Abstract
Insulin resistance is a component of the metabolic syndrome and Type 2 diabetes. It has been recently shown that in liver insulin resistance is not complete. This so-called selective insulin resistance is characterized by defective insulin inhibition of hepatic glucose output while insulin-induced lipogenesis is maintained. How this occurs and whether uncoupled insulin action develops in other tissues is unknown. Here we show in a model of chronic hyperinsulinemia that adipocytes develop selective insulin resistance in which translocation of the GLUT4 glucose transporter to the cell surface is blunted yet nuclear exclusion of the FoxO1 transcription factor is preserved, rendering uncoupled insulin-controlled carbohydrate and lipid metabolisms. We found that in adipocytes FoxO1 nuclear exclusion has a lower half-maximal insulin dose than GLUT4 translocation, and it is because of this inherent greater sensitivity that control of FoxO1 by physiological insulin concentrations is maintained in adipocytes with compromised insulin signaling. Pharmacological and genetic interventions revealed that insulin regulates GLUT4 and FoxO1 through the PI3-kinase isoform p110α, although FoxO1 showed higher sensitivity to p110α activity than GLUT4. Transient down-regulation and overexpression of Akt isoforms in adipocytes demonstrated that insulin-activated PI3-kinase signals to GLUT4 primarily through Akt2 kinase, whereas Akt1 and Akt2 signal to FoxO1. We propose that the lower threshold of insulin activity for FoxO1’s nuclear exclusion is in part due to its regulation by both Akt isoforms. Identification of uncoupled insulin action in adipocytes suggests this condition might be a general phenomenon of insulin target tissues contributing to insulin resistance’s pathophysiology.
Insulin resistance is a disorder in which peripheral tissues fail to properly respond to normal insulin concentrations, resulting in deregulated carbohydrate and lipid homeostasis, which contributes to the risk of developing cardiovascular disease and Type 2 diabetes mellitus (T2DM). Although insulin resistance is defined by impaired insulin action, in this condition not all insulin functions are equally affected (1). Complete blockade of hepatic insulin signaling, as observed in humans with inherited mutations of the insulin receptor, results in hyperinsulinemia and hyperglycemia but low plasma triglycerides. Intriguingly, patients with T2DM have impaired insulin regulation of glucose homeostasis, but they have enhanced insulin-mediated hepatic lipogenesis. This so-called selective deregulation or “uncoupling” of glucose and lipid metabolism results in the deleterious combination of hyperinsulinemia, hyperglycemia, and hypertriglyceridemia (2). Thus, during the development of hepatic insulin resistance, downstream effectors of the insulin signaling pathway are differentially impaired, giving rise to a phenomenon known as “selective insulin resistance.” To date, the molecular mechanisms mediating selective perturbation of insulin action in the insulin-resistant state remain unknown. Furthermore, whether selective insulin resistance is a liver-specific phenomenon or develops in other insulin responsive tissues has not been addressed.
Insulin’s metabolic action is largely mediated through the activation of the phosphatidylinositol 3-kinase (PI3)-kinase and its downstream effectors the Akt kinases (3). Consistent with a central role for this pathway in insulin action, deregulation of PI3-kinase/Akt signaling is a hallmark of insulin-resistant tissues (4). The PI3-kinase/Akt pathway is a complex signaling network (5). PI3-kinase is a heterodimer containing a regulatory subunit and a p110 catalytic subunit, both of which are a family of isoforms (6). Likewise, protein kinase Akt exists as three isoforms in mammalian cells, Akt1-3 (7). Recent studies indicate PI3-kinase/Akt isoform signaling specificity in insulin-regulated glucose metabolism. Among p110 isoforms, p110α activity is essential to insulin-regulated hepatic gluconeogenesis and glucose transport into fat and muscle cells (8, 9). Akt2 is the predominant Akt isoform mediating insulin’s control of glucose metabolism (10–14). A role for p110α and Akt2 in insulin-mediated lipid metabolism has also been described (15, 16). Thus, strong experimental evidence suggests that isoform signaling specificity within the PI3-kinase/Akt pathway mediates insulin’s metabolic regulation. Intriguingly, this diversity of signaling downstream of the insulin receptor could provide branch points for selective signaling deregulation during the development of insulin resistance.
Here we found that hyperinsulinemia-induced perturbation of PI3-kinase/Akt signaling results in selective insulin resistance in adipocytes, in which insulin-induced GLUT4 trafficking is impaired while FoxO1 nuclear exclusion is largely preserved. Our data indicate that distinct insulin sensitivity and Akt signaling requirements for GLUT4 translocation and FoxO1 nuclear exclusion might underlie the uncoupling of these processes upon chronic insulin exposure.
Results
Hyperinsulinemia Uncouples Insulin Regulation of GLUT4 and FoxO1.
