Abstract
The heteromeric inwardly rectifying Kir4.1/Kir5.1 K+ channel underlies the basolateral K+ conductance in the distal nephron and is extremely sensitive to inhibition by intracellular pH. The functional importance of Kir4.1/Kir5.1 in renal ion transport has recently been highlighted by mutations in the human Kir4.1 gene (KCNJ10) that result in seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME)/epilepsy, ataxia, sensorineural deafness, and renal tubulopathy (EAST) syndrome, a complex disorder that includes salt wasting and hypokalemic alkalosis. Here, we investigated the role of the Kir5.1 subunit in mice with a targeted disruption of the Kir5.1 gene (Kcnj16). The Kir5.1−/− mice displayed hypokalemic, hyperchloremic metabolic acidosis with hypercalciuria. The short-term responses to hydrochlorothiazide, an inhibitor of ion transport in the distal convoluted tubule (DCT), were also exaggerated, indicating excessive renal Na+ absorption in this segment. Furthermore, chronic treatment with hydrochlorothiazide normalized urinary excretion of Na+ and Ca2+, and abolished acidosis in Kir5.1−/− mice. Finally, in contrast to WT mice, electrophysiological recording of K+ channels in the DCT basolateral membrane of Kir5.1−/− mice revealed that, even though Kir5.1 is absent, there is an increased K+ conductance caused by the decreased pH sensitivity of the remaining homomeric Kir4.1 channels. In conclusion, disruption of Kcnj16 induces a severe renal phenotype that, apart from hypokalemia, is the opposite of the phenotype seen in SeSAME/EAST syndrome. These results highlight the important role that Kir5.1 plays as a pH-sensitive regulator of salt transport in the DCT, and the implication of these results for the correct genetic diagnosis of renal tubulopathies is discussed.
Keywords: kidney, homeostasis, acid–base balance
K+ channels play critical roles in renal tubular transport functions directly by providing a secretory pathway for K+ or indirectly by controlling membrane voltage and K+ recycling across the plasma membrane (1). Recent genetic and functional studies have shown that loss of renal K+ channel activity not only has an impact on K+ balance, but also affects other ion transport systems and the regulation of the acid–base balance. For instance, it has been clearly established that inherited mutations of the human KCNJ1 gene underlie type II Bartter syndrome, a severe disorder involving renal salt wasting and hypokalemic metabolic alkalosis (2). Likewise, inactivation of the Kcnk5 gene encoding the acid- and volume-sensitive TASK2 K+ channel induces renal HCO3− loss and metabolic acidosis in mice (3).
The cortical thick ascending limb (4, 5), distal convoluted tubule (DCT) (6, 7), and cortical collecting duct (CCD) principal cells (8, 9) are endowed with K+ channels displaying properties identical to inwardly rectifying heteromeric Kir4.1/Kir5.1 K+ channels (10). Recent studies have reported that rare homozygous missense mutations of the human KCNJ10 gene, which encodes the Kir4.1 subunit, underlie SeSAME/EAST syndrome, a rare disorder in which patients experience neurological and renal symptoms (11, 12). In the kidney, it is thought that these loss-of-function mutations in Kir4.1 impair the function of heteromeric Kir4.1/Kir5.1 channels (13, 14), thereby dramatically impairing salt reuptake from the DCT, and increasing downstream K+ and H+ secretion. This results in a Gitelman-like syndrome, characterized by salt wasting, hypocalciuria, hypomagnesaemia, and hypokalemic metabolic alkalosis (11, 12, 15).
The basolateral location of Kir4.1/Kir5.1 channels obviously suggests a role for these channels in maintenance of the basolateral membrane potential, and in the “recycling” across the basolateral membrane of any K+ that enters the cell via the basolateral Na+/K+ ATPase. The recycling of K+ is a crucial step for sustained transepithelial Na+ reabsorption. However, in the DCT of patients with seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME)/epilepsy, ataxia, sensorineural deafness, and renal tubulopathy (EAST) syndrome, this K+ recycling seems to be altered.
The expression of Kir5.1 alone does not produce detectable K+ currents in most recombinant expression systems (16). Instead, this “silent” subunit appears to heteromultimerize with Kir4.1 to produce novel Kir4.1/Kir5.1 channels (17–19) that have an extreme sensitivity to intracellular pH (pHi) within the physiological range (i.e., pKa of 7.4) (18, 20, 21). By contrast, homomeric Kir4.1 channels are only mildly sensitive to pHi (pKa, 6.0). This therefore raises the important question of how Kir5.1 contributes to renal ion transport and the role of these highly pH-sensitive Kir4.1/Kir5.1 channels in this process.
The present study addresses this issue by using a recently created strain of mice in which the Kir5.1(Kcnj16) gene has been deleted (22). We show that deletion of this subunit results in a pHi-insensitive, highly active K+ conductance in the DCT basolateral membrane, and a severe renal phenotype characterized by hypokalemic metabolic acidosis with hypercalciuria. Thus, instead of producing an effect similar to SeSAME/EAST syndrome mutations in Kir4.1, inactivation of Kir5.1 reduces the pHi sensitivity of the DCT basolateral K+ conductance and produces a mirror-image phenotype to SeSAME/EAST syndrome by increasing the mean basolateral K+ conductance in the distal nephron.
Results
Clinical Parameters of Kir5.1−/− Mice.
