Abstract
Lipopolysaccharides (LPS) and lipooligosaccharides (LOS) are the main lipid components of bacterial outer membranes and are essential for cell viability in most Gram-negative bacteria. Here we show that small molecule inhibitors of LpxC [UDP-3-O-(R-3-hydroxymyristoyl)-GlcNAc deacetylase], the enzyme that catalyzes the first committed step in the biosynthesis of lipid A, block the synthesis of LOS in the obligate intracellular bacterial pathogen Chlamydia trachomatis. In the absence of LOS, Chlamydia remains viable and establishes a pathogenic vacuole (“inclusion”) that supports robust bacterial replication. However, bacteria grown under these conditions were no longer infectious. In the presence of LpxC inhibitors, replicative reticulate bodies accumulated in enlarged inclusions but failed to express selected late-stage proteins and transition to elementary bodies, a Chlamydia developmental form that is required for invasion of mammalian cells. These findings suggest the presence of an outer membrane quality control system that regulates Chlamydia developmental transition to infectious elementary bodies and highlights the potential application of LpxC inhibitors as unique class of antichlamydial agents.
Keywords: anti-infectives
The obligate intracellular bacterium Chlamydia trachomatis is a widely disseminated human pathogen responsible for conjuctival diseases and a common cause of sexually transmitted infections. If left untreated, ocular and genital chlamydial infections can lead to blindness (trachoma), salpingitis, pelvic inflammatory disease, ectopic pregnancies, and infertility (1). Furthermore, genital chlamydial infections significantly increase susceptibility to infection with other sexually transmitted pathogens, including HIV (2).
Chlamydiae have a distinct biphasic developmental cycle consisting of two distinct morphological forms: the elementary body (EB) and the reticulate body (RB). Infection begins with the attachment of the metabolically inactive EB to the surface of epithelial cells, followed by its internalization and differentiation into the replicative RB (3). The RB replicates by binary fission within a membrane-bound vacuole termed an “inclusion” that is heavily modified with chlamydial proteins. Midway through the infectious cycle (18–24 h, depending on the serovar), RB replication becomes asynchronous, with some RBs differentiating back to the infectious EB form. EBs within the inclusion are eventually released into the extracellular space to initiate a new round of infection (4).
Lipopolysaccharide (LPS) is the principle component of the outer leaflet of the outer membrane of Gram-negative bacteria. It forms a tight permeability barrier that excludes cell-damaging agents such as detergents, proteases, bile salts, and hydrophobic antimicrobials. LPS consists of a hydrophobic membrane anchor lipid A, a nonrepeating core oligosaccharide, and a distal polysaccharide (O-antigen; reviewed in ref. 5). Chlamydia LPS is technically a lipooligosaccharide (LOS), because it only consists of a trisaccharide core of 3-deoxy-d-manno-oct-2-ulopyranosic acid (Kdo), linked to pentaacyl lipid A (6). In addition, chlamydial lipid A contains longer, nonhydroxylated fatty acids that significantly reduce its activity as an endotoxin (7). The Kdo linkage [α-Kdo-(2→8)-α-Kdo] was thought to be unique to Chlamydiaceae (8), although recent findings indicate that the Kdo core of Acinetobacter lwoffii F78 also shares this linkage and thus displays cross-reactivity to antichlamydial LOS monoclonal antibodies (9).
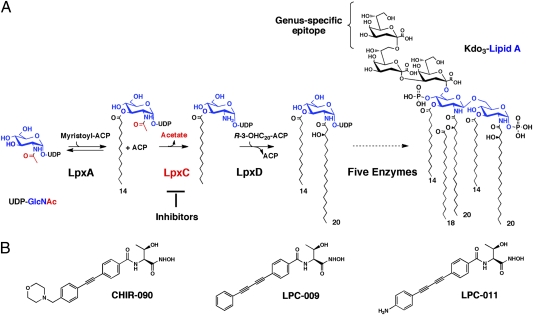
Because LPS is essential for the viability of most Gram-negative bacteria, components of the lipid A biosynthetic pathway are emerging targets for the development of new broad-spectrum antibiotics (10). One such enzyme is LpxC, a zinc-dependent cytoplasmic deacetylase that catalyzes the first committed step in lipid A biosynthesis (11) (Fig. 1A). Gene disruption experiments revealed that this enzyme is essential in Escherichia coli, and the first reported LpxC inhibitors displayed promising antimicrobial activities against E. coli (12–14). CHIR-090, a newer small-molecule inhibitor of LpxC with low nanomolar affinity, is as effective against Gram-negative pathogens as the DNA gyrase inhibitor ciprofloxacin (15). Structural and biochemical analysis have further revealed that the amino acid side chains in LpxC that are critical for substrate binding and catalysis are involved in the binding of CHIR-090 (16). These studies provided a template for the development of more potent LpxC inhibitors with a wider spectrum of antimicrobial activity. Based on CHIR-090 interactions with hydrophobic substrate-binding passage in Aquifex aeolicus LpxC, and on the molecular analysis of CHIR-090 resistance of the Rhizobium leguminosarum LpxC, two biphenyl diacetylene-based compounds (LPC-009 and LPC-011) with enhanced activity against LpxC were generated (16–18) (Fig. 1B).