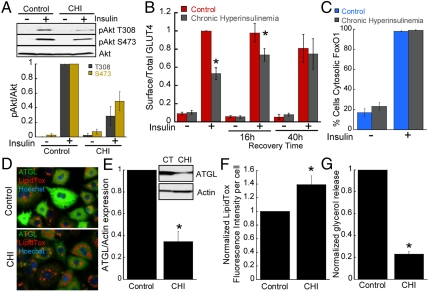
Hyperinsulinemia, a cause of insulin resistance in peripheral tissues, can be modeled by the chronic exposure of cultured 3T3-L1 adipocytes to high concentrations of insulin (17, 18). Here we use that model to investigate how deregulation of the PI3-kinase/Akt signaling affects insulin action. Adipocytes were incubated with 10 nM insulin for 16 h (referred to as chronic hyperinsulinemia) followed by 8 h incubation in serum-free medium to return cells to basal state, after which the effects of 1 nM insulin were assayed (19). One of the main acute effects of insulin is to induce the translocation of intracellular GLUT4 glucose transporter to the plasma membrane, thereby increasing glucose transport (20). To monitor GLUT4 behavior in adipocytes, we use a previously described HA-GLUT4-GFP reporter and quantitative fluorescence microscopy (Fig. S1A) (21). As shown in past studies, chronic hyperinsulinemia impaired both insulin-stimulated Akt phosphorylation and GLUT4 translocation to the plasma membrane (Fig. 1 A and B) (18, 19). The defect in GLUT4 translocation was fully reversed when cells were recultured for 40 h in growth medium without excess insulin, demonstrating that the adipocytes are not irreversibly damaged by the 16 h exposure to 10 nM insulin (Fig. 1B).
Fig. 1.
Hyperinsulinemia leads to selective insulin resistance in adipocytes. (A) Immunoblot analyses and densitometry of Akt phosphorylation from adipocytes unstimulated or stimulated with 1 nM insulin for 30 min. Each point is the mean ± SEM, n = 3. CHI, chronic hyperinsulinemia (10 nM insulin for 16 h). (B) Surface-to-total distribution of HA-GLUT4-GFP in unstimulated and 1 nM insulin-stimulated adipocytes. After exposure to 10 nM insulin for 16 h, cells were washed, serum-starved for 8 h, and 1 nM insulin-stimulated GLUT4 translocation assayed. Insulin-stimulated GLUT4 translocation was also measured in cells that after the 16 h incubation with 10 nM insulin were recultured in normal growth medium for 16 h or 40 h to measure reversibility of insulin resistance. Each point is the mean ± SEM, n≥3. (C) Percentage of adipocytes with cytosolic FoxO1-GFP. Each bar is the mean ± SEM, n = 5. (D) Micrographs of adipocytes stained for neutral lipids (LipidTox, red) and ATGL expression (green). Nuclei are stained with Hoechst 3322 (blue). (E) Immunoblot and densitometry analyses of ATGL expression. Chronic Hyperinsulinemia (CHI). Each point is the mean ± SEM, n = 5. (F) Neutral lipid accumulation in adipocytes. Neutral lipids were stained with LipidTox neutral lipid stain and the fluorescence intensity per cell was determined by quantitative fluorescence microscopy. Chronic Hyperinsulinemia (CHI). Each bar is the mean ± SE of five experiments. Approximately 200 cells per experiment were quantified. (G) Glycerol release in control adipocytes (CT) and adipocytes exposed to chronic hyperinsulinemia (CHI). Each bar is the mean ± SE of four experiments. *p < 0.05 versus control (t-test).
Exclusion of the transcription factor FoxO1 from the nucleus is another function of insulin downstream of Akt (Fig. S1B) (22). Interestingly, despite impaired Akt activation and blunted GLUT4 translocation, nuclear exclusion of FoxO1 stimulated by 1 nM insulin was preserved in cells exposed to chronic hyperinsulinemia (Fig. 1C). Insulin inactivates FoxO1 in adipocytes to control lipid metabolism, in part through the down-regulation of adipose triglyceride lipase (ATGL) and inhibition of lipolysis (23). Consistent with a negative role for FoxO1 in the control of lipolysis, we also found that FoxO1 knockdown caused an increase in neutral lipid accumulation concomitant with ATGL down-regulation (Fig. S1 C–E). To correlate the effect of chronic hyperinsulinemia on FoxO1 regulation and lipid metabolism we examined ATGL expression, lipid accumulation, and glycerol release. Immunofluorescence and Western blotting analyses revealed a reduction of ATGL following chronic hyperinsulinemia (Fig. 1 D and E). Concurrent with the reduction in ATGL, there was near 50% increase in neutral lipid accumulation and glycerol release was decreased by 70% in adipocytes exposed to chronic hyperinsulinemia (Fig. 1 D, F, and G). Although additional mechanisms might contribute to lipid accumulation in adipocytes exposed to chronic hyperinsulinemia (e.g., increased lipogenesis), our results indicate that preserved FoxO1 insulin responsiveness correlates with decreased lipolysis and enhanced lipid accumulation in this model of insulin resistance. Our analysis of GLUT4 translocation and FoxO1 exclusion from the nucleus establish a state of uncoupled insulin action in cultured adipocytes exposed to chronic hyperinsulinemia.
GLUT4 Translocation and FoxO1 Nuclear Exclusion Have Different Sensitivities to Insulin.
