Abstract
Recent work using an art-viewing paradigm shows that monetary sponsorship of the experiment by a company (a favor) increases the valuation of paintings placed next to the sponsoring corporate logo, an effect that correlates with modulation of the ventromedial prefrontal cortex (VMPFC). We used the same art-viewing paradigm to test a prevailing idea in the domain of conflict-of-interest: that expertise in a domain insulates against judgment bias even in the presence of a monetary favor. Using a cohort of art experts, we show that monetary favors do not bias the experts’ valuation of art, an effect that correlates with a lack of modulation of the VMPFC across sponsorship conditions. The lack of sponsorship effect in the VMPFC suggests the hypothesis that their brains remove the behavioral sponsorship effect by censoring sponsorship-dependent modulation of VMPFC activity. We tested the hypothesis that prefrontal regions play a regulatory role in mediating the sponsorship effect. We show that the dorsolateral prefrontal cortex (DLPFC) is recruited in the expert group. Furthermore, we tested the hypothesis in nonexpert controls by contrasting brain responses in controls who did not show a sponsorship effect to controls who did. Changes in effective connectivity between the DLPFC and VMPFC were greater in nonexpert controls, with an absence of the sponsorship effect relative to those with a presence of the sponsorship effect. The role of the DLPFC in cognitive control and emotion regulation suggests that it removes the influence of a monetary favor by controlling responses in known valuation regions of the brain including the the VMPFC.
Keywords: decision-making, functional MRI, preference, art-expertise
Recent behavioral and neuroimaging evidence demonstrates that decision-making is potentially exposed to bias by top-down variables in various domains, and furthermore that bias exerts control over identifiable neural responses (1–7). These top-down variables include price, brand knowledge, and monetary favors, to name a few. The nature, neural underpinnings, and behavioral dynamics of this bias are not well understood. Most institutions guard against bias by constructing rules that delimit the kinds of favors allowed and the channels by which such favors may or may not be received. The “spirit” of these rules is to allow judgments to be unbiased by external incentives, but a scientific understanding of the connection between favors and covert biases in judgment is largely missing, leaving open the possibility for many pathways to inadvertent bias. One major hypothesis concerning judgment bias is that expertise in a domain tends wholly or in part to insulate against the biasing influences of favors. In this article, we build upon previously published work (5) to test this idea directly.
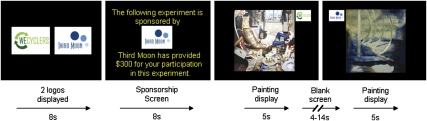
In a task designed to examine the effects of social gestures on subjective decision-making (5) we investigated the interaction of expertise with neural valuation processes. The task used company-logos, which served to act as sponsor for the participants in the experiment. In this paradigm, participants passively viewed digital images of art while undergoing an fMRI scan (Fig. 1). Participants were paid $300 and the funds were associated with one of two company logos. Two logos were initially presented and one of these was introduced as the sponsoring logo. The paintings were presented such that 50% were paired with a sponsoring company logo and the other 50% were paired with a nonsponsoring company logo. The paintings and logos were counterbalanced so that there were no changes in visual stimulation across participants. Postscanning, participants completed a second phase of the experiment consisting of behavioral preference ratings of the paintings to assess the influence of sponsorship on preference.
Fig. 1.
Art viewing paradigm. In the scanner participants were initially presented with two logos: a sponsoring and a nonsponsoring logo. One of two company-logos was associated with the funds ($300) that participants received for study compensation. The sponsorship screen was shown once at the beginning of the scanning cycle for 8 s. Participants were subsequently presented with 60 paintings that were displayed on different trials with either the sponsoring or the nonsponsoring logo during a passive scanning run. In a subsequent behavioral run, participants provided preference responses for each painting.
Several neuroimaging findings have established that value signals are encoded in the reward circuitry that includes the ventromedial prefrontal cortex (VMPFC) (8–20). Similar results have been found in monkey electrophysiology experiments (21–23). Neuroimaging studies have expanded these findings by demonstrating that value signals in the VMPFC can be modulated by cognitive inputs (1–5). We hypothesized that susceptibility to the sponsorship effect would modulate the response in the VMPFC in the two conditions (sponsor and nonsponsor), but that mitigation of the sponsorship effect would not lead to a modulation of the value signals computed in the VMPFC. We also speculated that mitigation of the sponsorship effect would engage the dorsolateral prefrontal cortex (DLPFC), based on this region's role in exerting cognitive control and in emotion regulation (24–26).