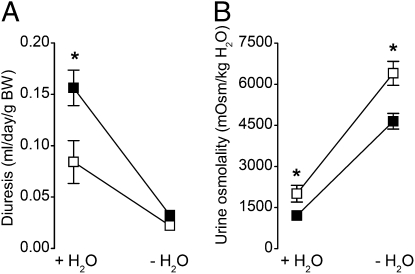
There was no obvious difference in the survival or gross physical appearance between Kir5.1+/+ and Kir5.1−/− mice maintained on a control diet, except for an approximately 15% lower body weight of the Kir5.1−/− mice (P < 0.0001; Table 1). Kir5.1−/− mice were slightly polydipsic, and exhibited polyuria and correspondingly lower urine osmolality. However, both groups of mice adapted normally to 24 h water restriction (Fig. 1), indicating that the urine-concentrating ability of Kir5.1−/− mice is not impaired. Furthermore, the hematocrit level of Kir5.1−/− mice was not increased (45.7 ± 1.42% vs. 45.5 ± 1.13% for Kir5.1+/+ and Kir5.1−/− mice, respectively; P = 0.4), indicating the absence of dehydration.
Table 1.
Biological parameters in Kir5.1+/+ and Kir5.1−/− mice
| Parameter | Kir5.1+/+ (n = 11) | Kir5.1−/− (n = 10) |
| Total BW, g | 27.9 ± 0.26 | 24.7 ± 0.57* |
| Water intake, μL/g BW/d | 167 ± 16 | 228 ± 14† |
| Systolic blood pressure, mm Hg | 109.8 ± 2.4 | 104 ± 2.6 |
| Urine | ||
| Uv, μL/g BW/d | 52 ± 11 | 105 ± 14† |
| Osmolality, mOsm/kg H2O | 2598 ± 247 | 1718 ± 167† |
| pH | 5.62 ± 0.04 | 5.68 ± 0.04 |
| Na+, mmol/mmol creatinine | 75.3 ± 4.8 | 67.6 ± 2.7 |
| K+, mmol/mmol creatinine | 8.23 ± 1.45 | 14.36 ± 1.77‡ |
| Cl−, mmol/mmol creatinine | 137.4 ± 10.2 | 136 ± 5.6 |
| Ca2+, mmol/mmol creatinine | 0.43 ± 0.06 | 1.82 ± 0.35‡ |
| Mg2+, mmol/mmol creatinine | 2.68 ± 0.25 | 3.42 ± 0.2§ |
| Aldosterone, μmol/mmol creatinine | 3.38 ± 0.54 | 2.71 ± 0.4 |
| Blood | ||
| pH | 7.35 ± 0.0.015 | 7.19 ± 0.015* |
| PCO2, mm Hg | 45.5 ± 1.1 | 45.7 ± 1.4 |
| [HCO3−], mM | 24.4 ± 0.5 | 16.9 ± 0.3* |
| Plasma | ||
| Osmolality (mOsm/kg H2O) | 305 ± 1.7 | 303 ± 5.2 |
| [Cl−], mM | 122.5 ± 0.7 | 130.6 ± 0.8* |
| [Na+], mM | 149.8 ± 2.4 | 153.1 ± 0.5 |
| [K+], mM | 4.4 ± 0.14 | 3.9 ± 0.12‡ |
| [Ca2+], mM | 1.26 ± 0.007 | 1.27 ± 0.009 |
| [Mg2+], mM | 1.66 ± 0.15 | 1.53 ± 0.37 |
Values are mean ± SEM for the numbers of animals given in brackets, except for fractional excretions in Kir5.1−/− mice, where n = 7, for plasma K+ concentration of Kir5.1+/+ and Kir5.1−/− mice, where n = 9 and n = 6, respectively, and for diastolic blood pressure of Kir5.1+/+ mice, where n = 12.
*P < 0.0001, †P < 0.005, ‡P < 0.05, and §P < 0.01.
Fig. 1.
Urine-concentrating ability of Kir5.1+/+ and Kir5.1−/− mice. Daily diuresis (A) and urine osmolality (B) of Kir5.1+/+ (□) and Kir5.1−/− (■) mice maintained in metabolic cages were first measured while the mice had free access to demineralized water (+H2O), and then after a 24-h period of water deprivation (−H2O). Weight loss induced by water deprivation averaged 7% and 9% for Kir5.1+/+ and Kir5.1−/− mice, respectively. Water deprivation tests showed that both groups of mice had similar urine-concentrating abilities. Values are mean values for 10 Kir5.1+/+ and eight Kir5.1−/− mice. Error bars represent SEM when larger than symbols. *P < 0.05, Kir5.1−/− versus Kir5.1+/+ mice.
Kir5.1−/− Mice Display Metabolic Acidosis and Hypokalemia.
Blood and plasma analyses revealed that Kir5.1−/− mice had metabolic hyperchloremic acidosis (Table 1). However, despite this acidosis, urinary NH4+ excretion was not increased (81 ± 3.5 mmol/mmol creatinine in 10 Kir5.1−/− mice vs. 99 ± 5.2 mmol/mmol creatinine in 11 Kir5.1+/+ mice). Also, glomerular filtration rates were similar in Kir5.1+/+ mice (308 ± 43 μL/min; n = 11) and Kir5.1−/− mice (272 ± 35 μL/min; n = 7; P = 0.54), indicating the absence of chronic renal failure. Our data therefore clearly show that Kir5.1−/− mice have renal tubular acidosis. However, this may also be associated with a respiratory component because the Kir5.1−/− mice do not display the expected compensatory decrease in blood PCO2 (Table 1), an effect that would be consistent with the respiratory phenotype recently reported in these mice (22).
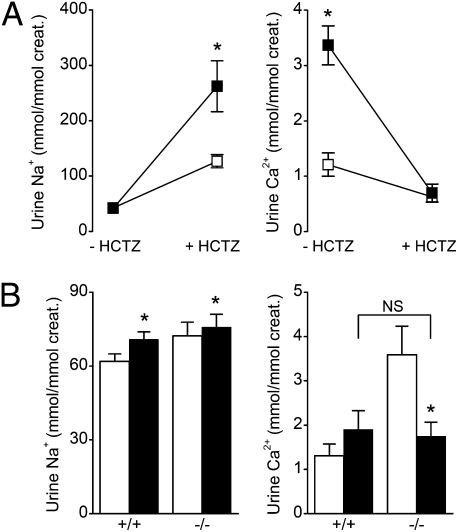
In addition to renal acidosis, the Kir5.1−/− mice also exhibited hypokalemia, associated with increased renal K+ excretion, as well as hypercalciuria and hypermagnesuria (Table 1). However, Kir5.1−/− mice were able to balance Na+ normally, and had normal blood pressure and 24-h urinary aldosterone excretion rates (Table 1).
