell number and density. Furthermore, we doubt that the prolonged period of maturation that we observed was due to participation in exercise because exercise accelerates the rate of new neuron maturation in rodents (14). We also doubt that mild hypoxia that might result from exercise led to aberrant DNA synthesis and false BrdU labeling of older, resident neurons (39). Such labeling is accompanied by a dramatic increase in NeuN+ co-labeled neurons, which conflicts with what we saw.
We have concluded that the decrease in cells with immature markers was related to maturation of these cells rather than to selective cell death. We base this conclusion on two observations. First, at the longest time point in our study, the percentages of immature and mature markers begin to converge. Second, we found a gradual increase in mature markers over time that is consistent with an increasing population of hippocampal granule cells in the adult M. mulatta (40).
DCX and βIII-tubulin are cytoskeletal proteins that play critical roles in the processes of cell migration, nuclear translocation, and dendritic growth (41–46). The continued expression of DCX and βIII-tubulin suggests that these processes were still occurring more than 19 wk longer than they do in rodents. The time frame of marker expression is consistent with our observations of the morphological development of the cell body and dendrites. The soma of cells expressing immature markers were fusiform at all time points, including 23 wk, suggesting that cell migration or nuclear translocation was continuing even at the end of our study. NeuN positive granule cells with round nuclei, an indication of maturity, were observed only at 23 wk (Fig. 4G). There was a continuous progression of dendritic arborization toward a mature morphology throughout the study. The time at which monkey granule neurons reach measurable points in maturation (e.g., when dendrites reach the molecular layer; extent of dendritic arborization) was delayed compared with rodents. In monkeys, the first appearance of higher order dendritic branching occurred at ∼11 wk. Establishment of functional connectivity in mice at 12–18 d (14) corresponds to the initiation of higher order dendritic branching and transition from immature to mature neuronal markers. Based on the rate of morphological maturation of these cells, we expect functional connectivity to be comparably delayed in the monkey.
A delay in maturation and functional connectivity has important clinical implications. For example, there has been much interest in links between neurogenesis and the effectiveness of antidepressant therapies (47, 48). The most commonly prescribed antidepressant medications, selective serotonin reuptake inhibitors and tricyclic antidepressants, have been shown to increase the rate of hippocampal neurogenesis in rodents (49, 50) and nonhuman primates (22). Antidepressants generally take 3–5 wk to have clinical efficacy on depression in humans (22, 51), a period that coincides with the maturation of adult born neurons in the hippocampus of the adult rat (14, 16, 18). However, the data we report here suggests that the time course of adult hippocampal neuronal maturation in a nonhuman primate is at least 6 times slower. Due to the increased size and longer developmental maturation of the human brain, we predict that the maturation period of adult generated neurons would be further lengthened. Thus, there is a marked mismatch between the onset of the clinical effects of antidepressants and the time that would be required for maturation of new hippocampal granule cells in humans. This mismatch argues against the hypothesis that hippocampal neurogenesis is a critical mechanism underlying the initiation of effectiveness of antidepressant medications.
In our study, the overall sequence of events that constitute adult hippocampal neurogenesis in monkeys is consistent with that reported in rodents; however, the relative numbers of new cells per hippocampus and the duration of developmental events is very different. Each hippocampus of an adult monkey of 5–9 y has ∼7.2 million granule cells (40), whereas adult rats have 1.2 million granule cells (52). We found, on average, 1,100 surviving BrdU+ cells/hippocampus in the GCL (Fig. 1) of young adult macaques after a single BrdU injection, which is ˜1.5 new cells/10,000 granule cells. In rats, there are an estimated 3,000 BrdU+ cells/hippocampus (15), which is ∼25 new cells/10,000 granule cells. Although caution is necessary when comparing BrdU findings between studies (53), our findings are consistent with other studies that demonstrate a lower rate of neurogenesis in monkeys (3). It has been shown recently that new granule cells gradually accumulate postnatally in monkey. There is a 25% increase in the first 3 mo and an additional, but diminishing, increase of 15% from 3 mo to young adulthood at 5–9 y of age (40). Our data support the idea that new cells continue to mature at a slow rate in monkeys of 5.6–10.2 y of age. While new neurons mature, they show unique properties that might provide a substrate for new memory formation in the hippocampus including increased excitability, easy induction of long term potentiation, and no prior stored information (5). We propose that adult granule cell maturation is analogous to embryonic neurogenesis, where a longer cell cycle together with a longer neurogenic period results in an overall larger population of neurons in monkeys (30). The long cell cycle together with long maturation of adult granule cells results in a substrate for memory formation that is appropriate for preservation of neural plasticity over the longer life span of primates, including humans.