To accomplish our experimental aim, we used participants with cognitive training within a specific knowledge domain to test if expertise serves to mitigate bias instigated by a sponsoring company compared with a control group (n = 20). We included a congruent expert group, namely art-experts (n = 20), to test directly if art-experts would be influenced by monetary favors during the art-viewing paradigm. Participants constituting this group were carefully selected based on various requirements, such as a formal education in a visual art-related area and a minimum of 5 years of experience working within a visual art-related area. Our hypothesis finds support in previous behavioral studies to the extent that is has been shown that aesthetic expertise modulates judgment of art (27–29).
Results
Behavioral Effect of Sponsorship.
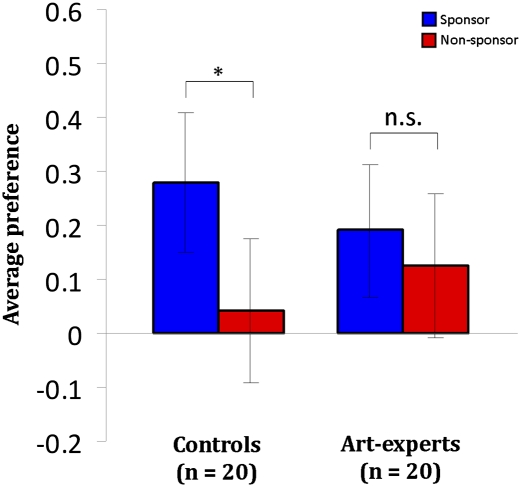
To assess if a monetary favor can bias subjective painting preference in both controls and art-experts, we calculated the average preference responses in the two conditions and in the two groups (Fig. 2). In the experimental set-up there is no explicit association between the logos and the paintings except for the visual presentation, and thus increased preference for paintings presented next to the sponsoring logo is considered a sponsorship effect. Controls showed greater average preference for sponsor compared with nonsponsor paintings (paired t = 3.02; df = 19; P < 0.006), demonstrating a sponsorship effect in line with our previous study (5). However, there was no effect of sponsorship within the expert group (paired t = 0.69; df = 19; P < 0.49). Familiarity ratings, collected postscanning, did not reveal significant interactions of sponsorship and familiarity in the two groups (Fig. S1). Finally, inspection of the distribution of preference responses within sponsor and nonsponsor conditions in the two groups did not display significant differences (Fig. S2 and Table S1).
Fig. 2.
Average preference responses across groups collected postscanning. Global average preference responses grouped into sponsoring (blue bars) and nonsponsoring (red bars) conditions presented separate for each of the two groups (controls and art-experts). The rating scale was a Likert-type scale (+3 to −3). Statistical analysis showed a significant difference between sponsor and nonsponsor conditions for controls (n = 20), which is denoted with an asterisk, but not for experts (n = 20). Error bars represent SE.
Modulation of VMPFC by Sponsorship.
In the neural data we sought to identify blood-oxygen level-dependent (BOLD) signals during the passive art-viewing paradigm that correlated with the behavioral sponsorship effect. Based on previous studies showing that the VMPFC encodes value signals across several sensory modalities (1–6, 8–12, 14–20), we made two predictions about the pattern of neural activity in the VMPFC. First, activity in the VMPFC should encode subjects’ preference ratings in both groups, regardless of condition-modality and expertise. Second, value signals in the VMPFC should be modulated by sponsorship in the control group but not exhibit sensitivity to sponsorship in the expert group.
