Abstract
In humans, the precursor to all steroid hormones, pregnenolone, is synthesized from cholesterol by an enzyme complex comprising adrenodoxin reductase (AdR), adrenodoxin (Adx), and a cytochrome P450 (P450scc or CYP11A1). This complex not only plays a key role in steroidogenesis, but also has long been a model to study electron transfer, multistep catalysis, and C–C bond cleavage performed by monooxygenases. Detailed mechanistic understanding of these processes has been hindered by a lack of structural information. Here we present the crystal structure of the complex of human Adx and CYP11A1—the first of a complex between a eukaryotic CYP and its redox partner. The structures with substrate and a series of reaction intermediates allow us to define the mechanism underlying sequential hydroxylations of the cholesterol and suggest the mechanism of C–C bond cleavage. In the complex the [2Fe-2S] cluster of Adx is positioned 17.4 Å away from the heme iron of CYP11A1. This structure suggests that after an initial protein–protein association driven by electrostatic forces, the complex adopts an optimized geometry between the redox centers. Conservation of the interaction interface suggests that this mechanism is common for all mitochondrial P450s.
Keywords: ferredoxin-hemeprotein electron transfer, hydroxysteroids, inner mitochondrial membrane, fusion protein
The cytochrome P450 enzymes comprise a superfamily of hemeproteins that participate in an array of metabolic processes. Members of this family are unified by a common fold and yet catalyze diverse reactions. In humans, there are at least 57 P450s that can be divided into classes based on their intracellular localization and requirement for redox partners, which provide electrons for the monooxygenase reaction. One class is localized in the inner mitochondrial membrane and receives electrons from adrenodoxin reductase (AdR), via the [2Fe-2S] ferredoxin, Adx. The molecular mechanism of complex formation and electron transport within this system have remained unclear: AdR, Adx, and P450 have been proposed to form 1∶1∶1 or 1∶2∶1 complexes (1, 2), but Adx has also been suggested to act as a shuttle, sequentially transporting one electron at a time from AdR to P450 (3–5). Both complex and shuttle models assume that interactions are mainly electrostatic.
The first step in steroid hormone biosynthesis is the conversion of cholesterol to pregnenolone and isocaproic aldehyde by mitochondrial CYP11A1. The reaction occurs in three steps: two stereospecific hydroxylations, with formation of 22R-hydroxycholesterol (22-HC) and 20R,22R-dihydroxycholesterol (20, 22-DHC) followed by a C–C bond cleavage (Fig. S1), which does not mechanistically typify a P450 reaction and so, is less understood.
Contrary to drug-metabolizing but similarly to P450s involved in physiological functions, CYP11A1 has narrow substrate specificity—limited to cholesterol, 7-dehydrocholesterol, and vitamin D (6–8) (Fig. S1 and Table S1). Notably, CYP11A1 hydroxylates but does not cleave the side chain of vitamin D3 producing biologically active 20-hydroxyvitamin D and minor di- and trihydroxylated derivatives (6, 7, 9, 10) (Fig. S1 and Table S1). In the absence of structural information, the molecular mechanisms that allow this enzyme to catalyze two different reactions while at the same time maintaining exquisite stereospecificity are unknown.
Results and Discussion
Structural Insights into Cholesterol Side-Chain Cleavage.
The substrate access channel is the most dynamic part of the P450 protein and provides recognition and guidance of the substrate from the surface to the active site. Cholesterol appears to be transferred to the active site directly from the inner mitochondrial membrane where CYP11A1 resides (3). A substrate access channel is oriented toward the membrane, with the residues of the F–G loop and the A′ helix comprising the hydrophobic surface involved in the interaction with the membrane (11, 12) (Fig. 1). The cholesterol is bound in the active site with its side chain bent at C20 and C22 positions (Fig. 2A). This conformation is induced by the active site residues Leu101, Trp87 (B′ helix), Phe202 (F helix), and Ile461 (β4-loop), which control the side chain positioning above the heme iron for subsequent hydroxylations. The distances between C22 and C20 carbons and the iron are 4.3 and 4.5 Å, respectively. When modeled, the C22 hydrogen points toward the oxyferryl oxygen (distance 2.3 Å), whereas the hydrogen at C20 projects slightly away (distance 2.6 Å), partially explaining a preferential hydroxylation at C22 to produce the R-isomer (22-HC). Polar and nonpolar residues of the binding pocket tightly enclose the four cholesterol rings (Fig. 2A). Notably, two methyl groups projecting from the β-face of cholesterol interact with the Ser352 side chain and its hydrogen-bonded water molecule, restricting sliding of cholesterol and placing the C22 carbon nearest to the heme iron. The 3β-hydroxyl of cholesterol does not interact directly with the enzyme residues but binds to two water molecules, which are part of a hydrogen-bonded network formed by additional water molecules and the polar residues His39, Tyr61, Asn210, Gln377 (Fig. 2A) and conserved in all ligand-bound CYP11A1 structures. This network may explain the vitamin D hydroxylation pattern; i.e., the formation of 20-OH-vitamin D3 as a first product. Vitamin D3 has an opened B-ring and mostly exists in an extended conformation. Binding of the vitamin D3 A-ring to the water-filled pocket would position the C20 carbon, but not C22, above the heme iron for hydroxylation. The water-filled pocket near the 3β-hydroxyl group provides a space to accommodate cholesterol esters, which can also be used as substrates (13).
