Abstract
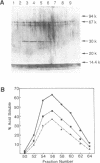
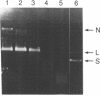
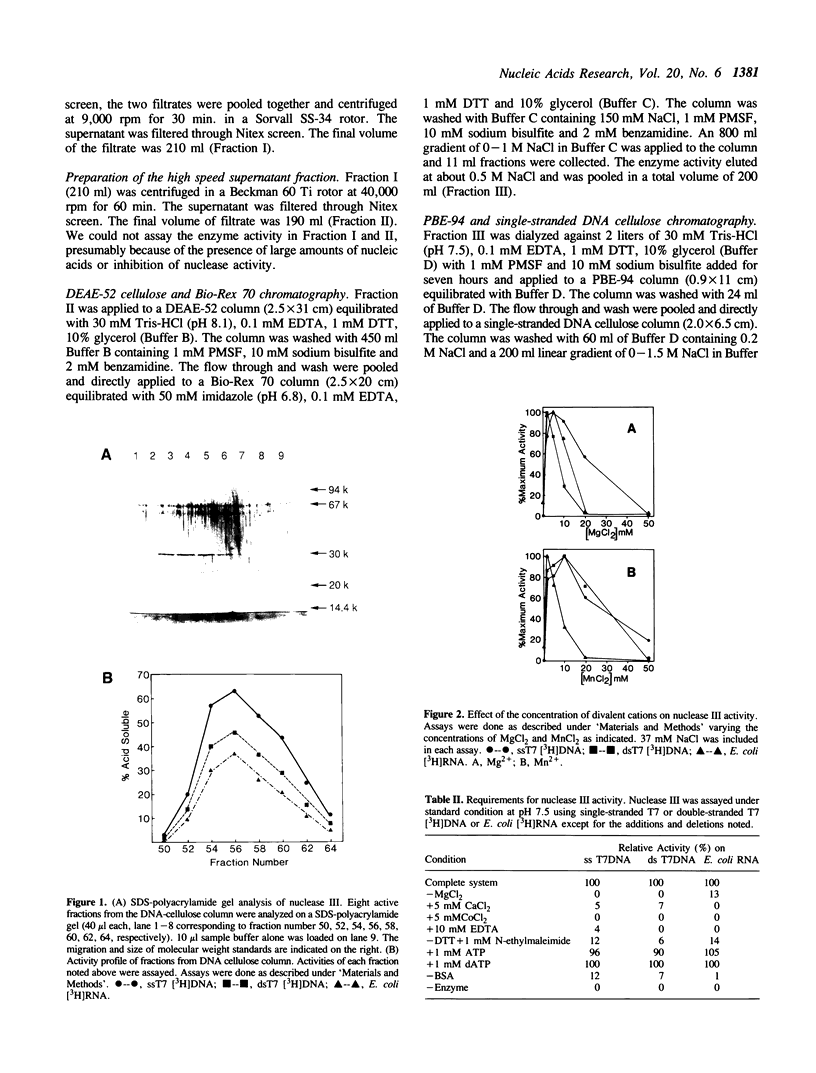
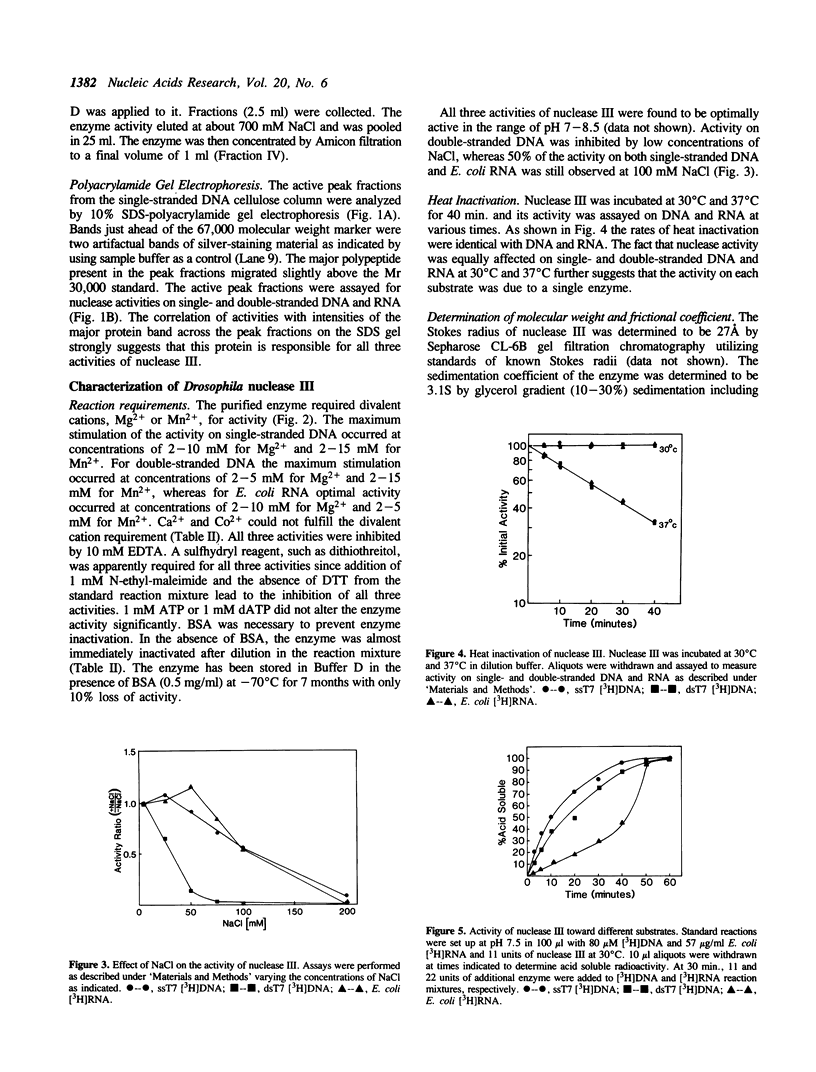
An endo-exonuclease (designated nuclease III) has been purified to near homogeneity from adult flies of Drosophila melanogaster. The enzyme degrades single- and double-stranded DNA and RNA. It has a sedimentation co-efficient of 3.1S and a strokes radius of 27A The native form of the purified enzyme appears to be a monomer of 33,600 dalton. It