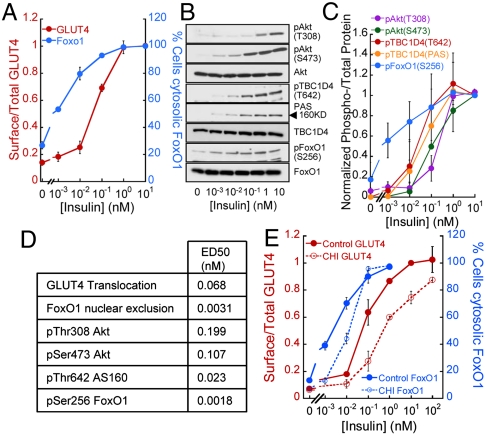
To further characterize the uncoupling of insulin-controlled GLUT4 translocation and FoxO1 nuclear exclusion, we determined the insulin-dose responses of these processes in adipocytes. FoxO1 nuclear exclusion was about 10 times more sensitive to insulin than GLUT4 translocation, with ED50s of approximately 3.1 pM ± 2.7 pM and 68 pM ± 20 pM insulin, respectively (Fig. 2 A and D). FoxO1 expression modulates insulin sensitivity in certain cellular contexts (22); therefore, to ensure that overexpression of the FoxO1-GFP was not affecting our measurements of FoxO1 insulin-dose response, we confirmed the increased sensitivity of FoxO1 to insulin by immunofluorescence of endogenous FoxO1 (Fig. S2).
Fig. 2.
GLUT4 translocation and FoxO1 nuclear exclusion display distinct sensitivities to insulin. (A) Insulin-dose response for HA-GLUT4-GFP translocation and FoxO1 nuclear exclusion. Each point is the mean ± SEM, n≥5. (B) Immunoblot of insulin signaling intermediates in adipocytes stimulated with insulin for 30 min. (C) Densitometry analyses of experiments like those shown in B. Each point is mean ± SEM, n = 3. Values were normalized to that of 10 nM insulin treated cells. (D) ED50s for insulin-induced GLUT4 plasma membrane translocation, FoxO1 nuclear exclusion, and Akt, TBC1D4, and FoxO1 phosphorylation. The values were derived from the data in A and C using a dose-response logistic curve fit. (E) Insulin-dose response for HA-GLUT4-GFP translocation and FoxO1-GFP nuclear exclusion in control adipocytes and adipocytes exposed to chronic hyperinsulinemia (CHI). Each point is the mean ± SEM, n≥3.
Insulin regulation of both GLUT4 and FoxO1 are dependent on Akt activation. Akt regulates GLUT4 translocation in part through the phosphorylation and inhibition of the RabGAP TBC1D4 (AS160), whereas FoxO1 is a direct substrate of Akt (24–26). Insulin induces dose-dependent phosphorylations of Akt, FoxO1, and TBC1D4 (Fig. 2 B and C). Interestingly, FoxO1 phosphorylation at Ser256, a gate-keeping site for FoxO1 nuclear exclusion, displayed enhanced insulin sensitivity compared to TBC1D4 phosphorylation (Fig. 2 C and D). These data are consistent with the observation that FoxO1 nuclear exclusion is more sensitive to insulin than GLUT4 plasma membrane translocation.
Chronic Hyperinsulinemia Rightward Shifts the Insulin-Dose Response for FoxO1 Nuclear Exclusion.
The increased sensitivity of FoxO1 to insulin prompted us to reexamine the effect of chronic hyperinsulinemia on exclusion of FoxO1 from the nucleus. FoxO1 nuclear exclusion stimulated by insulin concentrations ≥0.1 nM was not affected; however, the activities of lower insulin concentrations were indeed blunted (Fig. 2E). Thus, the insulin resistance of FoxO1 nuclear exclusion was revealed at low insulin concentrations, in contrast to the insulin resistance of GLUT4 translocation, which was detected at a wide range of insulin concentrations (Fig. 2E). Consequently, in adipocytes with impaired insulin signaling, low physiological doses of insulin that normally induced FoxO1 nuclear exclusion would no longer do so. However, at higher insulin concentrations (> 0.1 nM), FoxO1 will remain sensitive to insulin action while GLUT4 translocation will be impaired.
Different Thresholds of p110α Activity for GLUT4 Translocation and FoxO1 Nuclear Exclusion.
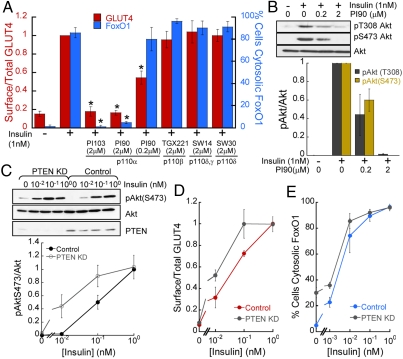
Activation of PI3-kinase is critical for insulin’s metabolic actions. Past studies demonstrated that insulin-stimulated glucose transport was solely dependent on p110α (8). Consistent with those findings, we observed that pharmacological inhibition of p110α, but not p110β, p110δ, or p110γ, blunted insulin-induced GLUT4 translocation (Fig. 3A). Furthermore, we found that insulin-stimulated FoxO1 nuclear exclusion was also solely dependent on p110α, supporting a key role for p110α in insulin control of adipocyte metabolism (Fig. 3A). Complete inhibition of p110α with PI90 (2 μM) completely inhibits insulin-stimulated phosphorylation of Akt and blocked insulin-stimulated FoxO1 nuclear exclusion (Fig. 3 A and B). However, a lower concentration of the PI90 (0.2 μM) that only partially blocked Akt phosphorylation impaired GLUT4 translocation but not FoxO1 nuclear exclusion (Fig. 3 A and B). These results are consistent with FoxO1 nuclear exclusion requiring less p110α activity than GLUT4 translocation.