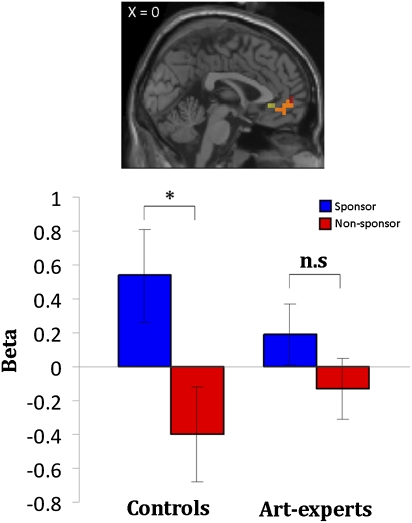
To test these predictions we used a parametric regression analysis. In this analysis we were particularly interested in identifying regions that correlated linearly with painting preference. We found that activity in the VMPFC correlated with a linear response profile when collapsing across conditions (sponsor and nonsponsor) in controls [Montreal Neurological Institute (MNI) coordinates −4 48 −6; P < 0.05, false-discovery rate (FDR), small-volume corrections (SVC)] and art-experts (0 48 −6; P < 0.05, FDR, SVC) (Fig. 3, Upper), supporting the prediction that the VMPFC encodes common value signals. In accordance with our a priori hypothesis, we applied SVC (30) to correct for multiple comparisons in reporting these results in the VMPFC. We identified the search volume using MNI coordinates (0 48 −16) that overlap with activity reported in two related studies (2, 5). No other brain regions correlated with preference across sponsorship in the two groups in a whole-brain analysis (P < 0.001, uncorrected). To determine the effects of sponsorship, we constructed a region of interest (ROI) in the VMPFC centered on identical coordinates as applied in the SVC. We extracted the average β-values from the VMPFC ROI and found that the sponsor condition displayed increased activity compared with the nonsponsor condition in the control group (paired t = 2.72; P < 0.01), supporting the second prediction that the VMPFC can be modulated by sponsorship (Fig. 3, Lower). In contrast, average β-estimates in the VMPFC ROI were not significantly modulated by sponsorship within the art-expert group.
Fig. 3.
Neural activity in the VMPFC encoding value signals and sponsorship bias. (Upper) The VMPFC display linear increase with preference responses collapsed across conditions (sponsor and nonsponsor) in controls and experts (P < 0.005, uncorrected). SVC (10-mm sphere; MNI: 0 48 −16) was applied to correct for multiple comparisons. Significant VMPFC voxels are displayed for controls, in yellow (−4 48 −6; 27 voxels; z = 2.64; P < 0.05, FDR, SVC) and for art-experts, in red (0 48 −6; 20 voxels; z = 2.49; P < 0.05, FDR, SVC). Overlapping voxels are displayed in orange. (Lower) ROI in the VMPFC. Average β-values extracted for each group in the defined ROI (10-mm mask; MNI: 0 48 −16) display higher β-values for sponsor (blue bars) than nonsponsor (red bars) conditions in controls (paired t = 2.72; P < 0.01), which is denoted with an asterisk. The VMPFC activity in art-experts was not modulated by stimuli modality. Error bars indicate SE.
DLPFC Involvement in Mitigating Judgment Bias.
We next investigated which neural regions were responsible for censoring sponsorship-dependent modulation of the VMPFC activity in the expert group. We hypothesized that regulating individual susceptibility to the sponsorship effect involves modulation by the DLPFC of the preference signals computed in the VMPFC. This hypothesis was based on the role of the DLPFC in executive control and emotion regulation (24–26), and the implication of the DLPFC in top-down modulation of valuation regions (3, 14, 16). Specifically, we predicted that the DLPFC should be more active for the sponsor condition in the binary comparison between the two groups: art-experts > controls. Note that there was no behavioral difference in average preference in sponsor trials across the two groups (two-sample t = 0.4; df = 38; P < 0.6). Thus, preference cannot account as a confounding factor in this contrast.
To test this hypothesis, we built a regressor using each participant's preference response for each painting in the sponsor condition and subtracted the control group from the expert group. Based on our prediction, we used SVC constraining our analysis to our a priori bilateral DLPFC region. Using this approach, activity reaching corrected significance was observed in the right DLPFC (z = 3.51; 36 29 27; P < 0.005, FDR, SVC) for the contrast, art-experts > controls. No other regions reached significance at the whole-brain level (P < 0.001, uncorrected). This result suggests that the expert group is engaging the right DLPFC to continuously regulate bias susceptibility (Fig. 4 A and B).
Fig. 4.