Fig. 1.
The C. trachomatis lipid A biosynthetic pathway and structures of LpxC inhibitors. (A) LOS biosynthesis begins with the acylation of UDP-N-acetylglucosamine catalyzed by LpxA, which is selective for myristoyl-ACP in C. trachomatis (42). The deacetylation of the product UDP-3-O-(myristoyl)-N-acetylglucosamine in C. trachomatis, catalyzed by LpxC, is considered the first committed and irreversible step (16). The relatively simple structure of the major species of C. trachomatis LOS, consisting of Kdo3-lipid A, is well characterized (6). The C. trachomatis genus-specific epitope (i.e., the additional outer Kdo residue not present in other bacteria) is recognized by anti-C. trachomatis LOS antibodies. (B) Structures of the LpxC inhibitors CHIR-090, LPC-009, and LPC-011.
C. trachomatis contains all of the genes necessary for LOS biosynthesis (Fig. 1A) (19), but its role in cell viability and pathogenesis is not known. Because the Chlamydia LpxC has a 38% identity and 55% similarity to the E. coli LpxC, we sought to determine if the chlamydial enzyme was sensitive to LpxC inhibitors and whether these reagents could be used to probe the role that LOS plays in Chlamydia cell integrity, development, and pathogenesis. Here, we report that CHIR-090 and two of its derivatives blocked LOS synthesis in C. trachomatis but did not hinder the formation of inclusions or RB replication. Instead, LpxC inhibitors efficiently blocked the developmental transition of RB to EB. As a result, infected cells accumulated large inclusions filled with RBs but not infectious progeny. Our findings suggest that LOS plays a major role in the developmental transition essential for Chlamydia virulence, and that LpxC inhibitors can be potentially used as antichlamydial agents.
Results
LpxC Inhibitors Do Not Restrict the Intracellular Replication of C. trachomatis.
Because LPS is essential for the viability of most Gram-negative bacteria (5), we tested the effectiveness of LpxC inhibitors as antichlamydial agents. We first determined if these inhibitors could cross mammalian membranes and target intracellular bacteria by testing their effectiveness against Salmonella enterica serovar Typhimurium, a facultative intracellular Gram-negative bacterial pathogen that causes food-borne gastroenteritis (reviewed in ref. 20). HeLa cells were infected with S. typhimurium, and intracellular bacterial replication over a 24-h incubation period was assessed in the presence of the membrane-impermeable aminoglycoside antibiotic gentamicin. The intracellular minimal inhibitory concentrations (MICs) for LPC-011, LPC-009, and CHIR-090 were 0.06, 0.12, and 0.25 μg/mL, respectively (Fig. S1A). In contrast, the MIC for LpxC inhibitors for extracellular Salmonella cultured in the same medium used to maintain HeLa cells (DMEM supplemented with FBS) was 0.96, 1.44, and 2.0 μg/mL, for LPC-011, LPC-009, and CHIR-090, respectively (Fig. S1B). Therefore, between 8- and 16-fold higher doses of LpxC inhibitors were required to limit the growth of extracellular Salmonella. These differences in sensitivity to LpxC inhibitors may reflect an increased accumulation of these compounds within mammalian cells or an increased reliance for Salmonella on the membrane-stabilizing properties of LPS for intracellular growth.