No Alteration in the Responses to Furosemide and Amiloride in Kir5.1−/− Mice.
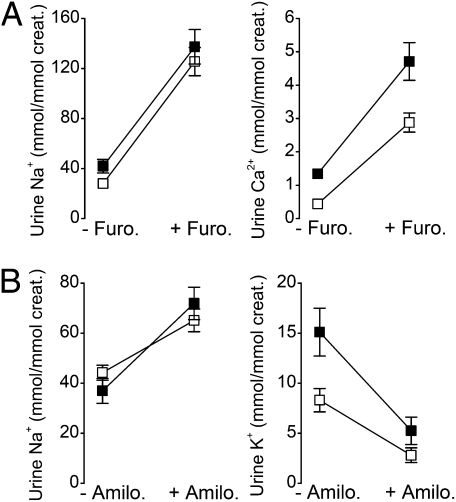
Expression of Kir5.1 is also seen in the TAL and DCT cells, as well as in CCD principal cells, where it coassembles with Kir4.1 to form Kir4.1/Kir5.1 channels in the basolateral membrane (5, 6, 9). We therefore investigated the effects of Kcnj16 disruption on NaCl transport in vivo by monitoring the response to diuretics in these nephron segments. TAL function was challenged with the loop diuretic furosemide, an inhibitor of the apical Na+-K+-2Cl− cotransporter. Fig. 2A shows that Kir5.1+/+ (n = 12) and Kir5.1−/− mice (n = 12) responded with similar increases in the urinary excretion of Na+ and Ca2+ 6 h after start of the treatment. Administration of the diuretic also similarly increased urine volume and urinary K+ and Mg2+ excretion (Table S1). Thus, NaCl absorption in the TAL is clearly not affected in Kir5.1−/− mice.
Fig. 2.
Effects of furosemide and amiloride. Kir5.1+/+ (□) and Kir5.1−/− (■) mice maintained in metabolic cages were subjected to furosemide (A) or amiloride (B) treatment for 6 h. Urinary Na+ and Ca2+ excretion were measured before (−Furo) and after (+Furo) furosemide treatment, and urinary Na+ and K+ excretion were measured before (−Amilo) and after (+Amilo) amiloride treatments. Values are means for 12 (A) or 10 (B) Kir5.1+/+ mice and 12 (A) or eight (B) Kir5.1−/− mice. Error bars represent SEM when larger than symbols. All diuretic-induced responses were statistically significant (P < 0.05).
We next investigated the effects of the K+-sparing diuretic amiloride, a blocker of the apical epithelial sodium channel (ENaC), which mediates sodium absorption in the aldosterone-sensitive distal nephron (ASDN). As expected, amiloride (Fig. 2B) increased urinary Na+ while decreasing urinary K+ excretion 6 h after its injection. However, the response to amiloride was the same in both groups of mice, indicating that ENaC-mediated Na+ absorption is not altered by deletion of Kir5.1.
Increased Response to Hydrochlorothiazide in Kir5.1−/− Mice.
Loss of function mutations in Kir4.1 predominantly affect DCT ion transport (11, 12). We therefore investigated the effects of hydrochlorothiazide (HCTZ), an inhibitor of the apical NaCl cotransporter in the DCT. Six hours after a single HCTZ injection, similar diuretic responses were observed in Kir5.1+/+ and Kir5.1−/− mice, with 3.5-fold increases in urine volume, and parallel increases in the excretion of K+ and Mg2+ (Table S2). However, as shown in Fig. 3A, HCTZ induced a significantly greater increase in Na+ urine excretion in Kir5.1−/− mice than in Kir5.1+/+ mice. HCTZ administration also normalized calciuria in Kir5.1−/− mice. These findings therefore demonstrate that genetic inactivation of Kir5.1 stimulates salt absorption in the DCT.
Fig. 3.
Short- and long-term effects of HCTZ. (A) Urinary Na+ and Ca2+ excretion of Kir5.1+/+ (□) and Kir5.1−/− (■) mice were measured before (−HCTZ) and after 6 h of treatment (+HCTZ). (B) Urinary Na+ (Left) and Ca2+ (Right) were measured in Kir5.1+/+ and Kir5.1−/− mice before (white bars) and after (black bars) a 4-d HCTZ treatment. Values are means ± SEM for 10 (A) or 12 (B) Kir5.1+/+ mice and nine (A) or 12 (B) Kir5.1−/− mice. *P < 0.05 versus control; NS, no significant difference at P < 0.05.
Interestingly, in type II pseudohypoaldosteronism (PHAII), WNK4 mutant mice also exhibit renal acidosis and hypercalciuria, an effect thought to be caused by excessive NaCl absorption in the DCT. This effect can also be corrected in the presence of thiazide (23, 24), and patients with PHAII are known to be highly sensitive to thiazide therapy (25, 26). This therefore prompted us to test the effects of chronic HCTZ treatment on Kir5.1+/+ and Kir5.1−/− mice. Interestingly, after treatment with HCTZ for 4 d, we observed a significant increase in blood pH and bicarbonate, showing that HCTZ abolished metabolic acidosis (Table 2). However, the effects of HCTZ treatment on urinary Ca2+ excretion were the same in both groups of mice (P = 0.37; Fig. 3B).
Table 2.