Materials and Methods
Subjects.
Fourteen female macaque monkeys, six M. mulatta (ages 5.6–7.1 y) and eight M. fascicularis (ages 8.3–10.2 y), were used in this study (Table 1). The monkeys were housed at the University of Pittsburgh until they were killed. The M. fascicularis were wild-caught and age estimates were based on radiographic determination of bone age when they were about 2 y of age (54). The monkeys were housed in pens ∼2 m × 4.5 m × 3.3 m high within a social living group of 2–3 similar aged pen mates or in individual cages. They were fed Purina Monkey Chow once daily. Animals living in pens had both natural and artificial lighting, making the light dark cycle equivalent to natural day length. Animals living in cages had lights on from 0700 to 1900 h. All animal care and use and tissue procedures were conducted in accord with protocols approved by the Institutional Animal Care and Use Committees of the University of Pittsburgh and the University of Illinois and in accordance with NIH standards and guidelines.
Experimental Design.
The thymidine analog BrdU was administered by i.p. injection either as a single dose or as a series of 10 weekly injections. Six monkeys (M. mulatta) were given a single injection of BrdU (100 mg/kg) under light sedation. Of these six, two monkeys were euthanized at each of three time points: 48 h, 2 wk, and 6 wk after the injection. Eight monkeys (M. fascicularis) were given 10 weekly injections of BrdU (75 mg/kg) and euthanized at one of two time points (11 and 23 wk) from the mid-injection point (n = 4/time point). All eight monkeys also participated in a 20-wk physical exercise protocol as part of a different study. We did not find that exercise significantly affected the proportion of BrdU+ cells that co-labeled with βIII-tubulin in this group compared with a sedentary control group (0.42 and 0.53, respectively, P = 0.2). Our multiple BrdU injection protocol was designed to optimize detection of new cells that were generated in response to early and long-term effects of exercise.
Perfusion and Tissue Preparation.
All monkeys were deeply i.v. anesthetized with sodium pentobarbital (30 mg/kg) and perfused intracardially with physiological saline containing heparin (5,000 U/L) and sodium nitrite (20 g/L) followed by cold 4% paraformaldehyde in PBS. The brain was removed and postfixed for 4 h in cold 4% paraformaldehyde in PBS followed by infusion with 20% glycerol in PBS. The brains were separated into blocks with coronal cuts at the rostral termination of the inferior optical sulcus and the temporal lobe removed at the lateral sulcus. The blocks of tissue were cryoprotected in 30% sucrose in Tris buffered saline (TBS) until sinking (∼4 wk; fresh solution weekly). Tissue was covered with tissue freezing media, frozen at −19 °C, and sectioned coronally at a thickness of 40 μm. Sections were collected in multiwell plates containing cryoprotectant (30% sucrose, 30% ethylene glycol in TBS) and stored at −20 °C.
Fluorescence Immunohistochemistry.
All immunohistochemistry was conducted on free floating sections in two sets (Set 1: BrdU, βIII-tubulin, and DCX; Set 2: BrdU, NeuN, and DCX). For BrdU immunohistochemistry, tissue sections were incubated for 30 min in 2N HCl at 47 °C to expose the BrdU followed by neutralization with 0.1 M borate at pH 8.62 in TBS. Sections were blocked and permeabilized for 1 h using a solution of 3% normal donkey serum and 0.01% Triton x-100 in TBS. Sections were incubated in a mixture containing the appropriate primary antibodies from the following list overnight at 4 °C except where noted: 1:400 rat anti Brdu (Accurate Chemical), 1:500 mouse anti-bIII tubulin 48 h (Promega), 1:400 mouse anti NeuN (Chemicon), and 1:400 goat anti c-19 DCX (Santa Cruz). Primary antibodies were washed and secondary antibodies made in donkey (Jackson Immuno Labs) conjugated to biotin (1:1,000), CY2, CY3, or CY5 (1: 250) were added overnight. All secondary antibodies had minimal cross reactivity with nontarget species in the mixture. Biotinylated antibodies were visualized using CY2 conjugated streptavidin 1.8 μg/mL (Jackson Immuno Labs). The sections were mounted on slides using Prolong Gold anti-fade mounting media (Molecular Probes).
Quantification of New Neurons.