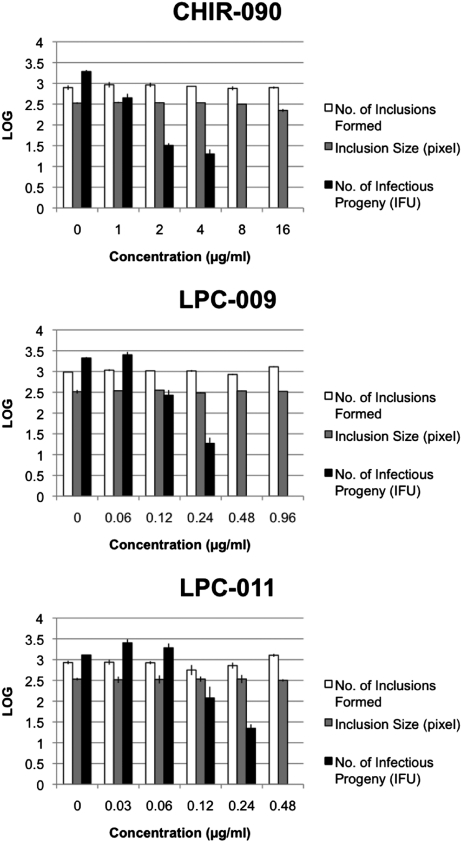
Having established that LpxC inhibitors can target intracellular bacteria, we next determined if LpxC inhibitors restricted chlamydial growth. HeLa cells were infected with C. trachomatis in the presence or absence of inhibitors for 36 h, and inclusion formation and sizes were assessed by indirect immunofluorescence microscopy. At the MICs required for containment of intracellular Salmonella, none of the three LpxC inhibitors led to any significant decreases in the number of inclusions formed or their size (Fig. 2). At significantly higher drug concentrations (128 μg/mL for CHIR-090, 7.68 μg/mL for LPC-011, and 15.36 μg/mL for LPC-009), inclusion formation was inhibited, although significant host cell toxicity was also observed, making it difficult to derive a true MIC value for these compounds.
Fig. 2.
LpxC inhibitors do not affect the formation of C. trachomatis inclusions but block the generation of infectious particles. Monolayers of HeLa cells were infected with C. trachomatis at a multiplicity of infection (MOI) of 2 in the absence or presence of increasing concentrations of LpxC inhibitors. Infected cells were fixed at 36 h postinfection (hpi) and labeled with polyclonal anti-Chlamydia antisera and a nucleic acid stain (Hoechst). The number and size of inclusions per well were determined with a Cellomics high-content image acquisition and analysis system. In parallel wells, the generation of infectious EB was determined by lysing infected cells in sterile water and determining the number of IFUs on fresh monolayers of HeLa cells.
LpxC Inhibitors Block LOS Synthesis in C. trachomatis.
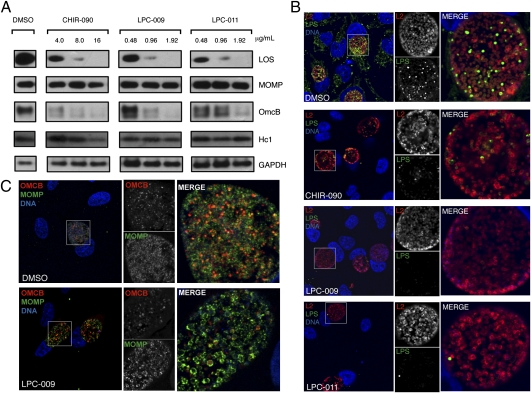
Our findings indicated that either LpxC inhibitors were ineffective against chlamydial LpxC or that Chlamydia does not require LOS for viability. To test these possibilities, we assessed the levels of LOS with a monoclonal anti–α-Kdo-(2→8)-α-Kdo antibody that specifically detects chlamydial LOS (21, 22). Treatment with all three LpxC inhibitors markedly decreased LOS levels in a dose-dependent manner, with no LOS detected by immunoblot analysis of chlamydiae-infected HeLa cells incubated in the presence of 16.0 μg/mL CHIR-090, 1.92 μg/mL LPC-011, or 1.92 μg/mL LPC-009 (Fig. 3A). The decrease in total LOS levels was not due to loss in bacterial viability, because the levels of major outer membrane protein (MOMP) were similar to the untreated control at these concentrations. Immunofluorescence assays with anti-LOS monoclonal antibodies in infected cells treated with LpxC inhibitors confirmed these results and revealed inclusions that were similar in size to DMSO-treated controls but showed little or no detectable LOS (Fig. 3B). Furthermore, the morphology and integrity of the inclusion membrane, as assessed by immunostaining for the inclusion membrane marker Cap1, appeared normal in inhibitor-treated cells (Fig. S2). Live microscopy of C. trachomatis-infected cells in the presence of LpxC inhibitors over a 20-h time period suggested that replication and inclusion expansion was indistinguishable from DMSO-treated controls (Movie S1). Interestingly, as previously reported (23, 24), antichlamydial LOS antibodies detected immunoreactive material in the host cytoplasm of infected and adjacent uninfected cells. There has been controversy as to whether LPS shedding in the cytosol of the host cell was an artifact of fixation. Treatment with LpxC inhibitors eliminated all anti-LOS immunostaining, suggesting that LOS accesses the host cell cytoplasm and may be delivered to adjacent uninfected cells.
Fig. 3.