Long-term effects of HCTZ on plasma and blood parameters of Kir5.1+/+ and Kir5.1−/− mice
|
Kir5.1+/+ |
Kir5.1−/− |
|||
| Parameter | −HCTZ | +HCTZ | −HCTZ | +HCTZ |
| pH | 7.24 ± 0.03 | 7.30 ± 0.02 | 7.09 ± 0.05 | 7.22 ± 0.04* |
| PCO2, mm Hg | 59.3 ± 2.5 | 56 ± 1.1 | 56.4 ± 2.64 | 55.6 ± 1.92 |
| [HCO3−], mM | 23.7 ± 1.8 | 27.3 ± 1* | 16.9 ± 1.4 | 22.3 ± 1.2† |
| [Cl−], mM | 119 ± 1 | 113 ± 0.3† | 126 ± 1.27 | 114 ± 0.96† |
| [Na+], mM | 153.7 ± 2.7 | 151.2 ± 1.29 | 156.2 ± 1.86 | 151 ± 1.76 |
| [Ca2+], mM | 1.21 ± 0.02 | 1.16 ± 0.03 | 1.17 ± 0.03 | 1.15 ± 0.02 |
Parameters were determined from retroorbital blood samples taken before and after a 4-d treatment with HCTZ. Values are means ± SEM, for 11 Kir5.1+/+ and 12 Kir5.1−/− mice.
*P < 0.05 and †P = 0.0005, Wilcoxon signed-rank test.
Expression of Transport Proteins.
According to our findings, Kir5.1−/− mice do not display a global sodium imbalance, even though salt transport is increased in their DCT. We therefore determined the relative levels of protein expression for a range of key transport proteins as an indirect measurement of their ion transport capacities in the proximal convoluted tubule, TAL, DCT, and ASDN. However, there was no difference in levels of expression for the NHE3 Na+/H+ exchanger, the Na+-K+-2Cl− (NKCC2) and Na+-Cl− (NCC) cotransporters, or the ENaC α-subunit (αENaC) between the two groups of mice (Fig. S1), indicating that the alteration of transport we observe probably depends on changes in regulation rather than on differences in the maximal transport capacity per se.
Distinct K+ Channels in the DCT Basolateral Membranes of Kir5.1+/+ and Kir5.1−/− Mice.
In the DCT, the basolateral K+ channel has been shown to be a heteromeric Kir4.1/Kir5.1 channel (6). Therefore, the loss-of-function mutations in Kir4.1 associated with SeSAME/EAST syndrome are predicted to reduce the functional activity of this basolateral K+ channel (13, 14, 27). However, the functional properties of heteromeric Kir4.1/Kir5.1 channels (10, 18, 21, 28, 29) predict that genetic inactivation of Kir5.1 subunit will have a different effect on this conductance because the partnering Kir4.1 subunits still remain, and can form functional homomeric channels. Furthermore, although these remaining Kir4.1 channels have a smaller single-channel conductance compared with Kir4.1/Kir5.1 channels, they have an increased activity and a significantly reduced pHi sensitivity, which would be predicted to increase the activity of this conductance at physiological pHi. We therefore next used patch-clamp analysis to investigate the functional properties of the basolateral K+ conductance in isolated DCTs of WT and mutant mice.
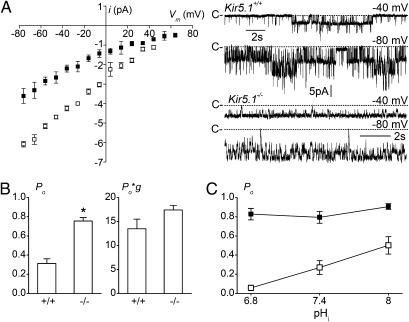
As previously described (6), and as expected from the reported properties of recombinant Kir4.1 and Kir4.1/Kir5.1 channels (10, 18, 21, 28, 29), only one type of basolateral K+ channel, with a conductance of 44.2 ± 1.1 pS (n = 15), was observed in DCT cells from Kir5.1+/+ mice (Fig. 4A), whereas in Kir5.1−/− mice, only a 23.3 ± 0.9 pS (n = 17) K+-selective channel was seen, with 145 mM K+ in the pipette (Fig. 4A). At the membrane resting potential, the average number of active channels per patch was similar in the two populations (5.7 ± 0.9 and 6.4 ± 1.2 channels in Kir5.1+/+ and Kir5.1−/− mice, respectively; P = 0.86). However, the open probability (Po) of these channels was much greater in Kir5.1−/− mice than in Kir5.1+/+ mice (0.74 ± 0.04 vs. 0.31 ± 0.05; P < 0.001; Fig. 4B). In this context, the time-averaged unit conductance Po*g was comparable in the two types of mice (Fig. 4B) despite a much lower single channel conductance in Kir5.1−/− mice. However, the calculated Po*g value in Kir5.1+/+ mice might actually be even lower in situ, as the DCT fragments used for patch-clamp experiments are not bathed in CO2/bicarbonate buffer, and so have a rather high pHi (7.57 ± 0.018; n = 4). Additional experiments with 15 mM K+ in the pipette also yielded a higher Po in Kir5.1−/− mice (n = 7) than in Kir5.1+/+ mice (n = 10; 0.70 ± 0.04 vs. 0.35 ± 0.06; P < 0.01). Furthermore, under this condition, Po*g was significantly higher in Kir5.1−/− mice than in Kir5.1+/+ mice (Fig. S2), a result that was caused in part by the fact that a 10-fold reduction in external [K+] had less influence on single-channel conductance in Kir5.1−/− mice than in Kir5.1+/+ mice (Fig. S2).
Fig. 4.