Cells were quantified using unbiased stereological sampling with a modified optical fractionator (55). Briefly, in accordance with stereological principles, the first section chosen for analysis was selected randomly from the 36 most anterior sections containing the GCL and every 36th section after that was evaluated, resulting in a total of 8 or 9 sections per animal for each of the two sets stained. BrdU positive cells were counted exhaustively within each section (using the bottom face of the tissue as the exclusion plain) and typed according to cell layer and cell-specific markers (total of 946 cells) using a Leica SP2 confocal microscope. SGZ was defined as a 2-cell body width layer inside the GCL. Nuclei that were transected by a straight-edge placed along the margin of the GCL were counted in GCL population. Dendritic branching of granule cells was assessed in only one of the two sets (total of 415 cells). Branching was quantified by counting the number of branch points in both GCL and molecular layer using the confocal microscope to follow DCX labeled dendrites from the cell body outwards in three dimensions. This approach assured that overlapping dendritic fields from two or more DCX labeled cells could be distinguished from one another. All quantification was done blind to the time points.
Analysis.
In study 1, in which single BrdU injections were made, the total number of BrdU+ cells was used for analysis (Fig. 1). To eliminate possible differences in the overall rate of neurogenesis due to age, housing conditions, activity level, and number of injections, the NeuN, DCX, and βIII-tubulin counts from both studies were presented in proportion to the total BrdU+ cells. These percentages where first calculated for the individual animals followed by calculation of means and SEs for each group. Statistics were performed using the SAS statistical package.
Acknowledgments
We thank Julie Markham, Justin Rhodes, and Neal Cohen for their feedback on earlier drafts of the manuscript. Support was contributed by National Institutes of Health Grant AG10154 and grants from the Spastic Paralysis and Allied Diseases of the Central Nervous System Research Foundation and the Retirement Research Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
References
- 1.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 2.Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- 3.Kornack DR, Rakic P. Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci USA. 1999;96:5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aimone JB, Deng W, Gage FH. Adult neurogenesis: integrating theories and separating functions. Trends Cogn Sci. 2010;14:325–37. doi: 10.1016/j.tics.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci. 2002;22:635–638. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur J Neurosci. 2003;18:215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- 8.Newton SS, Duman RS. Regulation of neurogenesis and angiogenesis in depression. Curr Neurovasc Res. 2004;1:261–267. doi: 10.2174/1567202043362388. [DOI] [PubMed] [Google Scholar]
- 9.Balu DT, Lucki I. Adult hippocampal neurogenesis: Regulation, functional implications, and contribution to disease pathology. Neurosci Biobehav Rev. 2009;33:232–252. doi: 10.1016/j.neubiorev.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markakis EA, Gage FH. Adult-generated neurons in the dentate gyrus send axonal projections to field CA3 and are surrounded by synaptic vesicles. J Comp Neurol. 1999;406:449–460. [PubMed] [Google Scholar]
- 11.Overstreet-Wadiche LS, Westbrook GL. Functional maturation of adult-generated granule cells. Hippocampus. 2006;16:208–215. doi: 10.1002/hipo.20152. [DOI] [PubMed] [Google Scholar]
- 12.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espósito MS, et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder JS, et al. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown JP, et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 17.Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 18.McDonald HY, Wojtowicz JM. Dynamics of neurogenesis in the dentate gyrus of adult rats. Neurosci Lett. 2005;385:70–75. doi: 10.1016/j.neulet.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 19.Aizawa K, Ageyama N, Yokoyama C, Hisatsune T. Age-dependent alteration in hippocampal neurogenesis correlates with learning performance of macaque monkeys. Exp Anim. 2009;58:403–407. doi: 10.1538/expanim.58.403. [DOI] [PubMed] [Google Scholar]
- 20.Jabès A, Lavenex PB, Amaral DG, Lavenex P. Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus. Eur J Neurosci. 2010;31:273–285. doi: 10.1111/j.1460-9568.2009.07061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koketsu D, et al. Increased number of new neurons in the olfactory bulb and hippocampus of adult non-human primates after focal ischemia. Exp Neurol. 2006;199:92–102. doi: 10.1016/j.expneurol.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Perera TD, et al. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gould E, Vail N, Wagers M, Gross CG. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc Natl Acad Sci USA. 2001;98:10910–10917. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ngwenya LB, Peters A, Rosene DL. Maturational sequence of newly generated neurons in the dentate gyrus of the young adult rhesus monkey. J Comp Neurol. 2006;498:204–216. doi: 10.1002/cne.21045. [DOI] [PubMed] [Google Scholar]