LpxC inhibitors block the synthesis of LOS in C. trachomatis. (A) HeLa cells were infected with C. trachomatis at MOI of 3 and incubated in the presence of the indicated concentrations of LpxC inhibitors or the DMSO solvent control. At 48 hpi, total protein lysates were prepared and subjected to immunoblot analysis with anti-GAPDH, antichlamydial LOS, anti-MOMP, and anti-OmcB antibodies. Host GAPDH levels are shown as a loading control. Note the marked decrease of LOS and OmcB levels. (B) HeLa cells were infected with C. trachomatis at MOI of 1 in media containing either DMSO only, 16 μg/mL of CHIR-090, 1.92 μg/mL of LPC-009, or 1.92 μg/mL of LPC-011. At 36 hpi, cells were fixed and immunolabeled with polyclonal anti-Chlamydia L2 antisera (red) and an anti-LOS monoclonal antibody (green). (C) Infected cells were treated as in B and immunostained with anti-OmcB (red) and anti-MOMP (green). Note the loss of LOS (B) and OmcB (C) signal in cells treated with LpxC inhibitors.
Overall, these findings indicate that C. trachomatis does not require LOS for intracellular replication. LpxC inhibitors also blocked LOS synthesis in distantly related Chlamydiales, including zoonotic pathogens such as Chlamydophila caviae and Chlamydophila pneumoniae, a widely disseminated respiratory human pathogen (Fig. S3). Unlike C. trachomatis, however, LpxC inhibitors decreased the size of the inclusion formed, suggesting a greater need for LOS for optimal replication of these pathogens.
LpxC Inhibitors Block the Developmental Transition from RB to EB.
One noticeable difference between infected cells treated with LpxC inhibitors and untreated cells was an increased abundance of C. trachomatis forms that resembled RBs (Fig. 3B and Movie S1). We tested if the absence of LOS impacted Chlamydia developmental transitions and the generation of EBs. HeLa cells were infected with C. trachomatis in the absence or presence of LpxC inhibitors and lysed at 48 hpi; the yield of infectious particles was then assessed by determining the number of inclusion-forming units (IFUs) after infection of fresh HeLa cell monolayers. Surprisingly, despite the formation of inclusions of normal size and robust replication, treatment of infected cells with LpxC inhibitors had a dramatic effect on the generation of infectious progeny. The concentration of inhibitors that lead to no IFUs being generated is considered the minimal chlamydiacidal concentration (MCC) (25). The MCCs were 0.48 μg/mL for both LPC-011 and LPC-009, and 8.0 μg/mL for CHIR-090 (Fig. 2). These concentrations were four- to 32-fold above the intracellular MIC of Salmonella and 16- to 32-fold below the concentrations that impacted host cell viability. Therefore, even though LOS does not appear to be required for chlamydial replication, it is essential for the generation of infectious particles. This effect is reversible, because removal of LpxC inhibitors restored the generation of IFUs (Fig. S4).
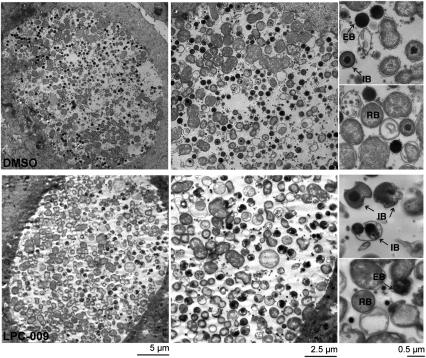
In response to cellular stresses, chlamydiae will stall the developmental cycle to generate enlarged, morphologically aberrant RBs (abRBs) that do not transition to EBs (26). This is unlikely to be the case for Chlamydia forms generated in the presence of LpxC inhibitors, because they were clearly distinct from abRB formed during treatment with ampicillin (10 μg/mL; Movie S1), indicating that loss of LOS did not induce the formation of chlamydial-persistent bodies. Next, we performed an ultrastructural analysis by transmission electron microscopy of HeLa cells infected for 36 h with Chlamydia in the presence or absence of LpxC inhibitors. Consistent with our observation by immunofluorescence and live cell microscopy, the LpxC inhibitors did not induce any major morphological or structural changes on inclusion or RB membranes (Fig. 4 and Movie S1). However, we noticed a clear lack of EB and an accumulation of aborted intermediate bodies (RBs in the process of transition to EBs), which were readily distinguished by EM by their size and compact nucleoids (Fig. 4). The occasional bacterial cell resembling an EB appeared misshapen.
Fig. 4.
LpxC inhibitors block the RB to EB developmental transition. C. trachomatis infected HeLa cells were incubated in media with DMSO only (Upper) or 1.92 μg/mL of LPC-009 (Lower). At 36 hpi, cells were fixed and processed for transmission electron microscopy. EB, elementary bodies; IB, intermediate bodies; RB, reticulate bodies. Note misshapen EB and IB in the inhibitor-treated samples.