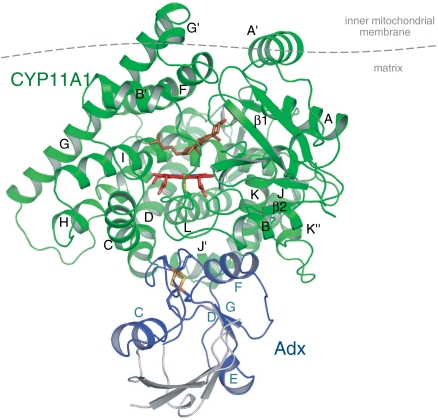
Fig. 1.
Crystal structure of the CYP11A1–Adx complex in a ribbon representation. CYP11A1 has a typical P450 fold with the heme buried inside a conserved protein core. Putative position of the membrane is indicated with a horizontal line. Adx (blue) is bound at the proximal surface of CYP11A1 (green), which is facing the mitochondrial matrix. The disordered regions of Adx are shown in gray based on bovine structure (PDB ID code 1AYF). The heme, the bound cholesterol molecule at the active site, and the [2Fe-2S] cluster are shown as sticks.
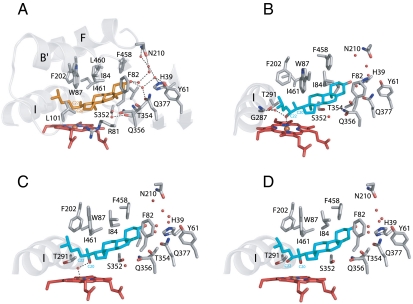
Fig. 2.
Active site of CYP11A1. (A) View of cholesterol binding (orange) within the sterol-binding pocket around the cholesterol 3β-hydroxyl group. Residues F82, L84, and L460 contact the substrate from the flat side (α-face); S352, V353, T354 and Q356 approach the side with two methyl groups (β-face); R81 and F458 side chains make contacts at its edges. CYP11A1 complexed with: (B) the reaction intermediate 22-HC (blue); (C) substrate 20-HC (blue); (D) reaction intermediate 20,22-DHC (blue). Water molecules are represented as small red spheres; dashed lines indicate hydrogen bonds.
To further understand the mechanism of sequential hydroxylation of the cholesterol side chain, we solved the structure in complex with 22-HC (Table S2). Continuous electron density is observed between the C22-hydroxyl group and the heme iron (distance 2.6 Å; Fig. S2). Close proximity to the heme iron was suggested for 22-HC earlier based on NMR studies (14). Crystallographic observation of the hydroxyl–heme iron interaction was made with the hydroxylated product of P450cam (15, 16). Upon 22-HC binding, the conformation of the Thr291 side chain is slightly changed so the interaction between the carbonyl oxygen of Gly287 and the hydroxyl group of Thr291 is loosened, which allows a new water molecule to bind in the I-helix groove (Fig. 2B). The carbonyl oxygen of Asp290 does not reorient to interact with Met294. A water molecule, which interacts with Thr291 and Gly287, is also hydrogen-bonded to the 22R-OH group (Fig. 2B). This network may contribute to the retention of the first stable intermediate, 22-HC, which displays very tight binding (17) and remains bound in the active site for the next round of hydroxylation; i.e., until the reduction and following oxygen binding (18).
The specific interaction we observed for 22-HC with CYP11A1 provides a rationale for developing unique high affinity compounds based on the hydroxyl moiety rather than the nitrogen of heterocycles as potential heme ligand for other drug target P450s that catalyze multiple hydroxylations.
As CYP11A1 predominantly 22-hydroxylates cholesterol, we used the alternative intermediate, 20S-OH-cholesterol (20-HC) to understand the proceeding of the reaction through 20,22-DHC. The structure in complex with 20-HC reveals that the 20-OH-group is positioned away from the heme iron (distance 3.7 Å) and is hydrogen-bonded to a water molecule coordinating the heme iron at the distal position (Fig. 2C). This finding is consistent with its spectral properties as a low spin inducer, but differs from other P450 substrate complexes in which water is excluded from the coordination with heme. Moreover, interaction between Ser352 and the two sterol methyl groups, as in the cholesterol complex, seems to control the movement of the side chain near the heme iron allowing hydroxylation only at C22 for 20-HC. Consistent with this finding, 20-OH-vitamin D3 as a secosteroid cannot be converted to a vicinal diol (7).