Analysis of channel currents in the basolateral membrane of DCT tubules. (A) Left: Current (i)–voltage (Vm) relationships for the K+ channels detected in the DCTs of Kir5.1+/+ (□) and Kir5.1−/− (■) mice in the cell-attached configuration. Tubules were bathed with physiological saline solution, and the pipette contained 145 mM KCl. Values are means of 15 patches from three Kir5.1+/+ mice and 17 patches from four Kir5.1−/− mice. Error bars represent SEM when larger than symbols. Right: Current traces recorded in the cell-attached mode at the designed potentials for the two groups. “C” indicates the closed current level. (B) Diagrams for the channel Po and the averaged unit conductance, as defined by the product of individual measurements of Po and g, for the two groups of mice. Values are means of the numbers patches in Kir5.1+/+ and Kir5.1−/− mice given in A. Error bars represent SEM; *P < 0.001. (C) Sensitivity to pHi as measured in inside-out patches at +60 to 80 mV for Kir5.1+/+ (□) and Kir5.1−/− (■) mouse DCTs. Each point is the mean of eight measurements in Kir5.1+/+ mice and nine measurements in Kir5.1−/− mice. Error bars represent SEM when larger than symbols.
The remaining channels in Kir5.1−/− mice were also significantly less pHi-sensitive. As illustrated in Fig. 4C, changes in pHi tested on inside-out patches dramatically affected the Po of the 45 pS K+ channel in Kir5.1+/+ mice (n = 8), but had no effect on the Po for 25 pS K+ channel of Kir5.1−/− mice, which was stable at the near maximum value of 0.8 over the pHi range of 6.8 to 8 (n = 9). These effects of pHi are consistent with previous results on native (6, 30, 31) and cloned (10, 17–20) channels and indicate that the remaining basolateral K+ conductance in Kir5.1−/− mice is probably homomeric Kir4.1. The reduced pH-sensitivity of the remaining current means that, despite deletion of the Kir5.1 channel, the relative basolateral K+ conductance in Kir5.1−/− mice is probably greater than that seen in WT mice.
Discussion
The critical role of the Kir4.1/Kir5.1 K+ channel in the distal nephron has recently been highlighted in SeSAME/EAST syndrome, which, together with neurological symptoms, is characterized by a renal tubulopathy consisting of compensated Na+ wasting, hypokalemic metabolic alkalosis, and hypocalciuria (11, 12). This syndrome is caused by mutations in the human KCNJ10 gene encoding the Kir4.1 subunit and results in a loss of function in both homomeric Kir4.1 and heteromeric Kir4.1/Kir5.1 channels (13, 14, 27). The clinical features of SeSAME/EAST syndrome are reminiscent of Gitelman syndrome, which is caused by mutations in the NCC in the DCT (32). In this study, we show that deletion of the Kcnj16 gene, which encodes the Kir5.1 subunit, also produces a severe renal phenotype in adult mice, characterized by metabolic acidosis, K+ wasting, and hypercalciuria. However, in contrast to SeSAME/EAST syndrome, we found that DCT function was stimulated in Kir5.1−/− mice. Thus, apart from the presence of hypokalemia, the phenotype observed in these mice is the reverse of that seen in neonatal Kcnj10 KO mice (11), and a mirror image of that seen in SeSAME/EAST syndrome.
Enhanced NCC activity is also the mark of PHAII, which includes metabolic acidosis and hypercalciuria, but is mainly characterized by hypertension, low aldosterone level, and hyperkalemia (23–26). HCTZ reverses all the abnormalities in patients with PHAII (26), suggesting that K+ and H+ secretion by the distal nephron is down-regulated in this disease. In contrast, chronic treatment of Kir5.1−/− mice with HCTZ rectified the metabolic acidosis and hypercalciuria, but not the hyperkaluria. In addition, ENaC-mediated Na+ absorption in the ASDN seems to be normal. This suggests that the metabolic acidosis and hypokalemia seen in Kir5.1−/− mice might have another, nondistal, origin. Nijenhuis et al. (33) postulated that a compensatory increase in Na+ absorption by the proximal tubule occurs during NCC inhibition, thus increasing passive Ca2+ absorption in this segment, a hypothesis also put forward by Bockenhauer et al. (11) for patients affected by EAST syndrome. Conversely, it has also been suggested that the metabolic acidosis in PHAII patients may be a result of reduced bicarbonate absorption and ammonia production in the proximal tubule (34). In the present study, the low urinary pH and low NH4+ excretion rate in Kir5.1−/− mice would be compatible with a down-regulated proximal tubule absorption.
Recombinant heteromeric Kir4.1/Kir5.1 and homomeric Kir4.1 channels display distinct properties in terms of their single-channel conductance (40–60 vs. 20 pS) (10, 16, 18, 21), Po (∼0.4 vs. ∼0.9 at pHi 7.4) (21), and, in particular, their sensitivity to pHi. This has enabled their identification in several native tissues, e.g., Kir4.1-like channels have been identified in native retinal Müller cells (31, 35) and retinal pigment epithelium (30), whereas heteromeric Kir4.1/Kir5.1-like channels have been proposed to resemble those found in the basolateral membrane of epithelia in the distal nephron (5, 6, 9). Therefore, our results provide further direct confirmation that the basolateral K+ conductance in the DCT is indeed produced by heteromeric Kir4.1/Kir5.1 channels.
Taken together, recent reports on SeSAME/EAST syndrome (11, 12) and the findings we report here show that DCT salt absorption can be dramatically altered by changes in Kir4.1/Kir5.1 K+ channel activity. Although the mechanisms involved remain elusive, a possible explanation may be found within the framework of the long-lived “pump and K+ conductance coupling” hypothesis (36–38). Transepithelial salt transport occurs according to a generally accepted model (Fig. 5A) in which the Na+/K+-ATPase maintains a favorable electrochemical gradient for Na+ influx at the apical membrane and provides an exit pathway for Na+ at the basolateral membrane. However, the continued functioning of Na+/K+-ATPase requires that K+ entering the cell via the Na+/K+-ATPase be recycled across the basolateral membrane via K+ channels. Accordingly, a decrease in basolateral K+ conductance would be expected to reduce transepithelial salt absorption, as observed in SeSAME/EAST syndrome, whereas an increase would up-regulate it (Fig. 5B), as we observe here with our Kir5.1−/− mice. In this regard, the fact that the phenotypic effects of disrupting the Kcnj10 or Kcnj16 genes are mainly observed in the DCT and not the TAL and CCD is consistent with our previous observations, which showed that Kir4.1/Kir5.1 is the only K+ channel species present in DCT basolateral membrane (39), whereas the TAL and CCD principal cells are endowed with additional basolateral K+ channels (9, 39).