If LpxC inhibitors block EB morphogenesis, we expected that the expression of markers of this developmental transition would be blocked. To test this, we assessed the levels of OmcB, a cysteine-rich outer membrane protein that is present only on the EB form (27), and Hc1, a histone-like protein that aids nucleoid condensation in EBs (28). We observed a dose-dependent decrease in OmcB levels, but not MOMP or Hc1, upon treatment with all LpxC inhibitors (Fig. 3A). These findings were corroborated by immunfluorescence microscopy, where we observed a drop in steady-state levels of OmcB in the presence of LpxC inhibitors (Fig. 3C) and the accumulation of intermediary bodies (IBs) with morphological signs of chromatin condensation (Fig. 4). Overall, these data suggest that LOS is required for the proper morphological transition of RBs to EBs, and that cell surface EB markers fail to be properly expressed or become unstable in the absence of LOS.
Discussion
The lipid A and Kdo components of LPS are essential for viability in most Gram-negative bacteria (reviewed in ref. 5). Disruption of any of the first six genes involved in lipid A assembly is lethal (Fig. 1). One exception is Neisseria meningitidis, where a deletion of lpxA, the first gene in the LPS biosynthetic pathway, results in viable lipid A-deficient bacteria (29). Here we provide evidence that Chlamydia can replicate in the presence of LpxC inhibitors at concentrations that resulted in undetectable amounts of LOS. Though lipid A-deficient N. meningitidis replicated at slower rates (30), absence of LOS did not significantly alter C. trachomatis growth or inclusion expansion (Fig. 2 and Movie S1). By electron microscopy, RBs in cells treated with LpxC inhibitors displayed normal outer and inner membranes, with no obvious differences in membrane thickness compared with untreated infected cells, as has been reported for N. meningitidis lpxA mutants (29). In these Neisseria mutants, the distribution of phosphatidylethanolamine and phosphatidylglycerol in inner and outer membranes was not affected, but their fatty acyl chains showed a shift toward shorter-chain saturated fatty acids (31). Future work will be aimed at determining if Chlamydia undergoes a similar adaptation upon treatment with LpxC inhibitors.
Inhibition of LOS biosynthesis did not induce the formation of enlarged abRBs that are observed during treatment with peptidoglycan assembly inhibitors or during immunological and nutritional stresses (iron and amino acid starvation) (26, 32, 33). The only apparent morphological consequence of LOS inhibition was the marked accumulation of RBs and the concomitant absence of EBs (Figs. 3 and 4). Consistent with this, very few infectious particles could be recovered from infected cells incubated in the presence of LpxC inhibitors (Fig. 2). LPS plays a role in the developmental transition in Myxococcus xanthus. Mutants lacking O-antigen are impaired in the formation of fruiting bodies and in the differentiation from rod-shaped cells to ovoid myxospores, most likely because the extensive remodeling of the bacterial cell surface may require the membrane environment provided by LPS (34). By analogy, we hypothesize that the osmotically fragile cell membrane of RBs must reorganize during development to form the rigid, highly disulfide cross-linked surface of EBs (35, 36). OmcB, OmcC, MOMP, and a large family of polymorphic membrane proteins, constitute the bulk of the Chlamydia outer membrane complex, a proteinaceous shell that provides structural integrity to the outer envelope of EBs (37, 38). OmcB and OmcC are developmentally regulated with maximum transcription and translation occurring late in the infectious cycle as RBs begin to transition to EBs (39, 40). We determined that OmcB, but not OmpA (MOMP), was poorly expressed in cells lacking LOS, suggesting that LOS is required for the stability, expression, or localization of EB-specific outer membrane proteins. However, the chromatin-condensing factor Hc1 was properly expressed and likely assembled on DNA, because IBs were readily apparent by electron microscopy. Nonetheless, these IBs did not fully progress to EB form, which raises the intriguing possibility that Chlamydia, like many organisms that undergo developmental changes, may possess checkpoints to ensure that gene and/or protein expression are coupled to morphogenesis.
Although LpxC inhibitors did not possess bactericidal activity in Chlamydia, they are potent antibiotics due to their ability to block RB to EB transition, which is an essential step in their pathogenesis. Currently, there are no available vaccines, and infection does not provide immunity toward subsequent reinfection. Because cell-mediated immunity is a strong component of antichlamydial immune responses (as reviewed in ref. 41), we postulate that inhibitors that target LOS biosynthesis may enhance such responses by allowing limited bacterial replication and antigen presentation, while preventing the spread of infectious particles. Future experiments will be aimed at determining if infected animals treated with LpxC inhibitors can elicit long-term immunity to subsequent chlamydial challenges.