Fig. 3.
GLUT4 and FoxO1 regulation by insulin display distinct sensitivities to p110α activity. (A) Surface-to-total distribution of HA-GLUT4-GFP and percentage of cells with cytosolic FoxO1-GFP in adipocytes treated with p110 isoform inhibitors (30 min) followed by 1 nM insulin stimulation (30 min). Each point is the mean ± SEM, n≥3. *p < 0.05 (t-test). (B) Immunoblot and densitometry analyses of Akt phosphorylation in adipocytes. Each bar is the mean ± SEM, n = 3. Cells were treated as in A. (C) Immunoblot and densitometry analyses of cells lysates from adipocytes treated with control or PTEN siRNA. (D) Surface-to-total distribution of HA-GLUT4-GFP in adipocytes treated with control or PTEN siRNA. Each point is the mean ± SEM, n = 3. (E) Percentage of control or PTEN KD adipocytes with cytosolic FoxO1. Each point is the mean ± SEM, n = 2–4.
To further investigate the differences in GLUT4 and FoxO1 dependency on PI3-kinase activity, we characterized the effect of transient knockdown of PTEN, a PI-3,4,5-trisphosphate 3-phosphatase with a role in counteracting PI3-kinase action (27). As anticipated, depletion of PTEN resulted in enhanced Akt phosphorylation (Fig. 3C), and a shift in the dose-responses for insulin-stimulated GLUT4 translocation and FoxO1 nuclear exclusion (Fig. 3 D and E), consistent with increased PI3-kinase activity. Interestingly, depletion of PTEN induced FoxO1 nuclear exclusion in the absence of insulin, while PTEN knockdown was not sufficient to promote GLUT4 plasma membrane translocation. Expression of a constitutively active form of p110 is sufficient to trigger GLUT4 translocation (28); therefore, the fact that depletion of PTEN is sufficient to mobilize FoxO1 but not GLUT4 further indicates that different levels of p110α activity are required to modulate those insulin effectors.
Akt Isoform Selectivity in GLUT4 Translocation but not FoxO1 Nuclear Exclusion.
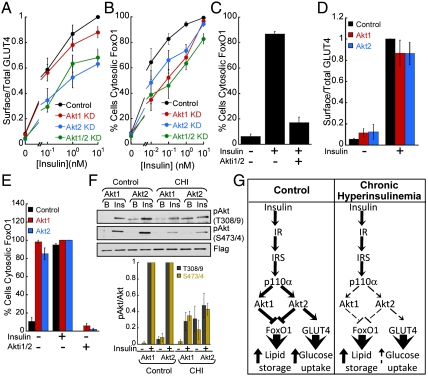
Downstream of p110α, multiple Akt kinase isoforms are activated by insulin in adipocytes. Akt2 is the predominant isoform required for GLUT4 translocation (13, 14). The Akt isoform requirements for FoxO1 nuclear exclusion in adipocytes are not known. Consistent with past studies, we found that down-regulation of Akt2 specifically impaired insulin-stimulated GLUT4 translocation, whereas Akt1 knockdown did not inhibit GLUT4 translocation nor did knockdown of both isoforms have a greater inhibitory effect than Akt2 knockdown (Fig. 4A). GLUT4 translocation requirement for Akt2 was not overcome by increasing doses of insulin. In contrast, knockdown of either Akt isoform rightward shifted the insulin-dose-response curve for FoxO1 nuclear exclusion, although Akt1 knockdown showed a trend toward a greater inhibitory effect (Fig. 4B). Thus, unlike Akt2 isoform-specific control of GLUT4, both Akt1 and Akt2 signal to FoxO1 in response to insulin. Interestingly, there was no significant additive effect in the inhibition of FoxO1 regulation when Akt1 and Akt2 were simultaneously knocked down (Fig. 4B). However, pharmacologic inhibition of Akt1 and Akt2 blocked insulin-stimulated FoxO1 nuclear exclusion (Fig. 4C). These data suggest that residual Akt1 and/or Akt2 in the double knockdown cells account for the residual activity of insulin on FoxO1 distribution (Fig. S3 A and B), although possible off-target effects of the Akt inhibitor cannot be excluded. Regardless, we found that Akt1 or Akt2 rescued the defect on FoxO1 regulation in Akt1 KD or Akt2 KD cells, demonstrating that Akt1 and Akt2 are interchangeable in regulating insulin-induced FoxO1 nuclear exclusion (Fig. S3 C and D).
Fig. 4.