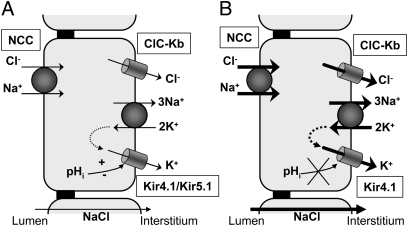
Fig. 5.
A cellular model for enhanced salt transport in DCT caused by deletion of Kcnj16. (A) Basolateral Kir4.1/Kir5.1 channels recycle K+ entering the cell via the Na+/K+ ATPase. The continuous functioning of the Na+/K+ ATPase maintains a chemical gradient favorable to Na+ and Cl− apical entry via the thiazide-sensitive electroneutral NCC. Kir4.1/Kir5.1 channels also maintain a negative basolateral membrane potential difference, thus providing a favorable electrochemical gradient to basolateral Cl− exit through ClC-Kb Cl− channels. Changes in pHi are sensed by Kir4.1/Kir5.1 channels and affect basolateral K+ conductance, thereby modulating salt reabsorption by DCT cells. (B) Kcnj16 deletion induces the formation of pHi-insensitive and constitutively highly active basolateral homomeric Kir4.1 channels. The resulting increase in basolateral K+ conductance is expected to enhance basolateral Cl− exit and Na+-K+ ATPase and NCC transport activities and thus to up-regulate overall salt reabsorption by DCT.
Interestingly, the pHi sensitivity of the DCT basolateral K+ conductance appears to be a key regulatory factor. Although homomeric Kir4.1 channels exhibit a mild sensitivity to inhibition by pHi (pKa, 6.0–6.4), heteromultimerization with Kir5.1 dramatically increases this pHi sensitivity and shifts it into the physiological range (pKa, 7.1–7.4) (10, 19–21, 29). The significant increase in pH sensitivity conferred by Kir5.1 means that, for homomeric Kir4.1 channels, their maximum activity is reached at a pHi of approximately 6.5 (18, 19), whereas for heteromeric Kir4.1/Kir5.1, it is reached at pHi values greater than 7.4. Several SeSAME/EAST mutations in Kir4.1 also shift the pHi sensitivity of heteromeric Kir4.1/Kir5.1 channels toward an even more alkaline pH, and so the overall effect of these mutations is to reduce channel activity at physiological pHi values. This is in marked contrast to the effect seen by deletion of the Kir5.1 subunit in these mice because the remaining homomeric Kir4.1 channels in the basolateral membrane possess a reduced pH sensitivity and so display a greater functional activity than that seen in WT Kir5.1+/+ mice. Small variations in pHi therefore have the potential to produce major changes in basolateral K+ conductance and so we may anticipate that under physiological conditions, changes in pHi might provide a regulatory link between NaCl uptake in the DCT and the acid/base status by reflecting the extracellular pH, as suggested by early studies (18).
Finally, although we observe a mirror-like phenotype to SeSAME/EAST syndrome in these Kir5.1−/− mice, this effect is a result of the complete absence of Kir5.1. In cases in which a stable but dysfunctional Kir5.1 subunit protein is produced, the phenotypic effects are likely to be very different because this subunit would have the potential to act as a dominant-negative subunit downregulating the overall functional activity of the basolateral K+ conductance and producing a phenotype more similar to that of SeSAME/EAST. Thus, future studies may reveal a complex range of related tubulopathies caused by mutations in KCNJ10 and KCNJ16 with differences being caused by the relative degree of functional activity of the basolateral K+ conductance at physiological pH.
Methods
Mice.
Kir5.1−/− mice were generated as previously described (22). Mutant and WT mice had free access to tap water, and maintained on standard (0.64% K+; 0.25% Na+) rodent chow (SAFE-A04; Usine d'Alimentation Rationnelle) until the studies began. All animals used in this study were handled in full compliance with the French government welfare policy. This work was performed under Permit 75–096 of the Veterinary Department of the French Ministry of Agriculture.
Metabolic Studies.
Kir5.1+/+ and Kir5.1−/− mice were individually housed in metabolic cages (Techniplast; Usine d'Alimentation Rationnelle), and their body weights, food and water intakes, and urine output were measured daily. Urine was collected under water-saturated mineral oil and in the presence of a few crystals of Thymol (VWR) to prevent bacterial degradation of NH4+. After a 5- to 7-d adaptation period with ad libitum access to water and purified powdered chow (SAFE A210; 0.27% Na+, 0.37% K+, 0.76% Ca2+), experiments were performed for a period of 5 to 10 d as appropriate. During days 1 to 5 of the experimental period, the mice were kept under control conditions. For the water-restriction experiments, demineralized water was substituted for mains water for 4 to 5 d before the 24-h water-deprivation period.
Methods for urine and blood analysis, immunoblot analysis, and arterial blood pressure measurements are detailed in SI Methods.
Diuretic Treatments.
Furosemide (Sigma-Aldrich) was given for 6 h at a dose of 90 μg/g body weight/d mixed with the powdered chow. The acute effects of HCTZ (Sigma-Aldrich) on urinary parameters of Kir5.1+/+ and Kir5.1−/− mice was studied 6 h after a single i.p. injection at a dose of 30 μg/g body weight. For chronic treatment, HCTZ was mixed with the powdered chow, and given for 4 d at a dose of 130 μg/g body weight/d (40). Amiloride (Sigma-Aldrich) was dissolved in physiological serum and injected s.c. at a single dose of 1.45 μg/g body weight. Its effects on urinary parameters were measured 6 h after injection.
