Abstract
Loss of cystic fibrosis transmembrane conductance regulator (CFTR) anion channel function causes cystic fibrosis (CF) lung disease. CFTR is expressed in airway epithelia, but how CF alters electrolyte transport across airway epithelia has remained uncertain. Recent studies of a porcine model showed that in vivo, excised, and cultured CFTR−/− and CFTRΔF508/ΔF508 airway epithelia lacked anion conductance, and they did not hyperabsorb Na+. Therefore, we asked whether Cl− and Na+ conductances were altered in human CF airway epithelia. We studied differentiated primary cultures of tracheal/bronchial epithelia and found that transepithelial conductance (Gt) under basal conditions and the cAMP-stimulated increase in Gt were markedly attenuated in CF epithelia compared with non-CF epithelia. These data reflect loss of the CFTR anion conductance. In CF and non-CF epithelia, the Na+ channel inhibitor amiloride produced similar reductions in Gt and Na+ absorption, indicating that Na+ conductance in CF epithelia did not exceed that in non-CF epithelia. Consistent with previous reports, adding amiloride caused greater reductions in transepithelial voltage and short-circuit current in CF epithelia than in non-CF epithelia; these changes are attributed to loss of a Cl− conductance. These results indicate that Na+ conductance was not increased in these cultured CF tracheal/bronchial epithelia and point to loss of anion transport as key to airway epithelial dysfunction in CF.
Keywords: chloride secretion, epithelial Na+ channels
Electrolyte transport by airway epithelia and submucosal glands controls the quantity and composition of airway surface liquid. The cystic fibrosis transmembrane conductance regulator (CFTR) anion channel is localized in the apical membrane of airway epithelia, and its loss in cystic fibrosis (CF) is thought to impair pulmonary host defense (1–7). However, how loss of CFTR function changes electrolyte transport across airway epithelia has been uncertain.
The first evidence of defective airway epithelial ion transport was the observation that the voltage (Vt) across nasal epithelia was more electrically negative in persons who have CF than in non-CF controls (8). Applying amiloride, an inhibitor of epithelial Na+ channels (ENaC), caused a greater reduction in nasal Vt (ΔVtamil) in CF epithelia. Subsequent studies revealed that CF epithelia were Cl− impermeable compared with non-CF epithelia (9, 10). Studies of excised nasal epithelia in Ussing chambers yielded varying results that, as the authors noted, might be attributed to epithelial location (CF polyps vs. non-CF turbinates), tissue injury, medications, small sample sizes, chronic infection, inflammation, and exclusion bias (11).
To circumvent some of these limitations and to facilitate studies of airway epithelial ion transport, many investigators established techniques for culturing human airway epithelial cells at the air–liquid interface so that they differentiate and can be studied in vitro (12). Loss of anion conductance has been apparent in nearly all studies of differentiated CF airway epithelial cultures (9, 10, 13, 14). In addition, some studies showed an increased short-circuit current (Isc) and voltage under basal conditions (basal Isc and basal Vt, respectively) and increased reductions with amiloride application (ΔIscamil and ΔVtamil, respectively) (7). The increased basal Vt, ΔVtamil, basal Isc, and ΔIscamil were interpreted to indicate that Na+ channel activity (i.e., Na+ conductance) was increased in CF epithelia (7, 15). Additional studies of recombinant CFTR and ENaC in MDCK cells and fibroblasts reported that CFTR inhibited ENaC channels (16), although other studies in oocytes suggested that CFTR did not inhibit ENaC (17). These reports plus additional studies led to the hypothesis that CFTR inhibits ENaC and that loss of CFTR increases ENaC Na+ conductance, resulting in Na+ hyperabsorption and reducing periciliary liquid depth to cause CF lung disease (for a review, see ref. 15).
To understand CF pathogenesis better, we recently developed pigs with either a targeted disruption of the CFTR gene or a ΔF508 mutation (18–20). CFTR−/− and CFTRΔF508/ΔF508 pigs spontaneously develop lung disease that closely resembles that in persons who have CF (20, 21). At birth, porcine CF airway epithelia are not inflamed, but they already exhibit defective host defense against bacteria. Moreover, newborn CFTR−/− and CFTRΔF508/ΔF508 pigs exhibit increased nasal Vt and ΔVtamil like that seen in persons who have CF. However, they do not have more Na+ absorption than controls, and the CF-induced elevations in basal Vt, basal Isc, ΔVtamil, and ΔIscamil are caused by the lack of apical Cl− conductance, not by increased Na+ conductance (20, 22).