Modulation of DLPFC activity relative to sponsorship bias. (A) Binary group-specific comparison restricted to sponsor trials (art-experts > controls) displayed significant activity in the right DLPFC (z = 3.51; 36 29 27; P < 0.005, FDR, SVC). (B) Average β-estimates from the right DLPFC ROI are shown for controls and art-experts in sponsor (blue) and nonsponsor (red) conditions. Linear regression showing a relationship between a behavioral measure of individual susceptibility to the sponsorship effect in (C) experts and (D) nonexperts and activity in the right DLPFC. Each datapoint represents a participant. The right DLPFC β-value represents individual peak voxels from the main effect (art-experts > controls) displayed in A. All error bars denote SE.
To capture the hypothesized regulatory role of the DLPFC in mediating the effects of sponsorship in greater resolution, we subsequently extracted the β-value in the right DLPFC region identified above. We estimated a linear regression of the impact of right DLPFC activity against a behavioral measure of each individual's susceptibility to the sponsorship effect. We performed the correlation separately for experts and controls. This analysis showed that the DLPFC correlated negatively with bias susceptibility in experts (regression coefficient = −0.63; P < 0.001) (Fig. 4C). This result provides credence to the hypothesis that the DLPFC mediates the influence of a monetary favor in domain expertise. In contrast, the correlation for the control group was nonsignificant (regression coefficient = −0.29; P < 0.1) (Fig. 4D), although the trend in the DLPFC seen in this group suggests that the DLPFC was elevated in those control participants who did not display a significant sponsorship bias. We further examined the possibility of a more general regulatory mechanism that insulates from biasing effects of a monetary favor in subsequent analyses.
Functional Connectivity Between the VMPFC and DLPFC.
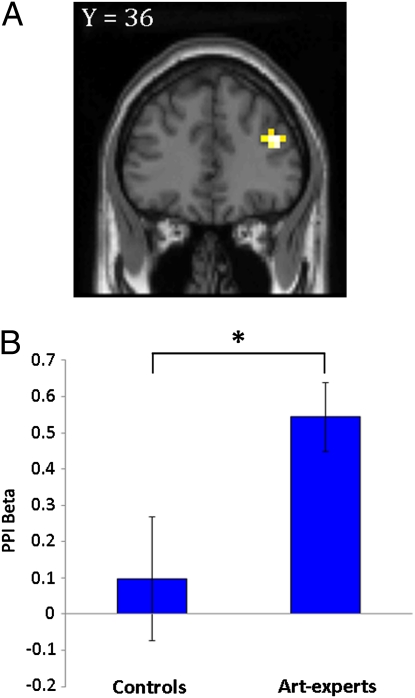
If a modulation of VMPFC by monetary favors were mediated by the DLPFC we would expect that VMPFC activity during sponsor trials should display a stronger coupling with DLPFC relative to nonsponsor trials. To test whether differential effects of sponsorship in the two groups were influenced by neural activity in DLPFC, we performed an effective connectivity analysis implemented as psychophysiological interactions (PPI) (30) using VMPFC as the seed region. Specifically, we assessed if the physiological coupling between the VMPFC and DLPFC changed relative to a modulation in the psychological parameter: sponsor > nonsponsor. This analysis exhibited increased connectivity with several regions (P < 0.05, FDR) (Table S2), including the right DLPFC in the expert group when using SVC in the a priori DLPFC region (z = 3.79; 44 36 24; P < 0.05, FDR, SVC) (Fig. 5A). Additionally, a post hoc analysis based on the average β-value in the right DLPFC showed that there was stronger connectivity between the VMPFC and right DLPFC in the expert group than in the control group (two-sample t = 2.2; df = 38; P < 0.03) (Fig. 5B).
Fig. 5.
Condition and group-specific changes in effective connectivity. (A) PPI displaying increased coupling between the VMPFC seed region and the right DLPFC in art-experts (z = 3.79; 44 36 24; P < 0.05, FDR, SVC). VMPFC activity in the control group did not exhibit significant connectivity with the DLPFC. (B) Average β-estimates from the right DLPFC measuring the correlation between BOLD activity in VMPFC and DLPFC in both groups. Statistical significance is denoted by an asterisk. Error bars are SE.