Materials and Methods
Reagents.
The LpxC inhibitors LPC-009, LPC-011, and CHIR-090 were solubilized in DMSO and added at the indicated concentrations (16, 17, 18). The following antibodies were used: rabbit anti-Chlamydia LGV-L2 (provided by P. Bavoil, University of Maryland, Baltimore), rabbit anti-MOMP (provided by K. Fields, University of Miami, Miami), mouse monoclonal anti-MOMP (ab20881; Abcam), rabbit anti-Cap1 (provided by A. Subtil, Pasteur Institute, Paris), rabbit anti-OmcB (provided by T. Hatch, University of Tennessee Health Science Center, Memphis, TN), and mouse antichlamydial genus-specific LOS (EVIH1, isotype IgG2a; provided by H. Caldwell, Rocky Mountain Laboratories, Hamilton, MT).
Strains and Chlamydia Infections.
HeLa cells (CCL-2; ATCC) were grown in DMEM supplemented with 10% FBS (CellGro; Mediatech, Inc.). Salmonella enterica serovar Typhimurium strain SMO22 is from our laboratory strain collection. C. trachomatis serovar LGV biovar L2 434/Bu, C. pneumoniae AR39, and C. caviae GPIC were obtained from R. Stephens (University of California, Berkeley, CA), H. Caldwell (Rocky Mountain Laboratories/National Institutes of Health), and R. Rank (University of Arkansas, Fayetteville, AR), respectively. EBs were purified on Omnipaque (GE Healthcare) density gradients and stored in sucrose/phosphate/glutamate (SPG; 0.25 M sucrose, 10 mM sodium phosphate, 5 mM l-glutamic acid) buffer. To titer IFUs, EBs were harvested by disrupting host cells with sterile water followed by addition of 5× SPG, and serial dilutions were added to HeLa cell monolayers seeded on 96-well plates. Cells were fixed and inclusions stained at 36 hpi with anti-Chlamydia LGV2 antibodies followed by Alexa 555 conjugated secondary antibodies. The plates were analyzed using a Cellomics ArrayScan Vti HCS automated fluorescent imaging system (ThermoFisher) to determine the size and number of inclusions.
Microscopy.
LGV-L2 infections were synchronized by centrifugation (2,700 × g for 30 min at 15 °C) of EB onto prechilled HeLa cell monolayers grown on glass coverslips and incubated for the indicated times. Cells were fixed with 3% formaldehyde/ 0.025%glutaraldehyde in PBS and permeabilized with 0.1% Triton X-100/PBS. After a blocking step with 5% BSA in PBS, cells were incubated with the indicated primary antibodies followed by Alexa conjugated secondary antibodies (Invitrogen). Host and bacterial DNA were stained with Hoechst (Invitrogen). For live cell imaging, see Movie S1.
For transmission electron microscopy, infected cells were fixed with 2.5% glutaraldehyde/0.05% malachite green (EMS) in 0.1 M sodium cacodylate buffer (pH 6.8) and then postfixed with the following stains: 0.5% osmium tetroxide/0.8% potassium ferricyanide in 0.1 M sodium cacodylate; 1% tannic acid; and 1% uranyl acetate. Samples were dehydrated with graded amounts of ethanol and embedded in Spur's resin. Ultrathin sections were processed, poststained with uranyl acetate and lead citrate, and imaged on a Tecnai G2 Twin microscope (FEI).
Immunoblot Analysis.
Infected HeLa cells were lysed with a cold lysis buffer [25 mM Tris (pH 7.4), 150 mM NaCl, 5 mM EDTA, 1% Triton X-100] supplemented with Complete EDTA-Free Protease Inhibitor Cocktail Tablets (Roche). Protein lysates were resolved by SDS/PAGE or tricine SDS/PAGE and blotted onto PDVF membranes (Bio-Rad) and probed with the indicated antibodies.
Supplementary Material
Acknowledgments
We thank H. Caldwell, A. Subtil, T. Hatch, K. Fields, R. Stephens, R. Rank, and P. Bavoil for the gift of strains and reagents. This work was supported by National Institutes of Health Grants AI085238 and AI081694 (to R.H.V.), GM-51310 (to C.R.H.R.), and AI055588 (to P.Z.). R.H.V. is a Burrough's Wellcome Scholar in the Pathogenesis of Infectious Diseases.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1107478108/-/DCSupplemental.