Electrophysiology.
Patch-clamp analysis of single K+ channels was performed as previously described (6). Briefly, microdissected DCTs were transferred to a physiological saline solution containing (in mM) 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, and 10 Hepes, and adjusted to pH 7.4 with NaOH. The patch pipettes were filled with a high-K+ solution containing (in mM) 145 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, and 10 Hepes, and adjusted to pH 7.4 with KOH. When appropriate, the potassium content of pipette solution was reduced to 15 mM by substituting 130 mM NaCl for KCl in the high-K+ solution. In the cell-excised configuration, the cytoplasmic side of the membrane patch was exposed to a nominally Ca2+- and Mg2+-free solution (6), which contained (in mM) 145 KCl, 5 K-EDTA, 10 glucose, and 10 Hepes, and the pH of which was adjusted to 6.8 to 8 with KOH. Experiments were carried out at room temperature (22–27 °C).
Statistics.
All data are reported as means ± SEM for n experiments. Comparisons of parameters between groups were performed with the Mann–Whitney rank-sum test or the Student t test for two independent variables. Comparisons of the parameters of two related samples were performed by using the Wilcoxon signed-rank test or Student t test for paired variables. P values lower than 0.05 were taken to represent statistically significant differences.
Supplementary Material
Acknowledgments
We thank Monika Ghosh for help with preparation of the manuscript, and Gregory Messaoudi and Marine Grégoire for contributions during their graduate studies at Université Pierre et Marie Curie Paris 6. We also thank Lijun Shang for help with genotyping, and Sara Wells and the staff at the Mary Lyon Centre, Medical Research Council Harwell, for help with the initial generation and maintenance of the mutant mice. This work was supported by Agence Nationale de la Recherche (ANR) Grant Physio 2007-RPVO7084. M.K. holds a PhD fellowship from the French Ministère de la Recherche. S.K.R. holds a PhD fellowship from the Erasmus Mundus External Cooperation Window Lot 15 India European scholarship program. M.P. is an Institut National de la Santé et de la Recherche Médicale researcher. S.J.T. was supported by the Royal Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101400108/-/DCSupplemental.
References
- 1.Wang W, Hebert SC, Giebisch G. Renal K+ channels: Structure and function. Annu Rev Physiol. 1997;59:413–436. doi: 10.1146/annurev.physiol.59.1.413. [DOI] [PubMed] [Google Scholar]
- 2.Lorenz JN, et al. Impaired renal NaCl absorption in mice lacking the ROMK potassium channel, a model for type II Bartter's syndrome. J Biol Chem. 2002;277:37871–37880. doi: 10.1074/jbc.M205627200. [DOI] [PubMed] [Google Scholar]
- 3.Warth R, et al. Proximal renal tubular acidosis in TASK2 K+ channel-deficient mice reveals a mechanism for stabilizing bicarbonate transport. Proc Natl Acad Sci USA. 2004;101:8215–8220. doi: 10.1073/pnas.0400081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurst AM, Duplain M, Lapointe JY. Basolateral membrane potassium channels in rabbit cortical thick ascending limb. Am J Physiol. 1992;263:F262–F267. doi: 10.1152/ajprenal.1992.263.2.F262. [DOI] [PubMed] [Google Scholar]
- 5.Paulais M, Lourdel S, Teulon J. Properties of an inwardly rectifying K(+) channel in the basolateral membrane of mouse TAL. Am J Physiol Renal Physiol. 2002;282:F866–F876. doi: 10.1152/ajprenal.00238.2001. [DOI] [PubMed] [Google Scholar]
- 6.Lourdel S, et al. An inward rectifier K(+) channel at the basolateral membrane of the mouse distal convoluted tubule: Similarities with Kir4-Kir5.1 heteromeric channels. J Physiol. 2002;538:391–404. doi: 10.1113/jphysiol.2001.012961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taniguchi J, Yoshitomi K, Imai M. K+ channel currents in basolateral membrane of distal convoluted tubule of rabbit kidney. Am J Physiol. 1989;256:F246–F254. doi: 10.1152/ajprenal.1989.256.2.F246. [DOI] [PubMed] [Google Scholar]
- 8.Gray DA, Frindt G, Zhang YY, Palmer LG. Basolateral K+ conductance in principal cells of rat CCD. Am J Physiol Renal Physiol. 2005;288:F493–F504. doi: 10.1152/ajprenal.00301.2004. [DOI] [PubMed] [Google Scholar]
- 9.Lachheb S, et al. Kir4.1/Kir5.1 channel forms the major K+ channel in the basolateral membrane of mouse renal collecting duct principal cells. Am J Physiol Renal Physiol. 2008;294:F1398–F1407. doi: 10.1152/ajprenal.00288.2007. [DOI] [PubMed] [Google Scholar]
- 10.Pessia M, Tucker SJ, Lee K, Bond CT, Adelman JP. Subunit positional effects revealed by novel heteromeric inwardly rectifying K+ channels. EMBO J. 1996;15:2980–2987. [PMC free article] [PubMed] [Google Scholar]
- 11.Bockenhauer D, et al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scholl UI, et al. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci USA. 2009;106:5842–5847. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sala-Rabanal M, Kucheryavykh LY, Skatchkov SN, Eaton MJ, Nichols CG. Molecular mechanisms of EAST/SeSAME syndrome mutations in Kir4.1 (KCNJ10) J Biol Chem. 2010;285:36040–36048. doi: 10.1074/jbc.M110.163170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams DM, et al. Molecular basis of decreased Kir4.1 function in SeSAME/EAST syndrome. J Am Soc Nephrol. 2010;21:2117–2129. doi: 10.1681/ASN.2009121227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandulik S, et al. The salt-wasting phenotype of EAST syndrome, a disease with multifaceted symptoms linked to the KCNJ10 K+ channel. Pflugers Arch Eur J Physiol. 2011;461:423–435. doi: 10.1007/s00424-010-0915-0. [DOI] [PubMed] [Google Scholar]
