Abstract
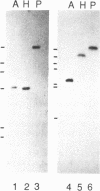
Although alternative splicing has been shown to give rise to isoforms of a number of transcription factors, such isoforms have not previously been detected for the POU homeodomain protein Pit-1. Screening of a rat pituitary GH3 cell cDNA expression library yielded a clone, termed pCMVPit-1a, encoding a 35.8 kD protein (Pit-1a) containing a 26 amino acid insert in the Pit-1 trans-activation domain. The position of the insert, plus Southern blot analysis, implied that Pit-1a mRNA arises by alternative splicing of the Pit-1 gene transcript. Pit-1a mRNA was detected in GH3 rat pituitary tumor cells at levels about 1/7 that of Pit-1 mRNA. Pit-1a mRNA-specific sequences were also detected in rat and mouse pituitary, and in mouse thyrotropic tumor TtT cells. DNA mobility shift assays showed that Pit-1a binds specifically to Pit-1 binding sites in the proximal prolactin promoter, but produces DNA-protein complexes of markedly different mobilities than Pit-1. In stably transfected CHO cells which accumulated approximately equal levels of either of the two proteins, Pit-1 trans-activated a prolactin promoter-driven CAT construct, while Pit-1a yielded no detectable transactivation, implying a trans-activation ratio for Pit-1a/Pit-1 of less than 0.05. Thus, the insertion of 26 amino acids of similar composition into the activation domain of Pit-1 has at once affected both the mode of binding of this protein and its ability to function as a trans-activator.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodner M., Castrillo J. L., Theill L. E., Deerinck T., Ellisman M., Karin M. The pituitary-specific transcription factor GHF-1 is a homeobox-containing protein. Cell. 1988 Nov 4;55(3):505–518. doi: 10.1016/0092-8674(88)90037-2. [DOI] [PubMed] [Google Scholar]
- Castrillo J. L., Theill L. E., Karin M. Function of the homeodomain protein GHF1 in pituitary cell proliferation. Science. 1991 Jul 12;253(5016):197–199. doi: 10.1126/science.1677216. [DOI] [PubMed] [Google Scholar]
- Ciudad C. J., Urlaub G., Chasin L. A. Deletion analysis of the Chinese hamster dihydrofolate reductase gene promoter. J Biol Chem. 1988 Nov 5;263(31):16274–16282. [PubMed] [Google Scholar]
- Ding Y., Lu W., Roberson M. S., Moye-Rowley W. S., Maurer R. A. The tissue-specific mammalian transcription factor, Pit-1, activates transcription in Saccharomyces cerevisiae. Mol Endocrinol. 1991 Sep;5(9):1239–1245. doi: 10.1210/mend-5-9-1239. [DOI] [PubMed] [Google Scholar]
- Erickson A. H., Blobel G. Cell-free translation of messenger RNA in a wheat germ system. Methods Enzymol. 1983;96:38–50. doi: 10.1016/s0076-6879(83)96007-x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fox S. R., Jong M. T., Casanova J., Ye Z. S., Stanley F., Samuels H. H. The homeodomain protein, Pit-1/GHF-1, is capable of binding to and activating cell-specific elements of both the growth hormone and prolactin gene promoters. Mol Endocrinol. 1990 Jul;4(7):1069–1080. doi: 10.1210/mend-4-7-1069. [DOI] [PubMed] [Google Scholar]
- Gershengorn M. C. Thyrotropin releasing hormone. A review of the mechanisms of acute stimulation of pituitary hormone release. Mol Cell Biochem. 1982 Jun 25;45(3):163–179. doi: 10.1007/BF00230085. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Gumbiner B., Kelly R. B. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982 Jan;28(1):51–59. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- Herr W., Sturm R. A., Clerc R. G., Corcoran L. M., Baltimore D., Sharp P. A., Ingraham H. A., Rosenfeld M. G., Finney M., Ruvkun G. The POU domain: a large conserved region in the mammalian pit-1, oct-1, oct-2, and Caenorhabditis elegans unc-86 gene products. Genes Dev. 1988 Dec;2(12A):1513–1516. doi: 10.1101/gad.2.12a.1513. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Chen R. P., Mangalam H. J., Elsholtz H. P., Flynn S. E., Lin C. R., Simmons D. M., Swanson L., Rosenfeld M. G. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988 Nov 4;55(3):519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Ingraham H. A., Flynn S. E., Voss J. W., Albert V. R., Kapiloff M. S., Wilson L., Rosenfeld M. G. The POU-specific domain of Pit-1 is essential for sequence-specific, high affinity DNA binding and DNA-dependent Pit-1-Pit-1 interactions. Cell. 1990 Jun 15;61(6):1021–1033. doi: 10.1016/0092-8674(90)90067-o. [DOI] [PubMed] [Google Scholar]