These observations led us to ask how lack of CFTR changes electrolyte transport in primary cultures of differentiated human airway epithelia. Our main goal was to determine if human CF epithelia have decreased Cl− conductance, increased Na+ conductance, or a combination of the two. Any of these alternatives might explain the changes in Vt and Isc observed in CF.
We began by examining the electrophysiological properties of primary cultures of differentiated airway epithelia and measured transepithelial conductance (Gt), Isc, and Vt. We first focused on Gt (the inverse of transepithelial resistance) for two main reasons. First, Gt measurements are related directly to the permeability of ion channels, and our two main questions are about Cl− and Na+ channels. Second, compared with Isc and Vt measurements in airway epithelia, Gt is much less affected by membrane voltages, ion concentrations, and ion flow in the physiologic range. Gt is the sum of the cellular (Gc) and paracellular (Gp) conductances, and Gc is determined by apical and basolateral conductances. If CF epithelia have only a reduced Cl− conductance, then Gt under basal conditions (basal Gt) should be decreased, and the converse should be true if increased Na+ conductance is the only abnormality. If CF epithelia have both reduced Cl− conductance and elevated Na+ conductance, then Gt changes will be more complex.
After considering Gt, we turned to Isc and Vt. Isc measures the net electrically conductive transepithelial flow of ions in the absence of ion concentration or voltage gradients. Thus, Isc and ΔIamil can reflect active Na+ transport. However, in addition to the effect of apical Na+ conductance, Isc and ΔIamil are influenced by other conductances in the apical membrane (for example, CFTR), by basolateral membrane conductances, by apical and basolateral voltages, and by transmembrane ion concentration gradients (23, 24). We also measured Vt because it is a valuable in vivo aid for diagnosis and for assessing therapeutic interventions.
Results
CF Epithelia Lack Cl− Conductance, and Na+ Conductance Is Not Increased.
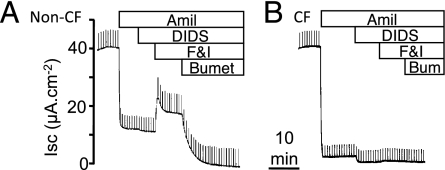
We studied primary cultures of tracheal/bronchial epithelia from 261 non-CF and 74 CF donors. We grew epithelia on permeable filter supports at the air–liquid interface and studied them after differentiation (25). The finding that freshly excised and cultured porcine airway epithelia have similar electrophysiological properties supports the use of differentiated primary cultures to assess CF/non-CF differences in ion transport (20, 22). We sequentially altered transport with (i) amiloride, which inhibits apical ENaC Na+ channels; (ii) 4,4′-diisothiocyanotostilbene-2,2′-disulfonic acid (DIDS), which inhibits most non-CFTR Cl− channels in the apical membrane; (iii) forskolin and 3-isobutyl-2-methylxanthine (IBMX), which increase cellular levels of cAMP leading to phosphorylation of CFTR by cAMP-dependent protein kinase; and (iv) bumetanide, which inhibits the basolateral Na+-K+-2Cl− cotransporter (NKCC). Fig. 1 shows examples of the effects of these compounds on Isc.
Fig. 1.
Representative tracings of Isc from differentiated cultures of human airway epithelia. (A) Non-CF epithelia. (B) CF epithelia. Regular pulses are current required to clamp Vt to 5 mV. Sequential additions were 100 μM apical amiloride (Amil), 100 μM apical DIDS, 10 μM apical forskolin plus 100 μM IBMX (F&I), and 100 μM basolateral bumetanide (Bumet).