To investigate whether the DLPFC was anatomically consistent across analyses, we carried out a conjunction analysis between the right DLPFC identified in the binary contrast (art-experts > controls) (Fig. 4A) and the region of DLPFC identified in the PPI analysis (Fig. 5A). The conjunction formally confirmed that the same region of the DLPFC was activated in both analyses (P < 0.0052, uncorrected) (Fig. S3).
These results demonstrate a coupling between the right DLPFC and VMPFC. This coupling was strongest in sponsor compared with nonsponsor conditions, providing further weight to the hypothesis that the DLPFC is implicated in regulating the influence of sponsorship on preference in the expert group.
We further wanted to establish whether the DLPFC play a general regulatory role in mediating sponsorship bias independent of expertise. To test this theory we carried out a second PPI analysis using identical parameters as in the first PPI: that is, seeding from the VMPFC to assess the physiological coupling between the VMPFC and DLPFC relative to a modulation in the psychological parameter: sponsor > nonsponsor. In this second PPI we excluded the expert group and included only control participants by amalgamating data from our previous study (SI Text). Participants were separated into those who displayed a significant sponsorship effect and those controls that did not display such an effect. We hypothesized that participants without judgment bias should display stronger coupling between the VMPFC seed region and the DLPFC relative to those participants who displayed a sponsorship influence.
The results showed increased connectivity with the right DLPFC in the controls without sponsorship bias, which reached corrected significance when applying SVC in this region (z = 3.23; 36 32 32; P < 0.05, FDR, SVC) (Fig. S4 and Table S3). Average β-values in the right DLPFC showed stronger connectivity between the VMPFC and right DLPFC in the controls without sponsorship bias than in controls that displayed a sponsorship bias in a direct comparison (two-sample t = 2.8; df = 47; P < 0.007) (Fig. S4).
This analysis validates and expands the results from the first PPI by demonstrating that individual differences in the connectivity parameters between the VMPFC and DLPFC drives the fundamental difference in the ability to being able to reduce the influence of a monetary favor. This finding sets forth the notion that the DLPFC is engaged in a regulatory mechanism that seems independent of expertise.
Discussion
In this study, we demonstrate that domain expertise mitigates the influence of a monetary favor on behavioral preference during an art-viewing paradigm. In contrast, controls show on average that a monetary favor increases the valuation of paintings placed next to the sponsoring corporate logo. The neural data demonstrates that the behavioral sponsorship effect correlates with a modulation of the VMPFC in controls, but not in experts. In contrast, the VMPFC is modulated by the DLPFC in experts. More specifically, the DLPFC correlates with individual susceptibility to the sponsorship effect, in the direction of being more engaged in those participants that lack the sponsorship bias. We demonstrate an increased coupling between the VMPFC and DLPFC in experts, and expand this group specific analysis by amalgamating data from our previous study (5), showing that the subset of controls that do not display a sponsorship effect have greater coupling between the VMPFC and DLPFC. These results demonstrate that increases in self-censuring can modulate neural valuation mechanisms including the VMPFC.
We show that the VMPFC computes value signals for experts and controls in accordance with the theory that the VMPFC is part of a general valuation mechanism (1–6, 15). However, the VMPFC is modulated by sponsorship in the control group, but not in the expert group. This finding is in line with several recent neuroimaging studies demonstrating that external parameters, including sponsorship, can modulate responses in the VMPFC, even when controlling for visual stimulation across conditions (1–6). The VMPFC exhibited stronger connectivity with the DLPFC in experts relative to controls. A recent study of the neurobiology of self-control (14) found that the VMPFC is modulated by areas of the DLPFC, which is in accordance with the present result and suggests that the DLPFC sends input to the VMPFC that is integrated to compute common value signals in the VMPFC. Taken together, these results shows that external parameters can modulate value signals encoded in the VMPFC, and furthermore that people with specific domain expertise, such as art-experts, mitigate the influence of value signals on the VMPFC by recruiting the DLPFC. These results are enlightening in the context of the established role of the DLPFC in top-down modulation during tasks requiring executive control (25) and emotion regulation (26).