With two adjacent carbons being oxidized, 20,22-DHC, a precleavage intermediate, was subsequently cocrystallized to follow the reaction pathway. The structure shows that both hydroxyls are close to the heme iron but without direct interactions (Fig. 2D). Such positioning for the substrate is unique leaving limited space for subsequent oxygen binding to the reduced heme iron. However, superposition of the cholesterol and 22-HC structures reveals the flexibility of the side chain, whereas the position of the ring system remains fixed (Fig. S3). Formation of the next intermediate, 20,22-DHC, brings the side chain back to the original position as observed for cholesterol but with two OH-groups now near the heme Fe. Thus, the shift in the position of the carbon atom, which will be hydroxylated, is achieved by an up and down movement of the side chain. So the room for the oxygen to bind to the heme iron at each step with the observed intermediates could be provided by this mechanism and might occur upon reduction (19).
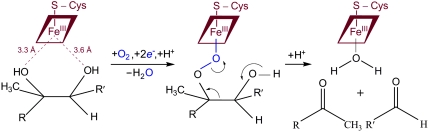
Although the mechanism of the CYP11A1 catalyzed reaction has been extensively studied for decades (20–25), it is still not clear how C–C bond cleavage occurs. Earlier experiments showing a retention of the C22 hydrogen and C20 hydroxyl oxygen in the products (22, 23) and ability of the enzyme to convert cholesterol analogs with a hydrogen substituent at C22 (24, 25) to pregnenolone indicate that C–C bond cleavage can proceed via a C20-peroxy species (Fig. 3). Our structures with hydroxylated complexes show that the C22 position is more dynamic than C20 (no interaction of the 20-OH-group with the heme iron is observed). This limitation might be required for direct interaction between the substrate C20 OH-group and compound I, which is more likely to occur based on the distance and angle of the 20-OH-group of 20R,22R-diol to the heme iron in our structure. The C20-peroxy intermediate is further rearranged to two carbonyl products, pregnenolone and isocaproic aldehyde. However, the ultimate oxidant for the CYP11A1 mediated C–C bond cleavage reaction remains unknown and further studies are required.
Fig. 3.
Proposed mechanism for the final step of the cholesterol side-chain cleavage reaction (C–C bond cleavage of 20R,22R-dihydroxycholesterol). R is the sterol nucleus and R′ is the aliphatic side chain—CH2CH2CH(CH3)2. Dashed lines indicate distances from the heme Fe to the oxygen atoms of 20,22-DHC.
The CYP11A1 active site architecture adapts to release reaction products via different routes presumably defined by their metabolic fates. Pregnenolone, a substrate for further conversions out of mitochondria, might be released into the membrane through the same access channel as for the cholesterol substrate. The smaller and more hydrophilic isocaproic aldehyde can exit through the water channel (channel surrounded by the B-C loop), which faces the mitochondrial matrix (Fig. S4).
Interaction of CYP11A1 with Redox Partner.
The cholesterol side-chain cleavage reaction requires efficient transfer of electrons from ferredoxin to the heme iron of CYP11A1 twice during each catalytic cycle: to the ferric substrate-bound form and to the ferrous dioxygen-bound form. Each electron-transfer reaction is realized through the formation of a donor—acceptor complex. To obtain the complex of substrate-bound CYP11A1 with Adx, which would normally be transient and hence structurally inaccessible, the fusion proteins comprising N-terminal Adx and C-terminal CYP11A1 were constructed. By varying the length and composition of the linker we were able to purify and crystallize catalytically competent fusion proteins (Table S3). Independent of the type of linker between proteins, which is not visible in the crystalline state, the Adx molecule is present in the structure and binds to the same site on the proximal surface of CYP11A1 (Fig. S5), although the regions distal from the interaction interface are not defined. We analyzed the cholesterol-bound CYP11A1-Adx structure (linker AAKKT), the highest resolution model reported here.
Adx binds at the proximal surface of CYP11A1 via its F-helix (interaction domain) contacting the K-helix of CYP11A1 and with the loop surrounding the [2Fe-2S] cluster (core domain) interacting with the heme-binding loop, C- and L-helices of CYP11A1 (Fig. 4A). The hydrogen bonding interactions predominate upon the Adx–CYP11A1 complex formation. The interface comprises two salt bridges, Lys339CYP11A1-Asp72Adx and Lys343CYP11A1-Asp76Adx, and is consistent with site-directed mutagenesis and chemical modification data (26–29). Residue Asp79 of Adx interaction domain, which affects Adx–CYP11A1 complex formation and activity (28–30), is not involved in direct contact but close (approximately 5 Å) to the “meander” region of CYP11A1, more specifically, to Lys406, the latter being implicated in redox partner binding (31) (Fig. 4A). It is interesting to note that the effect of replacement of Asp79Adx (28–30) is more profound than the effect of replacement of either Lys404, Lys406, or Arg411 of CYP11A1 (31, 32). We hypothesize that during complex formation, Asp79Adx can initially interact with either of these residues in accordance with conformational changes of the flexible meander region, which would sterically complement a proximal surface for the redox partner.