- Larkin S., Tait S., Treacy M., Martin F. Characterisation of tissue-specific trans-acting factor binding to a proximal element in the rat growth hormone gene promoter. Eur J Biochem. 1990 Aug 17;191(3):605–615. doi: 10.1111/j.1432-1033.1990.tb19164.x. [DOI] [PubMed] [Google Scholar]
- Li S., Crenshaw E. B., 3rd, Rawson E. J., Simmons D. M., Swanson L. W., Rosenfeld M. G. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990 Oct 11;347(6293):528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- Lufkin T., Bancroft C. Identification by cell fusion of gene sequences that interact with positive trans-acting factors. Science. 1987 Jul 17;237(4812):283–286. doi: 10.1126/science.3474782. [DOI] [PubMed] [Google Scholar]
- Mangalam H. J., Albert V. R., Ingraham H. A., Kapiloff M., Wilson L., Nelson C., Elsholtz H., Rosenfeld M. G. A pituitary POU domain protein, Pit-1, activates both growth hormone and prolactin promoters transcriptionally. Genes Dev. 1989 Jul;3(7):946–958. doi: 10.1101/gad.3.7.946. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Carothers A. M., Han J. H., Harding J. D., Kas E., Venolia L., Chasin L. A. Multiple transcription start sites, DNase I-hypersensitive sites, and an opposite-strand exon in the 5' region of the CHO dhfr gene. Mol Cell Biol. 1986 Feb;6(2):425–440. doi: 10.1128/mcb.6.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris A. E., Jiang Y. J., McChesney R. E., Jackson A. E., Bancroft C., Chasin L. A. Use of a selectable reporter for the isolation of mammalian regulatory genes. Gene. 1990 Oct 15;94(2):289–294. doi: 10.1016/0378-1119(90)90400-l. [DOI] [PubMed] [Google Scholar]
- Pan W. T., Liu Q. R., Bancroft C. Identification of a growth hormone gene promoter repressor element and its cognate double- and single-stranded DNA-binding proteins. J Biol Chem. 1990 Apr 25;265(12):7022–7028. [PubMed] [Google Scholar]
- Steinfelder H. J., Hauser P., Nakayama Y., Radovick S., McClaskey J. H., Taylor T., Weintraub B. D., Wondisford F. E. Thyrotropin-releasing hormone regulation of human TSHB expression: role of a pituitary-specific transcription factor (Pit-1/GHF-1) and potential interaction with a thyroid hormone-inhibitory element. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3130–3134. doi: 10.1073/pnas.88.8.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theill L. E., Castrillo J. L., Wu D., Karin M. Dissection of functional domains of the pituitary-specific transcription factor GHF-1. Nature. 1989 Dec 21;342(6252):945–948. doi: 10.1038/342945a0. [DOI] [PubMed] [Google Scholar]
- Theill L. E., Castrillo J. L., Wu D., Karin M. Dissection of functional domains of the pituitary-specific transcription factor GHF-1. Nature. 1989 Dec 21;342(6252):945–948. doi: 10.1038/342945a0. [DOI] [PubMed] [Google Scholar]
- Urlaub G., Chasin L. A. Isolation of Chinese hamster cell mutants deficient in dihydrofolate reductase activity. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4216–4220. doi: 10.1073/pnas.77.7.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urlaub G., Mitchell P. J., Kas E., Chasin L. A., Funanage V. L., Myoda T. T., Hamlin J. Effect of gamma rays at the dihydrofolate reductase locus: deletions and inversions. Somat Cell Mol Genet. 1986 Nov;12(6):555–566. doi: 10.1007/BF01671941. [DOI] [PubMed] [Google Scholar]
- Voss J. W., Yao T. P., Rosenfeld M. G. Alternative translation initiation site usage results in two structurally distinct forms of Pit-1. J Biol Chem. 1991 Jul 15;266(20):12832–12835. [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth T., Priess A., Annweiler A., Zwilling S., Oeler B. Multiple Oct2 isoforms are generated by alternative splicing. Nucleic Acids Res. 1991 Jan 11;19(1):43–51. doi: 10.1093/nar/19.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G. Z., Bancroft C. Mediation by calcium of thyrotropin--releasing hormone action on the prolactin promoter via transcription factor pit-1. Mol Endocrinol. 1991 Oct;5(10):1488–1497. doi: 10.1210/mend-5-10-1488. [DOI] [PubMed] [Google Scholar]
- Yan G. Z., Pan W. T., Bancroft C. Thyrotropin-releasing hormone action on the prolactin promoter is mediated by the POU protein pit-1. Mol Endocrinol. 1991 Apr;5(4):535–541. doi: 10.1210/mend-5-4-535. [DOI] [PubMed] [Google Scholar]