has a pH optimum of 7-8.5 and requires Mg2+ or Mn2+ but not Ca2+ or Co2+ for its activity. The enzyme activity on double-stranded DNA was inhibited 50% by 30 mM NaCl, while its activity on single-stranded DNA required 100 mM NaCl for 50% inhibition. Under the latter conditions, its activity on double-stranded DNA was inhibited approximately 98%. The enzyme degrades DNA to complete acid soluble products which are a mixture of mono- and oligonucleotides with 5'-P and 3'-OH termini. Supercoiled DNA was converted by the enzyme to nicked and subsequently to linear forms in a stepwise fashion under the condition in which the enzyme works optimally on single-stranded DNA. The amino acid composition and amino acid sequencing of tryptic peptides from purified nuclease III is also reported.
Full text
PDF

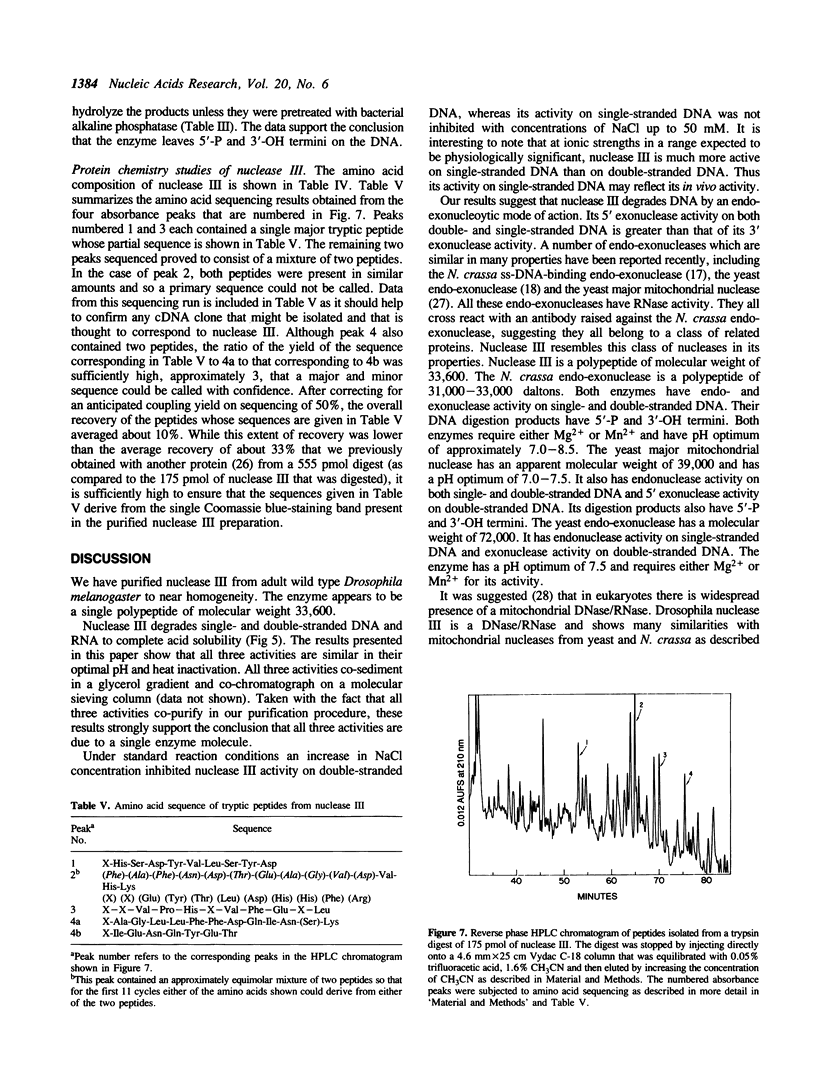

Images in this article
Selected References
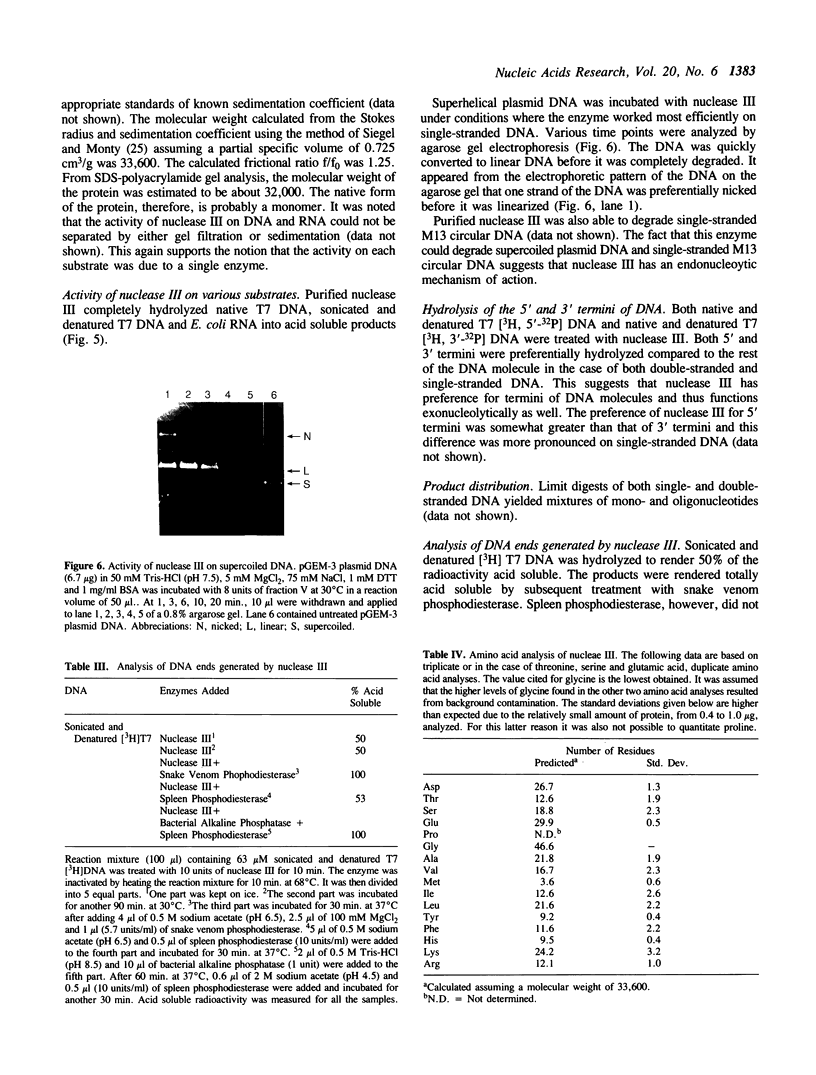
These references are in PubMed. This may not be the complete list of references from this article.