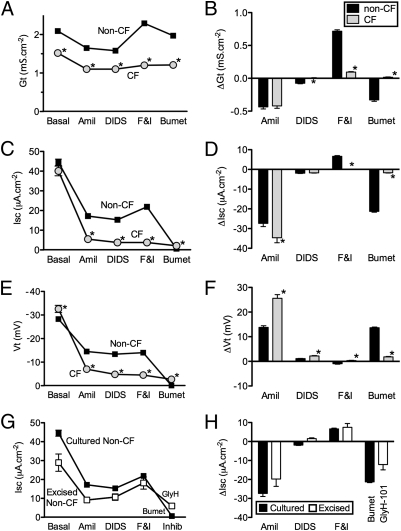
We made the following observations about Gt. (i) Basal Gt was lower in CF than in non-CF epithelia (Fig. 2A), although there was substantial variability among epithelia from different donors (Fig. S1A). (ii) Amiloride reduced Gt (ΔGtamil) to a similar extent in CF and non-CF epithelia. After amiloride was added, the non-CF Gt exceeded the CF Gt (Fig. 2 A and B). These Gt values allow three interpretations. First, the reduced basal Gt in CF epithelia is caused either by reduced Cl− conductance or by lower Gp; increased Na+ conductance in CF, on its own, could not account for the differences. Second, if CF epithelia had a larger Na+ conductance, then ΔGtamil should have been greater in CF. That was not what we found. Even if apical Na+ conductance was equal in CF and non-CF epithelia and other transport processes were identical, ΔGtamil should have been greater in CF epithelia (see ref. 22 and its supplemental online material). Third, finding a greater Gt after amiloride addition in non-CF vs. CF epithelia indicates the presence of CFTR Cl− channel activity under basal conditions, a conclusion consistent with the Isc measurements shown below and with earlier studies (26). Of note, the presence of Cl− conductance under basal conditions does not necessarily indicate the presence of Cl− secretion. (iii) Adding DIDS slightly but significantly reduced Gt in non-CF epithelia. (iv) Forskolin and IBMX increased Gt in non-CF epithelia with minimal change in CF epithelia. These data are consistent with phosphorylation-dependent activation of a CFTR Cl− conductance. (v) Bumetanide reduced Gt in non-CF but not in CF epithelia. Although inhibiting the electrically neutral basolateral NKCC might be expected not to alter Gt, a reduction has been observed previously after basolateral Cl− uptake was blocked in Cl−-secreting airway epithelia (27).
Fig. 2.
Transepithelial electrophysiological properties in non-CF and CF epithelia. Gt (A), Isc (C), and Vt (E) under basal conditions and following the sequential additions indicated in legend of Fig. 1. Changes in Gt (ΔGt) (B), Isc (ΔIsc) (D) and Vt (ΔVt) (F) with treatments shown in A, C, and E. Data are mean ± SEM from 261 non-CF donors and 74 CF donors; data for each donor were the average of measurements from 1–12 epithelia (three epithelia were measured for most donors). In some cases error bars are hidden by symbols. *Difference from non-CF, P < 0.05, unpaired t test. (G and H) Data as in C and D but from excised epithelia (n = 10). Cl− current was inhibited using 100 μM GlyH-101 rather than bumetanide, so values after addition of an inhibitor are not directly comparable. Data from cultured non-CF epithelia (C and D) are shown for comparison.
Apical Membrane Na+ Channel Activity Is Not Increased in CF Airway Epithelia.
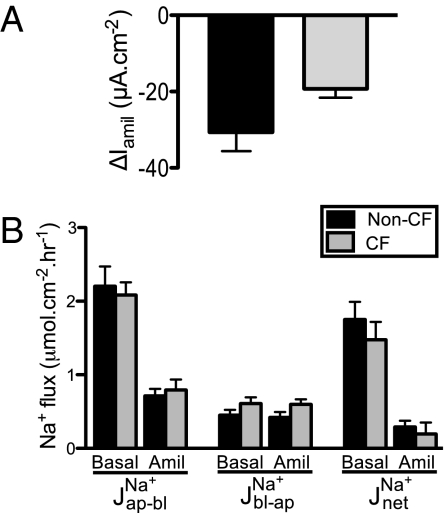
To test further whether apical Na+ channel activity was increased in CF epithelia, we generated an apical to basolateral Na+ concentration gradient, permeabilized the basolateral membrane with nystatin, and measured the resulting Na+ current. Amiloride addition decreased current, and the reduction was not greater in CF epithelia (Fig. 3A).
Fig. 3.
Apical Na+ current and Na+ fluxes in non-CF and CF epithelia. (A) Amiloride-sensitive current in epithelia with a permeabilized basolateral membrane and an apical-to-basolateral Na+ concentration gradient. n = 11 non-CF and 8 CF epithelia, all from different donors. (B) Open-circuit Na+ unidirectional and net Na+ flux rates determined isotopically under basal conditions and after addition of 100 μM amiloride apically. JNaap-bl indicates Na+ flux from the apical to the basolateral surface, JNabl-ap indicates flux in the opposite direction, and JNanet indicates net flux. n = 9 non-CF and 9 CF donors with one pair of epithelia from each donor. In matched epithelia, Vt was −20 ± 5 mV for non-CF and −24 ± 4 mV for CF epithelia; Isc was 44 ± 7 μA·cm−2 for non-CF and 47 ± 8 μA·cm−2 for CF; Gt was 2.2 ± 0.3 mS·cm−2 for non-CF and 1.8 ± 0.2 mS·cm−2 for CF.
Transepithelial 22Na+ Absorption in CF Epithelia Does Not Exceed That in Non-CF Epithelia.