- 16.Bond CT, et al. Cloning and expression of a family of inward rectifier potassium channels. Receptors Channels. 1994;2:183–191. [PubMed] [Google Scholar]
- 17.Pessia M, Imbrici P, D'Adamo MC, Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir4.2 potassium channels and their modulation by heteropolymerisation with Kir5.1. J Physiol. 2001;532:359–367. doi: 10.1111/j.1469-7793.2001.0359f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanemoto M, Kittaka N, Inanobe A, Kurachi Y. In vivo formation of a proton-sensitive K+ channel by heteromeric subunit assembly of Kir5.1 with Kir4.1. J Physiol. 2000;525:587–592. doi: 10.1111/j.1469-7793.2000.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Cui N, Yang Z, Qu Z, Jiang C. Modulation of kir4.1 and kir5.1 by hypercapnia and intracellular acidosis. J Physiol. 2000;524:725–735. doi: 10.1111/j.1469-7793.2000.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tucker SJ, Imbrici P, Salvatore L, D'Adamo MC, Pessia M. pH dependence of the inwardly rectifying potassium channel, Kir5.1, and localization in renal tubular epithelia. J Biol Chem. 2000;275:16404–16407. doi: 10.1074/jbc.C000127200. [DOI] [PubMed] [Google Scholar]
- 21.Yang Z, et al. Biophysical and molecular mechanisms underlying the modulation of heteromeric Kir4.1-Kir5.1 channels by CO2 and pH. J Gen Physiol. 2000;116:33–45. doi: 10.1085/jgp.116.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Adamo MC, et al. Genetic inactivation of Kcnj16 identifies Kir5.1 as an important neuronal CO2 chemoreceptor. J Biol Chem. 2011;296:192–198. doi: 10.1074/jbc.M110.189290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lalioti MD, et al. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet. 2006;38:1124–1132. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 24.Yang SS, et al. Molecular pathogenesis of pseudohypoaldosteronism type II: generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab. 2007;5:331–344. doi: 10.1016/j.cmet.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Furgeson SB, Linas S. Mechanisms of type I and type II pseudohypoaldosteronism. J Am Soc Nephrol. 2010;21:1842–1845. doi: 10.1681/ASN.2010050457. [DOI] [PubMed] [Google Scholar]
- 26.Mayan H, et al. Pseudohypoaldosteronism type II: Marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab. 2002;87:3248–3254. doi: 10.1210/jcem.87.7.8449. [DOI] [PubMed] [Google Scholar]
- 27.Reichold M, et al. KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc Natl Acad Sci USA. 2010;107:14490–14495. doi: 10.1073/pnas.1003072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takumi T, et al. A novel ATP-dependent inward rectifier potassium channel expressed predominantly in glial cells. J Biol Chem. 1995;270:16339–16346. doi: 10.1074/jbc.270.27.16339. [DOI] [PubMed] [Google Scholar]
- 29.Yang Z, Jiang C. Opposite effects of pH on open-state probability and single channel conductance of kir4.1 channels. J Physiol. 1999;520:921–927. doi: 10.1111/j.1469-7793.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kusaka S, et al. Expression and polarized distribution of an inwardly rectifying K+ channel, Kir4.1, in rat retinal pigment epithelium. J Physiol. 1999;520:373–381. doi: 10.1111/j.1469-7793.1999.00373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tada Y, Horio Y, Kurachi Y. Inwardly rectifying K+ channel in retinal Müller cells: comparison with the KAB-2/Kir4.1 channel expressed in HEK293T cells. Jpn J Physiol. 1998;48:71–80. doi: 10.2170/jjphysiol.48.71. [DOI] [PubMed] [Google Scholar]
- 32.Simon DB, et al. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 33.Nijenhuis T, et al. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest. 2005;115:1651–1658. doi: 10.1172/JCI24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nahum H, et al. Pseudohypoaldosteronism type II: proximal renal tubular acidosis and dDAVP-sensitive renal hyperkalemia. Am J Nephrol. 1986;6:253–262. doi: 10.1159/000167172. [DOI] [PubMed] [Google Scholar]
- 35.Ishii M, et al. Expression and clustered distribution of an inwardly rectifying potassium channel, KAB-2/Kir4.1, on mammalian retinal Müller cell membrane: their regulation by insulin and laminin signals. J Neurosci. 1997;17:7725–7735. doi: 10.1523/JNEUROSCI.17-20-07725.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dawson DC, Richards NW. Basolateral K conductance: Role in regulation of NaCl absorption and secretion. Am J Physiol. 1990;259:C181–C195. doi: 10.1152/ajpcell.1990.259.2.C181. [DOI] [PubMed] [Google Scholar]
- 37.Mauerer UR, Boulpaep EL, Segal AS. Regulation of an inwardly rectifying ATP-sensitive K+ channel in the basolateral membrane of renal proximal tubule. J Gen Physiol. 1998;111:161–180. doi: 10.1085/jgp.111.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsuchiya K, Wang W, Giebisch G, Welling PA. ATP is a coupling modulator of parallel Na,K-ATPase-K-channel activity in the renal proximal tubule. Proc Natl Acad Sci USA. 1992;89:6418–6422. doi: 10.1073/pnas.89.14.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paulais M, Lachheb S, Teulon J. A Na+- and Cl- -activated K+ channel in the thick ascending limb of mouse kidney. J Gen Physiol. 2006;127:205–215. doi: 10.1085/jgp.200509360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vallet M, et al. Pendrin regulation in mouse kidney primarily is chloride-dependent. J Am Soc Nephrol. 2006;17:2153–2163. doi: 10.1681/ASN.2005101054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.