GLUT4 and FoxO1 display distinct Akt isoform requirements and sensitivities in response to insulin. (A) Insulin-dose response for surface-to-total distribution of HA-GLUT4-GFP in adipocytes treated with control or Akt isoform-specific siRNAs. Each point is the mean ± SEM, n≥3. (B) Insulin-dose response for FoxO1-GFP nuclear exclusion in adipocytes treated with control or Akt isoform-specific siRNAs. Each bar is the mean ± SEM, n≥3. (C) Effect of the Akt inhibitor Akti1/2 (1 μM) on 1 nM insulin-mediated FoxO1 nuclear exclusion. Each bar is the mean ± SEM, n = 3. (D) Surface-to-total distribution of HA-GLUT4-GFP in adipocytes overexpressing either Flag-Akt1 or Flag-Akt2 by electroporation. Each bar is the mean ± SEM, n = 3. (E) Percentage of adipocytes overexpressing either Akt1 or Akt2 by electroporation displaying cytosolic FoxO1-GFP. Flag-Akt1 and Flag-Akt2 expression was more than 10-fold higher than the endogenous kinases. 1 μM Akt inhibitor Akti1/2 for 30 min as noted. Each bar is the mean ± SEM, n = 3. (F) Immunoblot and densitometry analyses of immunoprecipitated Flag-Akt1 and Flag-Akt2. CHI: chronic hyperinsulinemia. Each point is the mean ± SEM, n = 3. (G) Schematic of insulin signaling to GLUT4 and FoxO1 in control adipocytes and adipocytes exposed to chronic hyperinsulinemia. Dashed lines indicate impaired function.
To further characterize the role of Akt isoform-specific signaling in the regulation of GLUT4 and FoxO1, we studied the effects of overexpression of Akt1 and Akt2. Transient overexpression of either Akt isoform by electroporation, which results in more than 10-fold increase in the expression of these kinases (14), did not affect basal or insulin-induced GLUT4 plasma membrane localization but triggered FoxO1 phosphorylation and nuclear exclusion in basal adipocytes (Fig. 4 D and E and Fig. S3E). The effect on basal FoxO1 distribution was blocked by the Akti1/2 inhibitor, demonstrating that unstimulated activity of overexpressed Akt1 or Akt2 was sufficient to exclude FoxO1 from the nucleus (Fig. 4E). The failure of overexpression of Akt to promote GLUT4 translocation in basal adipocytes does not reflect a requirement for non-Akt signaling because overexpression of constitutively active forms of either Akt1 or Akt2 promotes GLUT4 translocation (14, 29). To further investigate the contribution of Akt isoform signaling to the differences in insulin sensitivity of GLUT4 translocation and FoxO1 nuclear exclusion, we performed dose-response studies of insulin-induced Akt1 and Akt2 activation. In vitro measurement of Akt isoform activity revealed a significantly higher catalytic activity of Akt1 compared to Akt2 at every insulin dose analyzed (Fig. S3F). Thus, although in vivo additional factors might contribute to Akt1 and Akt2 activity levels (i.e., expression levels, subcellular compartmentalization, substrate affinity), our data strongly indicate that phosphorylation of FoxO1 by Akt1 contributes to the enhanced insulin sensitivity of FoxO1 compared to GLUT4. Our data support the conclusion that GLUT4 and FoxO1 have distinct Akt isoform signaling requirements as well as distinct sensitivity to Akt activity levels in adipocytes.
Uncoupled Insulin Regulation of GLUT4 and FoxO1 Results from Distinct Thresholds of PI3-kinase/Akt Signaling Required to Modulate These Effectors.
The differences we have discovered in Akt isoform requirements for control of GLUT4 and FoxO1 could underlie uncoupled insulin regulation of these effectors in adipocytes exposed to chronic hyperinsulinemia. For example, chronic hyperinsulinemia could specifically affect insulin-activation of Akt2, which would have a greater effect on GLUT4. We tested the effect of chronic hyperinsulinemia on Akt isoform activation. Insulin-induced phosphorylation of Akt1 and Akt2 was similarly blunted in adipocytes chronically exposed to elevated insulin (Fig. 4F). Thus, chronic hyperinsulinemia affected the phosphorylation of both Akt isoforms equally, indicating that differences in the thresholds of PI3-kinase/Akt activity required to mobilize GLUT4 and FoxO1, rather than selective Akt isoform signaling deregulation, underlies the uncoupled insulin response of adipocytes exposed to chronic hyperinsulinemia.
Discussion
In this study we found that prolonged exposure to hyperinsulinemia results in “selective insulin-resistant” adipocytes, in which insulin-stimulated GLUT4 translocation is impaired while insulin-stimulated FoxO1 nuclear exclusion is maintained. Our data indicate that these changes are the result of inherent differences in insulin sensitivity of GLUT4 translocation and FoxO1 nuclear exclusion. A physiologically relevant insulin concentration, 1 nM insulin, which induces near maximal GLUT4 translocation in control adipocytes, fails to do so in insulin-resistant adipocytes. However, because FoxO1 nuclear exclusion is more sensitive to insulin than GLUT4 translocation, 1 nM insulin is still fully active for FoxO1 nuclear exclusion in the insulin-resistant adipocytes. Hyperinsulinemia-mediated perturbation of insulin signaling causes a rightward shift of the insulin-dose response for FoxO1 nuclear exclusion, resulting in impaired FoxO1 nuclear exclusion only at low physiological doses of insulin stimulation. The insulin resistance of FoxO1 could be relevant in states of adipose insulin resistance that are not accompanied by hyperinsulinemia. However, in the hyperinsulinemic state, characteristic of systemic insulin resistance, the differences in the sensitivities of GLUT4 and FoxO1 to insulin would lead to an uncoupling of insulin regulation of glucose transport and lipid storage in adipocytes. Therefore, uncoupled insulin action, a phenomenon associated with hepatic insulin resistance, might be a general characteristic of insulin-resistant tissues and contribute to the pathophysiology of insulin resistance.