The conclusion that Na+ conductance is not elevated in CF predicts that Na+ absorption in CF would not exceed that in non-CF epithelia. To test this prediction, we measured 22Na+ fluxes across the cultured epithelia under open-circuit conditions. Amiloride reduced apical-to-basolateral fluxes in both non-CF and CF epithelia (Fig. 3B). The net rate of Na+ absorption did not differ between non-CF and CF epithelia.
The Gt data, measurements of Na+ current when the basolateral membrane was permeabilized, and 22Na+ flux results indicate that Na+ conductance was not increased in CF epithelia.
Amiloride Causes Greater Isc and Vt Changes in CF Epithelia.
We compared Isc in CF and non-CF epithelia. (i) Basal Isc did not differ statistically between the two groups (Fig. 2C and Fig. S1B). (ii) Amiloride reduced Isc more in CF than in non-CF epithelia (Fig. 2 C and D). After amiloride was added, Isc was greater in non-CF than in CF epithelia because of the presence of Cl− secretion. This result is consistent with constitutive CFTR activity under basal conditions. These data also support the conclusion that the greater ΔIscamil in CF was caused by the lack of a Cl− conductance. (iii) DIDS had minimal effects in both CF and non-CF epithelia. (iv) Increasing cellular levels of cAMP increased Isc, and inhibiting basolateral Cl− entry with bumetanide reduced Isc in non-CF but not in CF epithelia. These observations have been made often and indicate CFTR-dependent Cl− secretion.
We also measured Vt. (i) Basal Vt was more electrically negative in CF epithelia than in non-CF epithelia (Fig. 2E and Fig. S1B). (ii) Amiloride reduced Vt almost twice as much in CF epithelia as in non-CF epithelia (Fig. 2 E and F). The lesser ΔVtamil in non-CF epithelia was caused largely by an amiloride-insensitive Vt from non-ENaC–mediated active ion transport. (iii) Adding forskolin and IBMX had minimal effects on Vt in non-CF epithelia. (iv) After bumetanide was added to inhibit Cl− entry into cells, and thereby transepithelial Cl− secretion, non-CF Vt fell to values close to those in CF epithelia, i.e., near zero.
Excised Non-CF Epithelia Show Properties Similar to Those in Cultured Epithelia.
We also studied bronchial epithelia excised from 10 nonsmoking persons without chronic obstructive lung disease; some of the lungs had been removed for transplantation but were not used for reasons that are unknown to us. Edge damage and potential injury during mounting can increase Gt markedly; the increase would be similar to an increase in Gp (28). Thus, this preparation reduces Vt. Edge damage also reduces the area of functioning epithelia in the Ussing chamber and thereby reduces Isc, but the effect on Isc is much less than on Gt. We found that values of basal Isc, ΔIscamil, and cAMP-induced ΔIsc were reasonably close to those of cultured epithelia (Fig. 2 G and H). The directions of Gt and Vt responses to the various interventions were consistent with (although much smaller than) those in cultured epithelia (Fig. S2). We did not evaluate excised CF bronchial epithelia, because the tissue we obtained usually was not suitable for study because of advanced disease.
Paradoxical Finding That ΔVtamil and ΔIscamil but Not Na+ Conductance Are Increased in CF.
It may seem paradoxical that these CF epithelia do not have increased Na+ conductance but have a greater basal Vt, ΔVtamil, and ΔIscamil than non-CF epithelia. Consider five points. (i) The non-CF epithelia have substantial CFTR Cl− conductance under basal conditions, consistent with earlier studies (26). That Cl− conductance is missing in CF. (ii) Adding a Cl− channel to a Na+-conductive apical membrane introduces another electromotive force generated by the apical Cl− concentration gradient, shunts voltage generated by Na+ channels, and alters the effect on the voltage of current generated at the basolateral membrane (23, 24). The resulting changes in apical (and basolateral) voltage then alter Vt and Isc. Subtracting a Cl− conductance has the opposite effects. (iii) Equivalent electrical circuit models show that loss of apical Cl− conductance can increase ΔVtamil and ΔIscamil even when apical Na+ conductance is held constant (23, 24). Although the responsible processes are complex, one of several factors is that amiloride hyperpolarizes the apical membrane, increasing the driving force for apical Cl− exit and thereby minimizing ΔIscamil compared with CF epithelia, which lack CFTR anion channels. (iv) In porcine nasal epithelia, CF/non-CF differences in basal Vt, Isc, ΔVtamil, and ΔIscamil are caused by the lack of CFTR Cl− conductance (22). These differences are absent or less striking in porcine tracheal/bronchial epithelia where CFTR Cl− conductance is minimal under basal conditions. (v) The paradoxical influence on electrophysiological properties of the absence of Cl− conductance is readily apparent in the sweat gland duct. In CF ducts, Na+ absorption is decreased by ∼80%, but absolute Vt can be increased 10-fold (29). Decreased Cl− conductance, not increased Na+ conductance, causes the increased Vt.