Fig. 4.
(A) CYP11A1–Adx interface. CYP11A1–Adx contacts involve the interaction domain (F helix) and loop surrounding the Adx [2Fe-2S] cluster (yellow) and occur on the proximal surface of CYP11A1 (green). Direct hydrogen bonds between residues Lys109CYP11A1-Ala45Adx,, Trp418CYP11A1-Leu80Adx, and water-mediated hydrogen bonds, including residues Met120CYP11A1-Thr49Adx and Met120CYP11A1-Ala51Adx are displayed. Dashed lines indicate salt bridges and H-bonding interactions; water molecules are represented as small red spheres. (B) Predicted electron-transfer pathway from the [2Fe-2S] cluster to the heme iron proceeds from Fe1 of the [2Fe-2S] cluster via side- and main-chain atoms of Cys52Adx and Ala51Adx, through-space jump (3 Å), Gln422CYP11A1, Cys423CYP11A1 to the heme iron.
The specific conserved motif C-GR/KR--E in mitochondrial P450s is important for interaction with Adx and enzymatic activity (Fig. S6) (31, 33). The residues Arg426, Arg427, and Glu430 of this motif form part of the interaction interface (Fig. 4A), but are not directly involved in the electrostatic interactions with Adx. Arg427CYP11A1 interacts with Glu430CYP11A1, the latter apparently facilitates Adx dissociation (33) possibly by repulsing the negative charge of Glu73Adx. Notably, ordered water molecules are enclosed within the interaction interface surrounding these charged residues, and may facilitate electronic coupling between redox centers. Taking together, the basic patch on the surface of P450, formed by Arg465Arg466 of the L-helix, can provide long range electrostatic steering for the recognition (guiding) of Adx, because a dipole moment of Adx does not contribute to electrostatic interactions (34).
Considering the number of positive and negative charges on the surface of CYP11A1 and Adx, respectively, the distance between redox centers, which has to be kept within a certain range (see below) and based on the analysis of the structure of the complex we suggest that following initial recognition and binding as directed electrostatically, Adx is slightly repositioned on the proximal surface of CYP11A1 to adopt an optimal orientation for efficient electron transfer. This notion contrasts other electron-transfer systems, where electrostatic interactions are believed to function primarily in precollisional orientation leading to encounter complexes with multiple geometries of similar free energy required for efficient electron transfer (35). The distance between the iron atom closest to the surface in the [2Fe-2S] cluster and the heme iron is 17.4 Å and is similar to that observed between the heme- and FMN-binding domains of the bacterial native fusion P450BM3 (36). The calculated electron-transfer pathway (Fig. 4B) provides a maximum electron-transfer rate of 24 s-1, which approximates the experimental value of 5 s-1 for bovine Adx–CYP11A1 complex (4).
To understand the mode of protein–protein interactions in the AdR–Adx–CYP11A1 system, we compared the CYP11A1–Adx complex with the AdR–Adx complex (37). Adx residues involved in the interaction interface with AdR and CYP11A1 greatly overlap (Fig. S7) providing evidence that an AdR–Adx–CYP11A1 ternary complex cannot form. The CYP11A1 proximal surface can accommodate only one molecule of Adx, and the activity and electron-transfer properties of the Adx–CYP11A1 fusion proteins (Table S3 and Fig. S8) suggest that formation of the quaternary AdR–Adx–Adx–CYP11A1 complex is also unlikely. Together, these results indicate that Adx functions as a mobile shuttle in electron transfer.
Methods
Protein Purification and Crystallization.
The human CYP11A1, Adx, and AdR cDNAs were purchased from the Mammalian Gene Collection collection (accession codes BC032329, BC010284, and BC002960, respectively) and subcloned into a modified pCW-LIC vector. The mature form of CYP11A1 (without the N-terminal mitochondrial signal peptide) with the C-terminal His6-tag was coexpressed with GroEL/ES in Escherichia coli JM109. Fusion proteins were designed as [Adx—linker—CYP11A1-His6] and cloned with the BD In-Fusion reaction (BD Biosciences) into the pCW-LIC vector. Three different linkers were used: the type I is the AAKKT tag usually added to the P450 N-terminus to improve the expression and solubility in E. coli (38, 39); the type II linker is AAVDAKASAGEAPAETLRGAKKT, from a natural fusion of the cytochrome P450 domain with the ferredoxin domain (CYP51 from Methylococcus capsulatus) (40); and the type III linker, VLHRHQPVTIGEPAAKKT, is from a natural fusion of the cytochrome P450 domain with the phthalate dioxygenase reductase-like domain (cytochrome P450 RhF from Rhodococcus sp. NCIMB 9784) (41). The fusions were coexpressed with GroEL/ES in E. coli and purified using metal affinity chromatography on a NiHiTrap-chelating column followed by anion-exchange chromatography on a SourceQ column. Fusion proteins were crystallized at concentration 15–20 mg/mL using the hanging drop vapor diffusion method at room temperature. The reservoir solution contained 10% PEG 8 K, 0.2 M calcium acetate, and 0.1 M Hepes pH 7.5. For cryoprotection, crystals were passed through the reservoir solution containing 20% glycerol, flash-frozen and stored in liquid nitrogen until data collection.