Hepatic insulin resistance is characterized by impaired insulin inhibition of gluconeogenesis due to deregulation of FoxO1, while insulin regulation of fatty acid and triglyceride biosynthesis through the transcription factor SERBP-1c is maintained, contributing to hyperglycemia and hypertriglyceridemia (30, 31). Intriguingly, we found that FoxO1 regulation is largely preserved in adipocytes exposed to chronic hyperinsulinemia, revealing tissue-specific differences in the regulation of FoxO1 by insulin. FoxO1’s insulin sensitivity and signaling requirements might differ in fat and liver due to: (i) the distinct functions of FoxO1 in those tissues. Hepatic FoxO1 modulates carbohydrate metabolism (30), while in the adipose FoxO1 contributes to lipid storage regulation (23, 32); (ii) the higher levels of insulin to which liver cells are exposed compared to adipocytes, both in the fasted and postprandial state (33). However, despite tissue-specific differences in molecular effectors controlling fat and carbohydrate metabolism, impaired insulin regulation of cellular metabolism in fat and liver both promote hyperglycemia. Thus, glucose metabolism is generally more sensitive to deregulation of insulin signaling than is lipid metabolism, consistent with our observations that a higher threshold of insulin signaling is required for regulation of glucose metabolism than for control of lipid metabolism in fat cells. Insulin lowers blood glucose via effects on liver, adipose, and muscle. The higher threshold for insulin’s blood glucose lowering effects might serve as a critical safe guard against hypoglycemia.
What are the consequences of uncoupled insulin action in fat cells? Adipose tissue has an important endocrine function in the regulation of whole body metabolism through the secretion of various of adipokines (34). Ablation of GLUT4 in adipocytes, and consequent changes in adipose glucose metabolism, results in whole-body insulin resistance (35). Thus, the perturbation of GLUT4 translocation in insulin-resistant adipocytes will not only promote hyperglycemia by reduced glucose flux into adipose tissue but it will also contribute to whole-body insulin resistance via alterations in adipose endocrine functions.
The contribution of adipose FoxO1 in insulin resistance is less clear. FoxO1 modulates energy homeostasis through the regulation of adipocyte size and gene expression (36). Insulin inhibition of FoxO1 might contribute to modulation of fat storages by down-regulating ATGL expression and lipolysis (23, 32). Our data show that adipocytes exposed to chronic hyeprinsulinemia maintain FoxO1 insulin responsiveness and consequent reduced ATGL expression, consistent with the observation that ATGL is down-regulated in animal models of insulin resistance (37). Moreover, reduced ATGL expression is related to the degree of insulin resistance and hyperinsulinemia in obese subjects (38). Thus, in chronic hyperinsulinemia, inhibition FoxO1 activity and the resultant decrease in ATGL might result in enhanced triglyceride accumulation in adipocytes. In the short-term enhanced lipid accumulation in fat cells might provide a protective response to elevated nutrients. However, in the long-term impaired lipid utilization in adipocytes might have detrimental consequences. For instance, enlarged adipocyte size has been correlated with changes in adipocyte function and metabolic disease (39, 40). Additionally, FoxO1 is a known regulator of the cellular redox state (41), and sustained inhibition of FoxO1 in insulin resistance might lead to enhanced oxidative stress further contributing to impaired insulin action in the fat cell.
How deregulation of the PI3-kinase/Akt signaling pathway relates to insulin resistance is only beginning to be elucidated. It has been proposed that selective hepatic insulin resistance might originate downstream of Akt (15). Here we find that selective insulin resistance in adipocytes derives from differences in insulin sensitivity and PI3-kinase/Akt signaling requirements of GLUT4 translocation and FoxO1 nuclear exclusion. Our data show that insulin-stimulated GLUT4 translocation and FoxO1 nuclear exclusion are controlled by p110α, consistent with p110α being the main PI3-kinase isoform activated by insulin in insulin-responsive tissues (8, 9, 16). However, a lower level of p110α activity is required to signal to FoxO1 than to GLUT4. Therefore, in normal physiologic conditions the distinct sensitivities to PI3-kinase activity of different pathways downstream of the insulin receptor could be critical for the proper integration of the metabolic functions of insulin, and these different thresholds for activation could provide the basis for the selective deregulation of insulin action in the pathologic condition of reduced insulin sensitivity.
Downstream of active p110α, the Akt kinases are critical signal transducers mediating insulin’s metabolic control. Insulin-stimulated GLUT4 translocation relies on Akt2 activity (13, 14), and here we show that Akt1 and Akt2 both contribute to FoxO1 regulation. Thus, the flux of insulin-stimulated p110α activity converges on FoxO1 through Akt1 and Akt2, while only p110α/Akt2 signaling translates into GLUT4 mobilization to the plasma membrane. We propose that this difference underlies the enhanced insulin sensitivity of FoxO1 compared to GLUT4. The Akt-isoform-signaling flexibility of FoxO1 regulation guarantees FoxO1 nuclear exclusion even when Akt1 and 2 signaling are partially impaired, such as in hyperinsulinemia (Fig. 4G).