To test further the conclusion that CF/non-CF differences in basal Vt, ΔVtamil, and ΔIscamil were caused by lack of CFTR Cl− conductance rather than by increased Na+ conductance, we did several additional studies.
Reducing Cl− Conductance Increases ΔIscamil and ΔVtamil in Non-CF Epithelia.
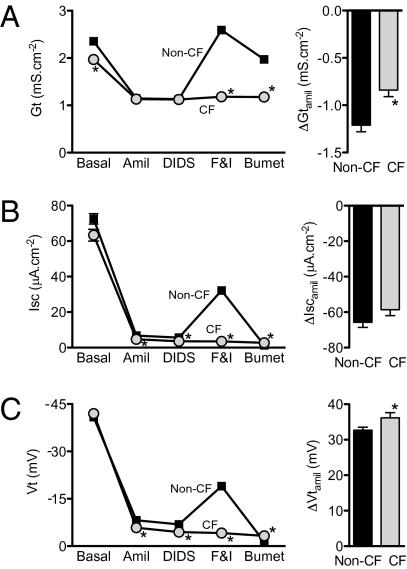
We predicted that reducing the CFTR Cl− conductance of non-CF epithelia would minimize CF/non-CF differences in Vt, ΔVtamil, and ΔIscamil. To test this prediction, we added forskolin and IBMX to culture medium for 16–24 h and then withdrew it when epithelia were mounted in Ussing chambers. Withdrawal of chronic cAMP stimulation minimizes basal CFTR activity, although it also increases Na+ conductance in both non-CF and CF epithelia (30), perhaps by increasing ENaC expression (31). After amiloride addition, Gt did not differ between CF and non-CF epithelia, consistent with minimal CFTR conductance in non-CF epithelia (Fig. 4A). Importantly, with the reduced Cl− conductance, values of basal Vt, ΔVtamil, and ΔIscamil did not exceed or were only slightly greater than those in non-CF epithelia (compare Fig. 4 B and C with Fig. 2).
Fig. 4.
Transepithelial electrophysiological properties in non-CF and CF epithelia following withdrawal of chronic cAMP stimulation. Left panels indicate Gt (A), Isc (B), and Vt (C) under basal conditions and after additions indicated in legend of Fig. 1. Right panels show changes with amiloride addition. In some cases error bars are hidden by symbols. n = epithelia from 261 non-CF donors and 105 CF donors; data for each donor were the average of measurements from 1–12 epithelia (three epithelia were measured for most donors). *P < 0.05, unpaired t test.
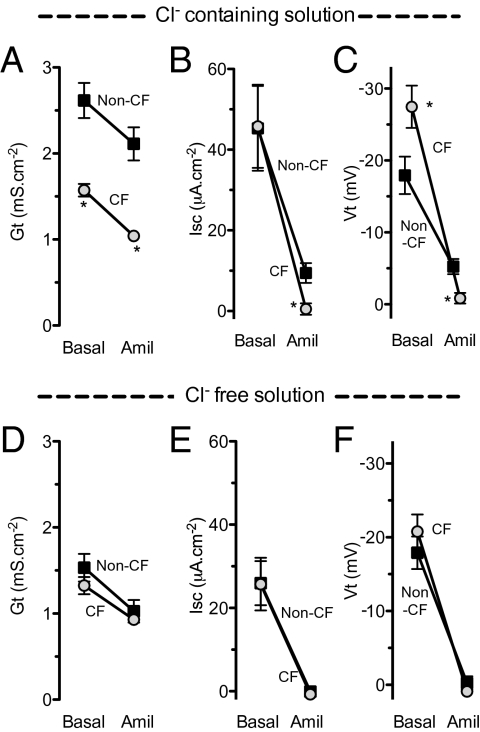
We predicted a similar effect if we removed Cl− from the bathing solutions. Fig. 5 shows that in Cl−-free solutions, basal Vt, ΔVtamil, and ΔIscamil did not differ between CF and non-CF epithelia. Of note, Cl−-free solutions reduced basal Isc in both non-CF and CF epithelia; we do not know the mechanism.
Fig. 5.
Effect of Cl−-free solution on the electrophysiological response to amiloride. Gt, Isc, and Vt under basal conditions and after amiloride addition in Cl−-containing (A–C) and Cl−-free (D–F) solutions. n = 12 non-CF and 13 CF epithelia for Cl−-containing solutions and 15 non-CF and 14 CF epithelia for Cl−-free solutions. *Difference in CF vs. non-CF epithelia, P < 0.05, unpaired t test.