References
- 1.Belland R, Ojcius DM, Byrne GI. Chlamydia. Nat Rev Microbiol. 2004;2:530–531. doi: 10.1038/nrmicro931. [DOI] [PubMed] [Google Scholar]
- 2.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 3.Dautry-Varsat A, Subtil A, Hackstadt T. Recent insights into the mechanisms of Chlamydia entry. Cell Microbiol. 2005;7:1714–1722. doi: 10.1111/j.1462-5822.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 4.Hybiske K, Stephens RS. Mechanisms of host cell exit by the intracellular bacterium Chlamydia. Proc Natl Acad Sci USA. 2007;104:11430–11435. doi: 10.1073/pnas.0703218104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raetz CRH, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rund S, Lindner B, Brade H, Holst O. Structural analysis of the lipopolysaccharide from Chlamydia trachomatis serotype L2. J Biol Chem. 1999;274:16819–16824. doi: 10.1074/jbc.274.24.16819. [DOI] [PubMed] [Google Scholar]
- 7.Heine H, Gronow S, Zamyatina A, Kosma P, Brade H. Investigation on the agonistic and antagonistic biological activities of synthetic Chlamydia lipid A and its use in in vitro enzymatic assays. J Endotoxin Res. 2007;13:126–132. doi: 10.1177/0968051907079122. [DOI] [PubMed] [Google Scholar]
- 8.Brade L, et al. Structural requirements of synthetic oligosaccharides to bind monoclonal antibodies against Chlamydia lipopolysaccharide. Glycobiology. 1997;7:819–827. doi: 10.1093/glycob/7.6.819. [DOI] [PubMed] [Google Scholar]
- 9.Hanuszkiewicz A, et al. Structural and immunochemical analysis of the lipopolysaccharide from Acinetobacter lwoffii F78 located outside Chlamydiaceae with a Chlamydia-specific lipopolysaccharide epitope. Chemistry. 2008;14:10251–10258. doi: 10.1002/chem.200800958. [DOI] [PubMed] [Google Scholar]
- 10.Barb AW, Zhou P. Mechanism and inhibition of LpxC: An essential zinc-dependent deacetylase of bacterial lipid A synthesis. Curr Pharm Biotechnol. 2008;9:9–15. doi: 10.2174/138920108783497668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whittington DA, Rusche KM, Shin H, Fierke CA, Christianson DW. Crystal structure of LpxC, a zinc-dependent deacetylase essential for endotoxin biosynthesis. Proc Natl Acad Sci USA. 2003;100:8146–8150. doi: 10.1073/pnas.1432990100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beall B, Lutkenhaus J. Sequence analysis, transcriptional organization, and insertional mutagenesis of the envA gene of Escherichia coli. J Bacteriol. 1987;169:5408–5415. doi: 10.1128/jb.169.12.5408-5415.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clements JM, et al. Antibacterial activities and characterization of novel inhibitors of LpxC. Antimicrob Agents Chemother. 2002;46:1793–1799. doi: 10.1128/AAC.46.6.1793-1799.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onishi HR, et al. Antibacterial agents that inhibit lipid A biosynthesis. Science. 1996;274:980–982. doi: 10.1126/science.274.5289.980. [DOI] [PubMed] [Google Scholar]
- 15.McClerren AL, et al. A slow, tight-binding inhibitor of the zinc-dependent deacetylase LpxC of lipid A biosynthesis with antibiotic activity comparable to ciprofloxacin. Biochemistry. 2005;44:16574–16583. doi: 10.1021/bi0518186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barb AW, Jiang L, Raetz CRH, Zhou P. Structure of the deacetylase LpxC bound to the antibiotic CHIR-090: Time-dependent inhibition and specificity in ligand binding. Proc Natl Acad Sci USA. 2007;104:18433–18438. doi: 10.1073/pnas.0709412104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CJ, et al. Species-specific and inhibitor-dependent conformations of LpxC: Implications for antibiotic design. Chem Biol. 2011;18:38–47. doi: 10.1016/j.chembiol.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang X, et al. Syntheses, structures and antibiotic activities of LpxC inhibitors based on the diacetylene scaffold. Bioorg Med Chem. 2011;19:852–860. doi: 10.1016/j.bmc.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephens RS, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 20.Ibarra JA, Steele-Mortimer O. Salmonella—the ultimate insider. Salmonella virulence factors that modulate intracellular survival. Cell Microbiol. 2009;11:1579–1586. doi: 10.1111/j.1462-5822.2009.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belunis CJ, Mdluli KE, Raetz CRH, Nano FE. A novel 3-deoxy-d-manno-octulosonic acid transferase from Chlamydia trachomatis required for expression of the genus-specific epitope. J Biol Chem. 1992;267:18702–18707. [PubMed] [Google Scholar]