Our findings have implications for an ongoing debate that individual decision-making is subject to bias across multiple domains. Although our control group substantiates the notion that decisions can be significantly biased by social gestures, the present study shows that expertise within a domain insulates a person against biasing maneuvers through modulation of activity in the prefrontal cortex. The standard maneuver for insulating someone from biased judgments is to publicly expose their financial obligations and connections. Although such public exposure does act to bring more explicit external scrutiny, the degree to which public exposure actually increases effective self-censuring remains an important and open question. The findings in this study do suggest that domain expertise is one route by which a person's judgment bias from external incentives may be reduced or even eliminated. It seems plausible that experts may not be able to “turn off” regions of the brain that insulate them from bias, making their valuation processes more selective than the general population.
Methods
Subjects.
Forty subjects participated in the study. The art-expert group consisted of 20 subjects (11 females/9 males; average age 38.8 y; age range 23–56; average level of experience: 9 y, std = 4.2). A separate age- and sex-matched control group (n = 20) was recruited who did not have a formal art-education (13 females/7 males; average age 36.2; age range 18–59). Social economic status did not differ between groups [controls: 51.2 (11.0); art-experts: 50.2 (7.6)]. All participants had normal or corrected-to-normal vision, and none had a history of neurological or psychiatric disorders. All procedures were conducted in accordance with the Institutional Review Board of the Baylor College of Medicine.
fMRI Task.
Before scanning, participants were told they would be sponsored by one of two companies. In the scanner subjects were initially presented with two company logos, followed by a screen indicating which of the two companies would be sponsoring them, as well as their amount of compensation ($300). Two groups (20 art-experts; 20 controls) participated in the task and were paid $300. On each trial a painting was presented centrally and the logos were positioned in the upper left and right corner of the screen. Each painting was paired with either the sponsor logo or another, nonsponsor logo. The procedure was presented in a pseudorandom fashion and counterbalanced across subjects. Likewise, the pairing of logo and sponsorship was counterbalanced across subjects. During the scanning session, subjects were instructed to passively view each painting. Postscanning, subjects were asked to complete a behavioral run of the paintings and to make a subjective preference rating of each image using a Likert-scale (+3 to −3). The exact participant instructions are given in SI Text. In the behavioral task, the paintings were displayed in a randomized order compared with the scanning session, but the painting-logo pairings were kept consistent across both phases. This two-phase set-up was selected to bifurcate action and planning associated with making a choice from the passive valuation of each painting in the scanner (31). The participants were not informed about the second phase (behavioral rating) of the experiment until after the first phase (scanning run). In a previous study (5) we were able to demonstrate that neural value signals were computed even during a passive viewing of paintings, demonstrating that value signals are generated independently of actual choice. Hence, we were confident about applying this two-phase set-up. Visual chromatic reproductions of original paintings served as stimuli. In total, 60 paintings (30 abstract and 30 representational) were selected from graduate work with permission from the Slade School of Art, University College London. Noncanonical, contemporary art made by art students was selected to serve as stimulus material to ensure that all paintings were unfamiliar to the participants. Familiarity ratings of paintings were collected postscanning using identical parameters as those applied to collect preference behavior (Fig. S1). The logos were unfamiliar to the participants in that logos were prefabricated by the experimenters without reference to existing brands. The experimental protocol consisted of an event-related design. On each trial, a stimulus appeared for 5 s followed by a jittered intertrial interval of 4 to 14 s (Fig. 1). The stimuli were presented at a screen resolution of 1,024 × 768 pixels and centered in a 500 × 500-pixel resolution surrounded by a black background. Stimuli were presented and responses collected using NEMO (Human Neuroimaging Laboratory, Baylor College of Medicine). The stimuli were back-projected via an LCD projector onto a transparent screen positioned over the subjects’ head and viewed through a tilted mirror fixed to the head coil.
fMRI Data Acquisition and fMRI Data Analysis.
See SI Text for specifics.
Supplementary Material
Acknowledgments
We thank Krystle Bartley, Carrie Howard, Christine Cortelyou, and Monica Alexander for data collection, Mark Ross for help scripting the experiment, Nathan Apple for the logo design, and Claudia Bracero for administrative assistance. This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS045790.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019332108/-/DCSupplemental.