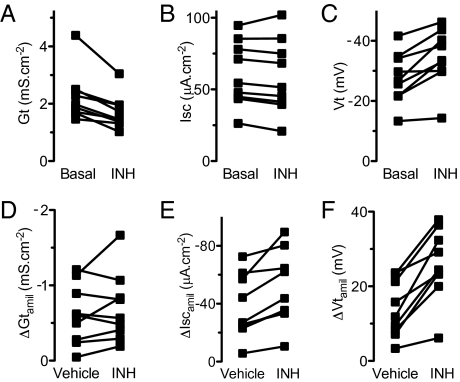
We predicted that inhibiting CFTR would increase Vt, ΔVtamil, and ΔIscamil. We applied CFTRinh-172, which inhibits CFTR (32) but also may have a small inhibitory effect on Na+ transport (33), and found that it reduced Gt as expected (Fig. 6A). This result, as well as the finding that removing Cl− reduced Gt more in non-CF than in CF epithelia (Fig. 5 A and D) indicates that, under basal conditions, CFTR channels show some constitutive activity. CFTRinh-172 also acutely increased basal Vt (Fig. 6C). Isc under basal conditions was not altered; that result was not surprising, because Isc was similar in non-CF and CF epithelia (Fig. 6B). CFTRinh-172 also increased ΔIscamil and ΔVtamil (Fig. 6 E and F). These results indicate that acutely inhibiting CFTR increases Vt, ΔVtamil, and ΔIscamil so that they are more similar to the values in CF epithelia.
Fig. 6.
Effect of CFTRinh-172 on electrophysiological properties of non-CF airway epithelia. (A–C) Gt, Isc, and Vt before and after addition of 100 μM CFTRinh-172 (INH). Each symbol indicates a different donor; n = 9. CFTRinh-172 decreased Gt and increased Vt but did not alter Isc (P = 0.0016, 0.0024, and 0.17, respectively, paired t test). Vehicle treatment (not shown) did not alter Gt, Vt, or Isc (all P > 0.27, paired t test). (D–F) ΔGtamil, ΔIscamil, and ΔVtamil in epithelia treated with vehicle or CFTRinh-172. Each set of data points indicates a pair of epithelia from an individual donor with one receiving CFTRinh-172 and the other vehicle. ΔIscamil and ΔVtamil but not ΔGtamil were greater in epithelia treated with CFTRinh-172 (P = 0.003, 0.0002, and 0.27, respectively, paired t test).
Each of these three maneuvers has limitations. Withdrawal of chronic cAMP stimulation also increases Na+ channel activity and probably affects other processes, removing Cl−-reduced basal Isc and possibly causing changes in cell volume, and CFTRinh-172 can have other effects in addition to inhibiting CFTR. It is possible that each of these interventions could affect non-CF and CF epithelia differently. However, consistent with our measurements of ΔGtamil, all the data indicate that non-CF/CF differences in values of basal Vt, ΔVtamil, and ΔIscamil are not the consequence of increased apical Na+ conductance but instead result from the presence/absence of an apical Cl− conductance.
Estimates of Gp in Non-CF and CF Epithelia.
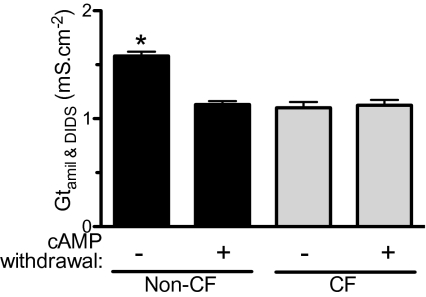
Because Gp influences Gt and thereby Vt, we asked if it was reduced in CF epithelia. We estimated Gp by measuring Gt after Gc was minimized as follows: CFTR anion conductance was decreased by withdrawal of chronic cAMP stimulation; ENaC was inhibited with amiloride; and other apical Cl− channels were inhibited with DIDS. Under these conditions, Gt was the same in non-CF and CF epithelia (Fig. 7). Those Gt values also were similar to those in epithelia genetically lacking CFTR function (i.e., CF) and treated with amiloride and DIDS but not by withdrawal of chronic cAMP stimulation. These data suggest that Gp did not differ between CF and non-CF epithelia. This conclusion also is consistent with passive (basolateral-to-apical) 22Na+ fluxes, which occur primarily through the paracellular pathway; these fluxes did not differ between non-CF and CF epithelia (Fig. 3). Passive Na+ fluxes also were similar in wild-type and CFTR−/− porcine epithelia (22).