Structure determination and refinement.
Diffraction data were collected at beamlines 19ID and 23ID at the Advanced Photon Source, Argonne National Laboratory, with the crystal kept at 100 K and processed using the program HKL2000 (42). The data collection statistics from the crystals of cholesterol-bound and three intermediate-bound CYP11A1 in complex with Adx are shown in Table S2. The structure of cholesterol-bound CYP11A1 complexed with Adx was solved by the molecular replacement (MR) method using Molrep in the CCP4 program suit (43). Human CYP3A4 [Protein Data Bank (PDB) ID code 1TQN) excluding the heme was used as a search model. Several iterations of manual building in the program O (44) and refinement in the program Refmac5 in the CCP4 suit gave almost complete structure of CYP11A1. To remove the model bias of the CYP11A1 structure, we performed the simulated annealing refinement in CNS (45). Consequent Fo-Fc difference map calculation brought out the density for cholesterol, heme and part of Adx including the Fe-S center (Fig. S9), which were absent from the CYP11A1 structure. We fitted cholesterol and heme into the difference map. To build the Adx model, we docked one molecule from bovine Adx structure (PDB ID code 1AYF) into the difference map as docking was guided by the shape of the Fe-S center difference map. This docked model was used as a reference for manual building of the Adx molecule in the CYP11A1—Adx complex. The structure of cholesterol-bound CYP11A1—Adx complex was finalized by additional iteration of model building in O and refinement in REFMAC5. Partially modeled Adx consists of two β-strands and three α-helices (αF is involved in recognition of CYP and AdR) and loops that include the [2Fe-2S] cluster-coordinating four cysteine residues.
The other three structures of CYP11A1 reported here were solved by the MR method using the cholesterol-bound CYP11A1—Adx complex as a search model excluding cholesterol, heme, and Adx. After MR solutions were found, the following Fo-Fc difference map showed the intermediates (20-HC, 22-HC and 20,22-DHC), heme and part of Adx, similar to the cholesterol-bound CYP11A1—Adx complex. Several iterations of model building in O and refinement in REFMAC5 finalized the structures. Their refinement statistics are shown in Table S2.
The crystals contain two CYP11A1—Adx complexes in the asymmetric unit. The crystal lattice is formed by CYP11A1, whereas the adrenodoxin molecule is present in the solvent channel making contacts only with the fused CYP11A1. This can explain the high thermal motion of Adx molecules and a poor density for the regions remote from the interaction interface of Adx. In the Adx–CYP11A1–cholesterol complex we were able to refine residues 28—95 of the Adx molecule. The activity of fusions is low compared to the nonfused CYP11A1, but the affinity to the substrates is not affected by the linker constraints (Table S3). In contrast, the ability of Adx in the fusions to reduce CYP11A1 (e.g., to transfer one electron) is dependent on the length of the linker (Fig. S8), indicating the requirement for Adx to function as a shuttle. Electron-transfer pathway from the [2Fe-2S] cluster of Adx to the heme iron of CYP11A1 was calculated by HARLEM (46).
Sterol Binding Spectral Determinations.
Ligand-induced spectral changes were monitored as a shift of the heme Soret peak: type I (blue shift from 418 nm) or reverse type II (blue shift from 425 nm). The apparent dissociation constant of the enzyme–sterol complex (Kd) was determined by differential spectral titration as described in ref. 47. The binding of sterols to human CYP11A1 was performed in 20 mM Hepes buffer (pH 7.2) with 0.1% Tween-20, 0.1 mM EDTA, and 50 mM NaCl at room temperature, with a final CYP11A1 concentration 1 μM. For the titration of sterol low spin inducers, 1 μM econazole (Kd for econazole 1 μM) was added to induce a type II shift (red-shift to 425 nm), and then the reverse type II spectral changes were monitored as a function of different sterol concentrations. The absence of denaturation during spectral titration was confirmed by the carbon monoxide difference spectra of reduced CYP11A1.
Enzyme Activity Assay.
Side-chain cleavage activity was measured in a reconstituted system containing CYP11A1 or fusion (0.5 μM), human Adx (1 μM), human AdR (0.125 μM), and NADPH regenerating system at 37 °C. Substrates were added from stock solutions in ethanol or in 45% 2-hydroxypropyl-beta-cyclodextrin to a final concentration of 100 μM. Steroids were extracted with methylene chloride and analyzed by reverse-phase HPLC with methanol as a mobile phase. The Δ5-steroids were incubated with cholesterol oxidase at 37 °C for 30 min before the extraction.