Apart from distinct Akt-isoform-signaling specificity, GLUT4 and FoxO1 also display distinct sensitivities to Akt activity levels, because overexpression of either Akt1 or Akt2 triggered FoxO1 nuclear exclusion but not GLUT4 translocation. Although we do not know the cause of those differences, it has been shown that subcellular compartmentalization of Akt activity contributes to substrate recognition and regulation (14, 42). GLUT4 regulation strongly relies on the total amount of Akt activity at the plasma membrane environment (14, 43), whereas it is likely that non-plasma-membrane-localized Akt phosphorylates FoxO1, and these different Akt pools might be differently affected in the insulin-resistant state.
In summary, our data reveal that in a model of chronic hyperinsulinemia adipocytes develop a state of selective insulin resistance. Our study indicates that uncoupled insulin action, a phenomenon first described in the insulin-resistant liver, might be indeed a general feature of insulin-resistant tissues consequent to deregulation of PI3-kinase/Akt signaling.
Materials and Methods
Antibodies, Drugs, cDNA Constructs, and siRNA.
For detailed information see SI Text.
Cell Culture, Adipocyte Differentiation, and Electroporation.
3T3-L1 fibroblast were cultured, differentiated into adipocytes, and electroporated as previously described (21, 44). For detailed information see SI Text. Insulin resistance was induced by incubating differentiated 3T3-L1 adipocytes in 10% fetal bovine serum-growth medium plus 10 nM insulin for 16 h (chronic hyperinsulinemia, CHI). Cells were then washed six times and incubated in serum-free medium for 4–8 h to allow the cells to return to unstimulated state. The cells were then challenged with insulin for 30 min as noted. Control cells were similarly treated except the medium was not supplemented with excess insulin during the 16 h incubation. The effect of chronic hyperinsulinemia on insulin signaling and GLUT4 and FoxO1 regulation was studied in parallel samples using biochemical and fluorescent microscopy analyses as described.
Immunoblot Analyses and Immunoprecipitation.
GLUT4 Translocation.
HA-GLUT4-GFP translocation has been described in detail (21). Surface-to-total GLUT4 distribution was normalized to that of control cells stimulated with the highest insulin concentration utilized in the experiment.
FoxO1 Subcellular Localization.
Nuclear or cytosolic localization of FoxO1-GFP was detected using fluorescence microscopy. Endogenous FoxO1 was detected using indirect immunofluorescence with an anti-FoxO1 antibody (Cell Signaling). Cells were scored for nuclear or cytosolic FoxO1 localization and results are expressed as the percentage of cells in which FoxO1 localized in the cytoplasm.
Neutral Lipid Accumulation.
Neutral lipid content in adipocytes was detected using LipidTox neutral lipid stain (Invitrogen). Cells were fixed in 3.7% formaldehyde for 10 min followed by staining with LipidTox neutral lipid stain for 30 min. Nuclei were counterstained using Hoechst 3342 (Invitrogen). Lipid accumulation was measured by fluorescence microscopy, corrected for autofluorescence, and expressed as normalized average fluorescence intensity per cell.
Fluorescence Quantification.
Fluorescence microscopy was performed and quantified as previously described (44, 45).
Statistical Analysis.
Student’s paired t-test analyses were used for data statistical analysis. Statistical significance was set at p < 0.05.
For additional materials and methods see SI Text.
Supplementary Material
Acknowledgments.
We thank Kevan Shokat for the isoform-specific PI3 kinase inhibitors. We thank Markus Schober and members of the McGraw lab for discussions and comments on the manuscript, and Jennifer Wen for excellent technical assistance. The work was supported by National Institutes of Health Grant DK52852 (T.E.M.) and American Diabetes Association mentor-based postdoctoral fellowship supporting D.M.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019268108/-/DCSupplemental.