Fig. 7.
Estimated paracellular conductance. Data are Gt in the presence of amiloride and DIDS for non-CF and CF epithelia. n = 261 non-CF and 74 CF epithelia under control conditions and 261 non-CF and 105 CF epithelia treated with withdrawal of chronic cAMP stimulation. Value for untreated non-CF epithelia differed from other conditions (P < 0.0001 one-way ANOVA and Bonferroni post test).
Discussion
CF Airway Epithelia Have Reduced Anion Conductance but Not Increased Na+ Conductance.
Our data allow four main conclusions about electrolyte transport in CF vs. non-CF airway epithelia.
First, CF airway epithelia have a markedly reduced transepithelial Cl− conductance under basal conditions. Moreover, there was no increase in Cl− conductance after cellular levels of cAMP were increased. These data are consistent with previous studies done in vivo, in excised tissue, and in cultured epithelia from humans, pigs, ferrets, and mice (1, 7, 20, 22, 34, 35). These results were expected, because CFTR is a phosphorylation-regulated anion channel (36).
Second, non-CF epithelia had some constitutive CFTR Cl− conductance under basal conditions. This result is consistent with previous observations (26).
Third, our measurements of Gt, apical Na+ current, and 22Na+ fluxes indicate that Na+ conductance in CF epithelia did not exceed that in non-CF epithelia.
Fourth, we found that basal Vt, ΔVtamil, and ΔIscamil were greater in CF than in non-CF epithelia. Our data indicate that loss of a Cl− conductance was responsible for these electrophysiological changes in CF epithelia. This conclusion was supported by studies that reduced the Cl− conductance of non-CF epithelia.
Comparison of Our Results with Previous Studies.
Our conclusion that CF epithelia do not have increased Na+ conductance contrasts with some previous interpretations that have been based, in part, on in vivo measurements of nasal Vt and measurements of Vt and Isc (or equivalent current) in cultured epithelia (7, 15). As our results here and in porcine epithelia indicate, using those values to make conclusions about Na+ conductance and Na+ absorption might lead to the wrong conclusion. However, other studies also have been done. With 22Na+ flux measurements made under short-circuit conditions, one study reported greater net Na+ absorption in CF epithelia, and another reported no difference between non-CF and CF epithelia (9, 11). However, 22Na+ flux rates performed under open-circuit conditions in human (37, 38) and porcine (22) epithelia showed no difference between non-CF and CF epithelia; those results agree with our data. Moreover, open-circuit conditions better reflect in vivo conditions than short-circuit conditions.
Measurements of the rate of liquid absorption, which depends in part on Na+ conductance, have shown increased (39, 40), similar (41), and reduced (30) rates in CF vs. non-CF epithelia. Although we did not measure liquid absorption, our data predict that CF absorption would not exceed non-CF absorption rates. The explanation for differences between studies might relate to culture conditions, the state of epithelial differentiation, the time of study after seeding, and the activity of CFTR (30). It also is noteworthy that in some reports (40) Gt did not differ between the two groups of epithelia, whereas we found that basal Gt was reduced in CF.
After liquid was added to the surface of airway epithelia, periciliary liquid depth has been reported to be decreased in cultured CF epithelia under equilibrium conditions (39, 41); however, such reductions do not necessarily indicate Na+ hyperabsorption and also might result from lack of Cl− secretion or alterations in other processes. A comparison of CF and non-CF cultured airway epithelia under equilibrium conditions found no difference in periciliary liquid depth (42). Moreover, periciliary liquid depth was not reduced in the trachea of newborn CFTR−/− pigs (22). In humans with CF, there was a tendency for a reduced airway surface liquid height, although disease was advanced at the time of the study, and the comparison with non-CF was not statistically significant (43). Finally, the inherent variability among epithelia from different donors can limit conclusions based on epithelia from small numbers of donors.
This Study Has Advantages and Limitations.
This study has some advantages. (i) We studied differentiated primary cultures of airway epithelia, avoiding potential limitations of studying cell lines in which genetic drift is possible. This approach also eliminates the possibility that recombinant CFTR might be expressed in cell types and at levels different from the endogenous protein. (ii) Study of cultured epithelia minimizes acute effects of inflammatory mediators. (iii) We report values for all the non-CF and CF epithelial cultures from our laboratories; we applied no specific criteria in selecting epithelia for study, a process that could introduce unintended biases.