Enzymatic and Chemical Reduction.
Reduction of fusion proteins and CYP11A1 was performed in the reconstituted system with a CYP11A1:AdR:Adx protein ratio of 1∶0.5∶2 μM and 250 μM NADPH with or without 50 μM cholesterol. For the fusions, 2 μM of external Adx and 0.5 μM AdR were added. The enzymatic reduction was quantified by recording the CO-difference spectra at 450 nm and calculated as a percentage following chemical reduction with sodium dithionite (1 mg/mL).
Supplementary Information.
See SI Appendix (Tables S1–S3 and Figs. S1–S9) for activity of human CYP11A1 and fusion proteins; crystallographic data table, schematic illustration of the CYP11A1 catalyzed reactions; initial electron density for the interaction of the 22R-hydroxycholesterol with the heme; superposition of the substrate and intermediates structures; access and egress channels; superposition of the fusions with different linker length; sequence alignment of mitochondrial P450s; molecular surface of Adx involved in interaction with CYP11A1 and AdR; reduction of fusion proteins; electron density of Adx molecule in the interaction interface.
Supplementary Material
Acknowledgments.
We thank R. Tuckey, U. Oppermann and A. Edwards for helpful discussions and reading the manuscript; A. Yantsevich and W. Tempel for excellent technical assistance; and R. Tuckey for providing 20,22-dihydroxycholesterol. The Structural Genomics Consortium is a registered charity (number 1097737) and receives funds from the Canadian Institutes for Health Research, the Canadian Foundation for Innovation, Genome Canada through the Ontario Genomics Institute, GlaxoSmithKline, Karolinska Institutet, the Knut and Alice Wallenberg Foundation, the Ontario Innovation Trust, the Ontario Ministry for Research and Innovation, Merck and Co. Inc., the Novartis Research Foundation, the Swedish Agency for Innovation Systems, the Swedish Foundation for Strategic Research, and the Wellcome Trust.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org [PDB ID code 3N9Y (CYP11A1–Adx in complex with cholesterol), 3N9Z (CYP11A1–Adx in complex with 22-HC), 3NA0 (CYP11A1–Adx in complex with 20,22-DHC), and 3NA1 (CYP11A1–Adx in complex with 20-HC)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019441108/-/DCSupplemental.
References
- 1.Kido T, Kimura T. The formation of binary and ternary complexes of cytochrome P-450scc with adrenodoxin and adrenodoxin reductase. adrenodoxin complex. The implication in ACTH function. J Biol Chem. 1979;254:11806–11815. [PubMed] [Google Scholar]
- 2.Turko IV, Adamovich TB, Kirillova NM, Usanov SA, Chashchin V. Cross-linking studies of the cholesterol hydroxylation system from bovine adrenocortical mitochondria. Biochim Biophys Acta. 1989;996:37–42. doi: 10.1016/0167-4838(89)90091-5. [DOI] [PubMed] [Google Scholar]
- 3.Hanukoglu I, Jefcoate CR. Mitochondrial cytochrome P-450scc. Mechanism of electron transport by adrenodoxin. J Biol Chem. 1980;255:3057–3061. doi: 10.1016/S0021-9258(19)85851-9. [DOI] [PubMed] [Google Scholar]
- 4.Lambeth JD, Seybert DW, Lancaster JR, Jr, Salerno JC, Kamin H. Steroidogenic electron transport in adrenal cortex mitochondria. Mol Cell Biochem. 1982;45:13–31. doi: 10.1007/BF01283159. [DOI] [PubMed] [Google Scholar]
- 5.Beilke D, et al. A new electron transport mechanism in mitochondrial steroid hydroxylase systems based on structural changes upon the reduction of adrenodoxin. Biochemistry. 2002;41:7969–7978. doi: 10.1021/bi0160361. [DOI] [PubMed] [Google Scholar]
- 6.Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc Natl Acad Sci USA. 2003;100:14754–14759. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuckey RC, et al. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008;275:2585–2596. doi: 10.1111/j.1742-4658.2008.06406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morisaki M, Duque C, Ikekawa N, Shikita M. Substrate specificity of adrenocortical cytochrome P-450scc--I. Effect of structural modification of cholesterol side-chain on pregnenolone production. J Steroid Biochem. 1980;13:545–550. doi: 10.1016/0022-4731(80)90211-3. [DOI] [PubMed] [Google Scholar]