- 22.Nano FE, Caldwell HD. Expression of the chlamydial genus-specific lipopolysaccharide epitope in Escherichia coli. Science. 1985;228:742–744. doi: 10.1126/science.2581315. [DOI] [PubMed] [Google Scholar]
- 23.Giles DK, Whittimore JD, LaRue RW, Raulston JE, Wyrick PB. Ultrastructural analysis of chlamydial antigen-containing vesicles everting from the Chlamydia trachomatis inclusion. Microbes Infect. 2006;8:1579–1591. doi: 10.1016/j.micinf.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Karimi ST, Schloemer RH, Wilde CE., 3rd Accumulation of chlamydial lipopolysaccharide antigen in the plasma membranes of infected cells. Infect Immun. 1989;57:1780–1785. doi: 10.1128/iai.57.6.1780-1785.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suchland RJ, Geisler WM, Stamm WE. Methodologies and cell lines used for antimicrobial susceptibility testing of Chlamydia spp. Antimicrob Agents Chemother. 2003;47:636–642. doi: 10.1128/AAC.47.2.636-642.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skilton RJ, et al. Penicillin induced persistence in Chlamydia trachomatis: High quality time lapse video analysis of the developmental cycle. PLoS ONE. 2009;4:e7723. doi: 10.1371/journal.pone.0007723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke IN, Ward ME, Lambden PR. Molecular cloning and sequence analysis of a developmentally regulated cysteine-rich outer membrane protein from Chlamydia trachomatis. Gene. 1988;71:307–314. doi: 10.1016/0378-1119(88)90047-9. [DOI] [PubMed] [Google Scholar]
- 28.Barry CE, III, Brickman TJ, Hackstadt T. Hc1-mediated effects on DNA structure: A potential regulator of chlamydial development. Mol Microbiol. 1993;9:273–283. doi: 10.1111/j.1365-2958.1993.tb01689.x. [DOI] [PubMed] [Google Scholar]
- 29.Steeghs L, et al. Meningitis bacterium is viable without endotoxin. Nature. 1998;392:449–450. doi: 10.1038/33046. [DOI] [PubMed] [Google Scholar]
- 30.van der Ley P, Steeghs L. Lessons from an LPS-deficient Neisseria meningitidis mutant. J Endotoxin Res. 2003;9:124–128. doi: 10.1179/096805103125001522. [DOI] [PubMed] [Google Scholar]
- 31.Steeghs L, et al. Outer membrane composition of a lipopolysaccharide-deficient Neisseria meningitidis mutant. EMBO J. 2001;20:6937–6945. doi: 10.1093/emboj/20.24.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raulston JE. Response of Chlamydia trachomatis serovar E to iron restriction in vitro and evidence for iron-regulated chlamydial proteins. Infect Immun. 1997;65:4539–4547. doi: 10.1128/iai.65.11.4539-4547.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beatty WL, Belanger TA, Desai AA, Morrison RP, Byrne GI. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect Immun. 1994;62:3705–3711. doi: 10.1128/iai.62.9.3705-3711.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowden MG, Kaplan HB. The Myxococcus xanthus lipopolysaccharide O-antigen is required for social motility and multicellular development. Mol Microbiol. 1998;30:275–284. doi: 10.1046/j.1365-2958.1998.01060.x. [DOI] [PubMed] [Google Scholar]
- 35.Hatch TP, Allan I, Pearce JH. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol. 1984;157:13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bavoil P, Ohlin A, Schachter J. Role of disulfide bonding in outer membrane structure and permeability in Chlamydia trachomatis. Infect Immun. 1984;44:479–485. doi: 10.1128/iai.44.2.479-485.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Afrane M, Clemmer DE, Zhong G, Nelson DE. Identification of Chlamydia trachomatis outer membrane complex proteins by differential proteomics. J Bacteriol. 2010;192:2852–2860. doi: 10.1128/JB.01628-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambden PR, Everson JS, Ward ME, Clarke IN. Sulfur-rich proteins of Chlamydia trachomatis: Developmentally regulated transcription of polycistronic mRNA from tandem promoters. Gene. 1990;87:105–112. doi: 10.1016/0378-1119(90)90500-q. [DOI] [PubMed] [Google Scholar]
- 40.Belland RJ, et al. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc Natl Acad Sci USA. 2003;100:8478–8483. doi: 10.1073/pnas.1331135100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roan NR, Starnbach MN. Immune-mediated control of Chlamydia infection. Cell Microbiol. 2008;10:9–19. doi: 10.1111/j.1462-5822.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 42.Sweet CR, Lin S, Cotter RJ, Raetz CRH. A Chlamydia trachomatis UDP-N-acetylglucosamine acyltransferase selective for myristoyl-acyl carrier protein. Expression in Escherichia coli and formation of hybrid lipid A species. J Biol Chem. 2001;276:19565–19574. doi: 10.1074/jbc.M101868200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.