- Alfageme C. R., Zweidler A., Mahowald A., Cohen L. H. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. J Biol Chem. 1974 Jun 25;249(12):3729–3736. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chase J. W., Rabin B. A., Murphy J. B., Stone K. L., Williams K. R. Escherichia coli exonuclease VII. Cloning and sequencing of the gene encoding the large subunit (xseA). J Biol Chem. 1986 Nov 15;261(32):14929–14935. [PubMed] [Google Scholar]
- Chow T. Y., Fraser M. J. Purification and properties of single strand DNA-binding endo-exonuclease of Neurospora crassa. J Biol Chem. 1983 Oct 10;258(19):12010–12018. [PubMed] [Google Scholar]
- Chow T. Y., Resnick M. A. Purification and characterization of an endo-exonuclease from Saccharomyces cerevisiae that is influenced by the RAD52 gene. J Biol Chem. 1987 Dec 25;262(36):17659–17667. [PubMed] [Google Scholar]
- Dake E., Hofmann T. J., McIntire S., Hudson A., Zassenhaus H. P. Purification and properties of the major nuclease from mitochondria of Saccharomyces cerevisiae. J Biol Chem. 1988 Jun 5;263(16):7691–7702. [PubMed] [Google Scholar]
- Elgin S. C., Hood L. E. Chromosomal proteins of Drosophila embryos. Biochemistry. 1973 Nov 20;12(24):4984–4991. doi: 10.1021/bi00748a026. [DOI] [PubMed] [Google Scholar]
- Fleischmann G., Pflugfelder G., Steiner E. K., Javaherian K., Howard G. C., Wang J. C., Elgin S. C. Drosophila DNA topoisomerase I is associated with transcriptionally active regions of the genome. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6958–6962. doi: 10.1073/pnas.81.22.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser M. J., Koa H., Chow T. Y. Neurospora endo-exonuclease is immunochemically related to the recC gene product of Escherichia coli. J Bacteriol. 1990 Jan;172(1):507–510. doi: 10.1128/jb.172.1.507-510.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T., Brutlag D. ATP-dependent DNA topoisonmerase from D. melanogaster reversibly catenates duplex DNA rings. Cell. 1980 Aug;21(1):115–125. doi: 10.1016/0092-8674(80)90119-1. [DOI] [PubMed] [Google Scholar]
- Hsieh T. Knotting of the circular duplex DNA by type II DNA topoisomerase from Drosophila melanogaster. J Biol Chem. 1983 Jul 10;258(13):8413–8420. [PubMed] [Google Scholar]
- Ingles C. J., Biggs J., Wong J. K., Weeks J. R., Greenleaf A. L. Identification of a structural gene for a RNA polymerase II polypeptide in Drosophila melanogaster and mammalian species. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3396–3400. doi: 10.1073/pnas.80.11.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Rabin B. A., Chase J. W. DNA ligase from Drosophila melanogaster embryos. Substrate specificity and mechanism of action. J Biol Chem. 1987 Oct 15;262(29):14105–14111. [PubMed] [Google Scholar]
- Rabin B. A., Hawley R. S., Chase J. W. DNA ligase from Drosophila melanogaster embryos. Purification and physical characterization. J Biol Chem. 1986 Aug 15;261(23):10637–10645. [PubMed] [Google Scholar]
- Resnick M. A. The repair of double-strand breaks in DNA; a model involving recombination. J Theor Biol. 1976 Jun;59(1):97–106. doi: 10.1016/s0022-5193(76)80025-2. [DOI] [PubMed] [Google Scholar]
- Richardson C. C. The 5'-terminal nucleotides of T7 bacteriophage deoxyribonucleic acid. J Mol Biol. 1966 Jan;15(1):49–61. doi: 10.1016/s0022-2836(66)80208-5. [DOI] [PubMed] [Google Scholar]
- Sakaguchi K., Boyd J. B. Purification and characterization of a DNA polymerase beta from Drosophila*. J Biol Chem. 1985 Sep 5;260(19):10406–10411. [PubMed] [Google Scholar]
- Shelton E. R., Osheroff N., Brutlag D. L. DNA topoisomerase II from Drosophila melanogaster. Purification and physical characterization. J Biol Chem. 1983 Aug 10;258(15):9530–9535. [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Vincent R. D., Hofmann T. J., Zassenhaus H. P. Sequence and expression of NUC1, the gene encoding the mitochondrial nuclease in Saccharomyces cerevisiae. Nucleic Acids Res. 1988 Apr 25;16(8):3297–3312. doi: 10.1093/nar/16.8.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]