- 9.Slominski A, et al. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J. 2006;273:2891–2901. doi: 10.1111/j.1742-4658.2006.05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zbytek B, et al. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol. 2008;128:2271–2280. doi: 10.1038/jid.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Headlam MJ, Wilce MC, Tuckey RC. The F-G loop region of cytochrome P450scc (CYP11A1) interacts with the phospholipid membrane. Biochim Biophys Acta. 2003;1617:96–108. doi: 10.1016/j.bbamem.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Pikuleva IA. Putative F-G loop is involved in association with the membrane in P450scc (P450 11A1) Mol Cell Endocrinol. 2004;215:161–164. doi: 10.1016/j.mce.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Tuckey RC, Lawrence J, Cameron KJ. Side-chain cleavage of cholesterol esters by human cytochrome P-450(scc) J Steroid Biochem Mol Biol. 1996;58:605–610. doi: 10.1016/0960-0760(96)00071-4. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs RE, Singh J, Vickery LE. NMR studies of cytochrome P-450scc. Effects of steroid binding on water proton access to the active site of the ferric enzyme. Biochemistry. 1987;26:4541–4545. doi: 10.1021/bi00388a056. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Narasimhulu S, Havran LM, Winkler JD, Poulos TL. Crystal structure of cytochrome P450cam, complexed with its catalytic product, 5-exo-hydroxycamphor. J Am Chem Soc. 1995;117:6297–6299. [Google Scholar]
- 16.Schlichting I, et al. The catalytic pathway of cytochrome p450cam at atomic resolution. Science. 2000;287:1615–1622. doi: 10.1126/science.287.5458.1615. [DOI] [PubMed] [Google Scholar]
- 17.Lambeth JD, Kitchen SE, Farooqui AA, Tuckey R, Kamin H. Cytochrome P-450scc-substrate interactions. Studies of binding and catalytic activity using hydroxycholesterols. J Biol Chem. 1982;257:1876–1884. [PubMed] [Google Scholar]
- 18.Tuckey RC, Kamin H. Kinetics of O2 and CO binding to adrenal cytochrome P-450scc. Effect of cholesterol, intermediates, and phosphatidylcholine vesicles. J Biol Chem. 1983;258:4232–4237. [PubMed] [Google Scholar]
- 19.Heyl BL, Tyrrell DJ, Lambeth JD. Cytochrome P-450scc-substrate interactions. Role of the 3 beta- and side chain hydroxyls in binding to oxidized and reduced forms of the enzyme. J Biol Chem. 1986;261:2743–2749. [PubMed] [Google Scholar]
- 20.Lieberman S, Lin YY. Reflections on sterol sidechain cleavage process catalyzed by cytochrome P450(scc) J Steroid Biochem Mol Biol. 2001;78:1–14. doi: 10.1016/s0960-0760(01)00068-1. [DOI] [PubMed] [Google Scholar]
- 21.Ortiz de Montellano PR, De Voss JJ. In: Cytochrome P450: Structure, Mechanism, and Biochemistry. 3rd Ed. Ortiz de Montellano PR, editor. New York: Kluwer; 2005. pp. 183–245. [Google Scholar]
- 22.Byon CY, Gut M. Steric considerations regarding the biodegradation of cholesterol to pregnenolone. Exclusion of (22S)-22-hydroxycholesterol and 22-ketocholesterol as intermediates. Biochem Biophys Res Commun. 1980;94:549–552. doi: 10.1016/0006-291x(80)91266-8. [DOI] [PubMed] [Google Scholar]
- 23.Duque C, Morisaki M, Ikekawa N, Shikita M, Tamaoki B. The final step of side-chain cleavage of cholesterol by adrenocortical cytochrome P-450(scc) studied with [22(-18)O]20,22-dihydroxycholesterols, [18O]isocaproaldehyde, [18O]water and atmospheric [18O]oxygen. Biochem Biophys Res Commun. 1978;85:317–325. doi: 10.1016/s0006-291x(78)80045-x. [DOI] [PubMed] [Google Scholar]
- 24.Hochberg RB, McDonald PD, Feldman M, Lieberman S. Studies on the biosynthetic conversion of cholesterol into pregnenolone. Side chain cleavage of some 20-p-tolyl analogs of cholesterol and 20 alpha-hydroxycholesterol. J Biol Chem. 1974;249:1277–1285. [PubMed] [Google Scholar]
- 25.Larroque C, van Lier JE. Hydroperoxysterols as a probe for the mechanism of cytochrome P-450scc-mediated hydroxylation. Homolytic versus heterolytic oxygen-oxygen bond scission. J Biol Chem. 1986;261:1083–1087. [PubMed] [Google Scholar]
- 26.Adamovich TB, Pikuleva IA, Chashchin VL, Usanov SA. Selective chemical modification of cytochrome P-450SCC lysine residues. Identification of lysines involved in the interaction with adrenodoxin. Biochim Biophys Acta. 1989;996:247–253. doi: 10.1016/0167-4838(89)90254-9. [DOI] [PubMed] [Google Scholar]
- 27.Wada A, Waterman MR. Identification by site-directed mutagenesis of two lysine residues in cholesterol side chain cleavage cytochrome P450 that are essential for adrenodoxin binding. J Biol Chem. 1992;267:22877–22882. [PubMed] [Google Scholar]