- 25.Rhyu IJ, et al. Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience. 2010;167:1239–1248. doi: 10.1016/j.neuroscience.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rakic P. A small step for the cell, a giant leap for mankind: A hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. doi: 10.1016/0166-2236(95)93934-p. [DOI] [PubMed] [Google Scholar]
- 27.Caviness VS, Jr, Takahashi T, Nowakowski RS. Numbers, time and neocortical neuronogenesis: A general developmental and evolutionary model. Trends Neurosci. 1995;18:379–383. doi: 10.1016/0166-2236(95)93933-o. [DOI] [PubMed] [Google Scholar]
- 28.Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- 29.Rakic P, Nowakowski RS. The time of origin of neurons in the hippocampal region of the rhesus monkey. J Comp Neurol. 1981;196:99–128. doi: 10.1002/cne.901960109. [DOI] [PubMed] [Google Scholar]
- 30.Kornack DR, Rakic P. Changes in cell-cycle kinetics during the development and evolution of primate neocortex. Proc Natl Acad Sci USA. 1998;95:1242–1246. doi: 10.1073/pnas.95.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi T, Nowakowski RS, Caviness VS., Jr The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J Neurosci. 1995;15:6046–6057. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 33.Mandyam CD, Harburg GC, Eisch AJ. Determination of key aspects of precursor cell proliferation, cell cycle length and kinetics in the adult mouse subgranular zone. Neuroscience. 2007;146:108–122. doi: 10.1016/j.neuroscience.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prickaerts J, Koopmans G, Blokland A, Scheepens A. Learning and adult neurogenesis: survival with or without proliferation? Neurobiol Learn Mem. 2004;81:1–11. doi: 10.1016/j.nlm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Hayes NL, Nowakowski RS. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Brain Res Dev Brain Res. 2002;134:77–85. doi: 10.1016/s0165-3806(01)00324-8. [DOI] [PubMed] [Google Scholar]
- 36.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 37.Brown J, et al. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- 38.Rhodes JS, et al. Exercise increases hippocampal neurogenesis to high levels but does not improve spatial learning in mice bred for increased voluntary wheel running. Behav Neurosci. 2003;117:1006–1016. doi: 10.1037/0735-7044.117.5.1006. [DOI] [PubMed] [Google Scholar]
- 39.Burns KA, et al. Nestin-CreER mice reveal DNA synthesis by nonapoptotic neurons following cerebral ischemia hypoxia. Cereb Cortex. 2007;17:2585–2592. doi: 10.1093/cercor/bhl164. [DOI] [PubMed] [Google Scholar]
- 40.Jabès A, Lavenex PB, Amaral DG, Lavenex P. Postnatal development of the hippocampal formation: a stereological study in macaque monkeys. J Comp Neurol. 2011;519:1051–1070. doi: 10.1002/cne.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.des Portes V, et al. A novel CNS gene required for neuronal migration and involved in X-linked subcortical laminar heterotopia and lissencephaly syndrome. Cell. 1998;92:51–61. doi: 10.1016/s0092-8674(00)80898-3. [DOI] [PubMed] [Google Scholar]
- 42.Francis F, et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- 43.Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- 44.Meyer G, Perez-Garcia CG, Gleeson JG. Selective expression of doublecortin and LIS1 in developing human cortex suggests unique modes of neuronal movement. Cereb Cortex. 2002;12:1225–1236. doi: 10.1093/cercor/12.12.1225. [DOI] [PubMed] [Google Scholar]
- 45.Mattson MP. Establishment and plasticity of neuronal polarity. J Neurosci Res. 1999;57:577–589. [PubMed] [Google Scholar]
- 46.Feng Y, Walsh CA. Protein-protein interactions, cytoskeletal regulation and neuronal migration. Nat Rev Neurosci. 2001;2:408–416. doi: 10.1038/35077559. [DOI] [PubMed] [Google Scholar]
- 47.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 48.Eisch AJ, et al. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28:11785–11791. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santarelli L, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 51.Blier P. The pharmacology of putative early-onset antidepressant strategies. Eur Neuropsychopharmacol. 2003;13:57–66. doi: 10.1016/s0924-977x(02)00173-6. [DOI] [PubMed] [Google Scholar]
- 52.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in thesubdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 53.Leuner B, Glasper ER, Gould E. Thymidine analog methods for studies of adult neurogenesis are not equally sensitive. J Comp Neurol. 2009;517:123–133. doi: 10.1002/cne.22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clifton DK, Bremner WJ, Steiner RA. An automated technique for the radiographic determination of bone age. J Med Primatol. 1982;11:147–154. [PubMed] [Google Scholar]
- 55.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]