References
- 1.de Araujo IE, Rolls ET, Velazco MI, Margot C, Cayeux I. Cognitive modulation of olfactory processing. Neuron. 2005;46:671–679. doi: 10.1016/j.neuron.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 2.Plassmann H, O'Doherty J, Shiv B, Rangel A. Marketing actions can modulate neural representations of experienced pleasantness. Proc Natl Acad Sci USA. 2008;105:1050–1054. doi: 10.1073/pnas.0706929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClure SM, et al. Neural correlates of behavioral preference for culturally familiar drinks. Neuron. 2004;44:379–387. doi: 10.1016/j.neuron.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Kirk U, Skov M, Hulme O, Christensen MS, Zeki S. Modulation of aesthetic value by semantic context: An fMRI study. Neuroimage. 2009;44:1125–1132. doi: 10.1016/j.neuroimage.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Harvey AH, Kirk U, Denfield GH, Montague PR. Monetary favors and their influence on neural responses and revealed preference. J Neurosci. 2010;30:9597–9602. doi: 10.1523/JNEUROSCI.1086-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grabenhorst F, Rolls ET, Bilderbeck A. How cognition modulates affective responses to taste and flavor: Top-down influences on the orbitofrontal and pregenual cingulate cortices. Cereb Cortex. 2008;18:1549–1559. doi: 10.1093/cercor/bhm185. [DOI] [PubMed] [Google Scholar]
- 7.Robinson TN, Borzekowski DL, Matheson DM, Kraemer HC. Effects of fast food branding on young children's taste preferences. Arch Pediatr Adolesc Med. 2007;161:792–797. doi: 10.1001/archpedi.161.8.792. [DOI] [PubMed] [Google Scholar]
- 8.Aharon I, et al. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron. 2001;32:537–551. doi: 10.1016/s0896-6273(01)00491-3. [DOI] [PubMed] [Google Scholar]
- 9.O'Doherty J, et al. Beauty in a smile: The role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia. 2003;41:147–155. doi: 10.1016/s0028-3932(02)00145-8. [DOI] [PubMed] [Google Scholar]
- 10.Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: Characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- 11.Kringelbach ML, O'Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 12.Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc Natl Acad Sci USA. 2001;98:11818–11823. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rangel A, Camerer C, Montague PR. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci. 2008;9:545–556. doi: 10.1038/nrn2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 15.Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M. An automatic valuation system in the human brain: Evidence from functional neuroimaging. Neuron. 2009;64:431–439. doi: 10.1016/j.neuron.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Delgado MR, Phelps EA. How instructed knowledge modulates the neural systems of reward learning. Proc Natl Acad Sci USA. 2011;108:55–60. doi: 10.1073/pnas.1014938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47:763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Philiastides MG, Biele G, Heekeren HR. A mechanistic account of value computation in the human brain. Proc Natl Acad Sci USA. 2010;107:9430–9435. doi: 10.1073/pnas.1001732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28:5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hampton AN, Bossaerts P, O'Doherty JP. The role of the ventromedial prefrontal cortex in abstract state-based inference during decision making in humans. J Neurosci. 2006;26:8360–8367. doi: 10.1523/JNEUROSCI.1010-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallis JD, Miller EK. Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. Eur J Neurosci. 2003;18:2069–2081. doi: 10.1046/j.1460-9568.2003.02922.x. [DOI] [PubMed] [Google Scholar]
- 22.Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441:223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Padoa-Schioppa C, Assad JA. The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nat Neurosci. 2008;11:95–102. doi: 10.1038/nn2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 25.Wagner AD, Maril A, Bjork RA, Schacter DL. Prefrontal contributions to executive control: fMRI evidence for functional distinctions within lateral Prefrontal cortex. Neuroimage. 2001;14:1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- 26.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 27.Hekkert P, van Wieringen PC. The impact of level of expertise on the evaluation of original and altered versions of post-impressionistic paintings. Acta Psychol (Amst) 1996a;94:117–131. [Google Scholar]
- 28.Hekkert P, van Wieringen PC. Beauty in the eye of expert and nonexpert beholders: A study in the appraisal of art. Am J Psychol. 1996b;109:389–407. [Google Scholar]
- 29.O'Hare DPA. Individual differences in perceived similarity and preference for visual art: A multidimensional scaling analysis. Percept Psychophys. 1976;20:445–452. [Google Scholar]
- 30.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 31.Montague R. Why Choose This Book. How We Make Decisions. New York: Penguin Publishing; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.