References
- 1.Brown MS, Goldstein JL. Selective versus total insulin resistance: A pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 2.Biddinger SB, Kahn CR. From mice to men: Insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–158. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- 3.Whiteman EL, Cho H, Birnbaum MJ. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab. 2002;13:444–451. doi: 10.1016/s1043-2760(02)00662-8. [DOI] [PubMed] [Google Scholar]
- 4.Cusi K, et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105:311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: Insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 6.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 7.Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem Soc Trans. 2007;35:231–235. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- 8.Knight ZA, et al. A pharmacological map of the PI3-K family defines a role for p110alpha in insulin signaling. Cell. 2006;125:733–747. doi: 10.1016/j.cell.2006.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foukas LC, et al. Critical role for the p110alpha phosphoinositide-3-OH kinase in growth and metabolic regulation. Nature. 2006;441:366–370. doi: 10.1038/nature04694. [DOI] [PubMed] [Google Scholar]
- 10.Cho H, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292:1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 11.Garofalo RS, et al. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest. 2003;112:197–208. doi: 10.1172/JCI16885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang ZY, et al. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci USA. 2003;100:7569–7574. doi: 10.1073/pnas.1332633100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae SS, et al. Isoform-specific regulation of insulin-dependent glucose uptake by Akt/protein kinase B. J Biol Chem. 2003;278:49530–49536. doi: 10.1074/jbc.M306782200. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez E, McGraw TE. Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling. Proc Natl Acad Sci USA. 2009;106:7004–7009. doi: 10.1073/pnas.0901933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leavens KF, et al. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab. 2009;10:405–418. doi: 10.1016/j.cmet.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sopasakis VR, et al. Specific roles of the p110alpha isoform of phosphatidylinsositol 3-kinase in hepatic insulin signaling and metabolic regulation. Cell Metab. 2010;11:220–230. doi: 10.1016/j.cmet.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haruta T, et al. A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol. 2000;14:783–794. doi: 10.1210/mend.14.6.0446. [DOI] [PubMed] [Google Scholar]
- 18.Hoehn KL, et al. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 2008;7:421–433. doi: 10.1016/j.cmet.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiong W, et al. GLUT4 is sorted to vesicles whose accumulation beneath and insertion into the plasma membrane are differentially regulated by insulin and selectively affected by insulin resistance. Mol Biol Cell. 2010;21:1375–1386. doi: 10.1091/mbc.E09-08-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007;5:237–252. doi: 10.1016/j.cmet.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Karylowski O, et al. GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes. Mol Biol Cell. 2004;15:870–882. doi: 10.1091/mbc.E03-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakae J, et al. Regulation of insulin action and pancreatic beta-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet. 2002;32:245–253. doi: 10.1038/ng890. [DOI] [PubMed] [Google Scholar]
- 23.Chakrabarti P, Kandror KV. FoxO1 controls insulin-dependent adipose triglyceride lipase (ATGL) expression and lipolysis in adipocytes. J Biol Chem. 2009;284:13296–13300. doi: 10.1074/jbc.C800241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sano H, et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J Biol Chem. 2003;278:14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 25.Eguez L, et al. Full intracellular retention of GLUT4 requires AS160 Rab GTPase activating protein. Cell Metab. 2005;2:263–272. doi: 10.1016/j.cmet.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Brunet A, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 27.Stambolic V, et al. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 28.Martin SS, et al. Activated phosphatidylinositol 3-kinase is sufficient to mediate actin rearrangement and GLUT4 translocation in 3T3-L1 adipocytes. J Biol Chem. 1996;271:17605–17608. doi: 10.1074/jbc.271.30.17605. [DOI] [PubMed] [Google Scholar]
- 29.Kohn AD, et al. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto M, et al. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Shimomura I, et al. Decreased IRS-2 and increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6:77–86. [PubMed] [Google Scholar]
- 32.Eguchi J, et al. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011;13:249–259. doi: 10.1016/j.cmet.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nurjhan N, et al. Insulin dose-response characteristics for suppression of glycerol release and conversion to glucose in humans. Diabetes. 1986;35:1326–1331. doi: 10.2337/diab.35.12.1326. [DOI] [PubMed] [Google Scholar]
- 34.Halberg N, Wernstedt-Asterholm I, Scherer PE. The adipocyte as an endocrine cell. Endocrinol Metab Clin North Am. 2008;37:753–768. doi: 10.1016/j.ecl.2008.07.002. x-xi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abel ED, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 36.Nakae J, et al. Forkhead transcription factor FoxO1 in adipose tissue regulates energy storage and expenditure. Diabetes. 2008;57:563–576. doi: 10.2337/db07-0698. [DOI] [PubMed] [Google Scholar]
- 37.Villena JA, et al. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: Ectopic expression of desnutrin increases triglyceride hydrolysis. J Biol Chem. 2004;279:47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- 38.Jocken JW, et al. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. J Clin Endocrinol Metab. 2007;92:2292–2299. doi: 10.1210/jc.2006-1318. [DOI] [PubMed] [Google Scholar]
- 39.O’Connell J, et al. The relationship of omental and subcutaneous adipocyte size to metabolic disease in severe obesity. PLoS ONE. 2010;5:e9997. doi: 10.1371/journal.pone.0009997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bluher M, et al. Role of insulin action and cell size on protein expression patterns in adipocytes. J Biol Chem. 2004;279:31902–31909. doi: 10.1074/jbc.M404570200. [DOI] [PubMed] [Google Scholar]
- 41.Nemoto S, Finkel T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science. 2002;295:2450–2452. doi: 10.1126/science.1069004. [DOI] [PubMed] [Google Scholar]
- 42.Schenck A, et al. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell. 2008;133:486–497. doi: 10.1016/j.cell.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 43.Ng Y, et al. Cluster analysis of insulin action in adipocytes reveals a key role for Akt at the plasma membrane. J Biol Chem. 2010;285:2245–2257. doi: 10.1074/jbc.M109.060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeigerer A, et al. GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps. Mol Biol Cell. 2002;13:2421–2435. doi: 10.1091/mbc.E02-02-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez E, McGraw TE. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol Biol Cell. 2006;17:4484–4493. doi: 10.1091/mbc.E06-07-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.