- 28.Coghlan VM, Vickery LE. Site-specific mutations in human ferredoxin that affect binding to ferredoxin reductase and cytochrome P450scc. J Biol Chem. 1991;266:18606–18612. [PubMed] [Google Scholar]
- 29.Coghlan VM, Vickery LE. Electrostatic interactions stabilizing ferredoxin electron transfer complexes. Disruption by “conservative” mutations. J Biol Chem. 1992;267:8932–8935. [PubMed] [Google Scholar]
- 30.Beckert V, Bernhardt R. Specific aspects of electron transfer from adrenodoxin to cytochromes p450scc and p45011beta. J Biol Chem. 1997;272:4883–4888. doi: 10.1074/jbc.272.8.4883. [DOI] [PubMed] [Google Scholar]
- 31.Usanov SA, et al. Probing the interaction of bovine cytochrome P450scc (CYP11A1) with adrenodoxin: Evaluating site-directed mutations by molecular modeling. Biochemistry. 2002;41:8310–8320. doi: 10.1021/bi0255928. [DOI] [PubMed] [Google Scholar]
- 32.Strushkevich NV, Azeva TN, Lepesheva GI, Usanov SA. Role of positively charged residues lys267, lys270, and arg411 of cytochrome p450scc (CYP11A1) in interaction with adrenodoxin. Biochemistry (Mosc) 2005;70:664–671. doi: 10.1007/s10541-005-0167-3. [DOI] [PubMed] [Google Scholar]
- 33.Strushkevich NV, Harnastai IN, Usanov SA. Mechanism of steroidogenic electron transport: role of conserved Glu429 in destabilization of CYP11A1-adrenodoxin complex. Biochemistry (Mosc) 2010;75:570–578. doi: 10.1134/s0006297910050056. [DOI] [PubMed] [Google Scholar]
- 34.Hannemann F, et al. The dipole moment of the electron carrier adrenodoxin is not critical for redox partner interaction and electron transfer. J Inorg Biochem. 2009;103:997–1004. doi: 10.1016/j.jinorgbio.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Xu X, et al. Dynamics in a pure encounter complex of two proteins studied by solution scattering and paramagnetic NMR spectroscopy. J Am Chem Soc. 2008;130:6395–6403. doi: 10.1021/ja7101357. [DOI] [PubMed] [Google Scholar]
- 36.Sevrioukova IF, Li H, Zhang H, Peterson JA, Poulos TL. Structure of a cytochrome P450-redox partner electron-transfer complex. Proc Natl Acad Sci USA. 1999;96:1863–1868. doi: 10.1073/pnas.96.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller JJ, Lapko A, Bourenkov G, Ruckpaul K, Heinemann U. Adrenodoxin reductase–adrenodoxin complex structure suggests electron transfer path in steroid biosynthesis. J Biol Chem. 2001;276:2786–2789. doi: 10.1074/jbc.M008501200. [DOI] [PubMed] [Google Scholar]
- 38.Williams PA, Cosme J, Sridhar V, Johnson EF, McRee DE. Mammalian microsomal cytochrome P450 monooxygenase: structural adaptations for membrane binding and functional diversity. Mol Cell. 2000;5:121–131. doi: 10.1016/s1097-2765(00)80408-6. [DOI] [PubMed] [Google Scholar]
- 39.Wester MR, Stout CD, Johnson EF. Purification and crystallization of N-terminally truncated forms of microsomal cytochrome P450 2C5. Methods Enzymol. 2002;357:73–79. doi: 10.1016/s0076-6879(02)57667-9. [DOI] [PubMed] [Google Scholar]
- 40.Jackson CJ, et al. A novel sterol 14alpha-demethylase/ferredoxin fusion protein (MCCYP51FX) from Methylococcus capsulatus represents a new class of the cytochrome P450 superfamily. J Biol Chem. 2002;277:46959–46965. doi: 10.1074/jbc.M203523200. [DOI] [PubMed] [Google Scholar]
- 41.Roberts GA, Grogan G, Greter A, Flitsch SL, Turner NJ. Identification of a new class of cytochrome P450 from a Rhodococcus sp. J Bacteriol. 2002;184:3898–3908. doi: 10.1128/JB.184.14.3898-3908.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 43.Collaborative Computational Project Number 4. The CCP4 suite: Programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 44.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47:(Pt 2)–110. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 45.Brunger AT, et al. Crystallography and NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 46.Kurnikov IV. Pittsburgh, PA: University of Pittsburgh; 2000. HARLEM, Version 1.0, Department of Chemistry. [Google Scholar]
- 47.Strushkevich N, Usanov SA, Park HW. Structural basis of human CYP51 inhibition by antifungal azoles. J Mol Biol. 2010;397:1067–1078. doi: 10.1016/j.jmb.2010.01.075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.