Our study also has limitations. (i) We used cultured and excised epithelia rather than studying epithelia in vivo. However, methodology to measure Isc and Gt in vivo are not available, and in vivo CF epithelia are subject to inflammation, infection, and remodeling. (ii) Epithelia were produced with cells removed from lungs that often were diseased; it is unknown whether disease status might have consequences for electrolyte transport after the cells are cultured. (iii) ENaC channel activity is regulated by proteolysis of its extracellular domain and insertion into the membrane (44, 45). Our study might not detect effects of the in vivo CF environment that modify ENaC or its regulators (46). (iv) We did not study HCO3− transport, which may play an important role in CF airway pathogenesis. (v) We did not study several other channels, pumps, and transporters that likely play important roles in airway epithelial function and CF pathophysiology. (vi) Culture methods can influence electrophysiological properties (12, 47). However, methods that generate differentiated airway epithelia, as ours do (25), do not markedly alter responses to amiloride and cAMP-elevating agents (12). Moreover, our cultured and excised non-CF epithelia had relatively similar basal Isc and responses to pharmacological interventions. In addition, we recently found similar properties for porcine airway epithelia studied in vivo, as freshly excised tissue, and as differentiated cultures (20, 22). These comparisons provide some assurance that our culture procedures can provide an informative model for investigating ion transport. Nevertheless, we recognize that there may be differences between CF and non-CF human epithelia that are not reflected in primary cultures. (vii) Airway region can influence electrophysiological properties, and our study was limited to epithelia cultured from large bronchi and trachea. As an example, basal Isc and ΔIscamil were greater in CF than in non-CF porcine nasal epithelia but did not differ between CF and non-CF tracheal/bronchial epithelia (22). Those differences were caused by greater CFTR-mediated Cl− conductance under basal conditions in nasal vs. tracheal/bronchial epithelia and by quantitative differences in other transport processes, including basolateral K+ channels.
Implications for CF.
Several hypotheses about the pathogenesis of CF airway disease postulate that abnormal electrolyte transport across airway epithelia and submucosal glands makes an important contribution to disease. Our data indicate that loss of anion conductance is a key abnormality in CF airway epithelia. The increased CF epithelial Vt and Isc responses to amiloride that sometimes are interpreted to mean that CF epithelia hyperabsorb Na+ could be attributed to loss of Cl− conductance rather than to increased activity of Na+ channels. Thus, our data correspond to studies of newborn CF pigs done at a time when they manifest a defect in host defense against bacteria but not secondary manifestations of the disease (21, 22). Our results also are consistent with studies of CF sweat gland ducts and airway submucosal glands, which show reduced anion transport but not Na+ hyperabsorption (2, 48, 49). Although our studies do not address the possibility that increased Na+ conductance might be important at some stage of the disease or that inhibiting ENaC might benefit patients with CF, these results focus attention on defective anion transport in CF lung disease.
Materials and Methods
Procedures for developing primary cultures of human airway epithelia and obtaining excised epithelia are similar to those previously reported (25). Electrophysiological and Na+ flux measurements are similar to those previously described (22). Please see SI Materials and Methods for a detailed description of the methods.
Supplementary Material
Acknowledgments
We thank Tami Nesselhauf, Janice Launspach, and Theresa Mayhew for excellent assistance. We appreciate the help and assistance of the Iowa Donor Network and are deeply grateful to the persons who donated their lungs for these studies. We appreciate the valuable assistance of the University of Iowa In Vitro Models and Cell Culture Core supported in part by Grants R458-CR07 from the Cystic Fibrosis Foundation, HL51670 and HL61234 from the National Heart, Lung, and Blood Institute, and DK54759 from the National Institute of Diabetes and Digestive and Kidney Diseases. This work was supported by Grant HL51670 from the National Heart, Lung, and Blood Institute. O.A.I. was a recipient of an Iowa Cardiovascular Interdisciplinary Research Fellowship (HL007121). P.H.K. is a Research Specialist, and M.J.W. is an Investigator of The Howard Hughes Medical Institute.
Footnotes
Conflict of interest statement: M.J.W. is a co-founder of Exemplar Genetics, which is licensing materials and technology related to this work.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106695108/-/DCSupplemental.
References
- 1.Welsh MJ, Ramsey BW, Accurso F, Cutting GR. Cystic fibrosis. In: Scriver CR, et al., editors. The Metabolic and Molecular Basis of Inherited Disease. 8th Ed. New York: McGraw-Hill; 2001. [Google Scholar]
- 2.Quinton PM. Physiological basis of cystic fibrosis: A historical perspective. Physiol Rev. 1999;79(1, Suppl):S3–S22. doi: 10.1152/physrev.1999.79.1.S3. [DOI] [PubMed] [Google Scholar]
- 3.Wine JJ. The genesis of cystic fibrosis lung disease. J Clin Invest. 1999;103:309–312. doi: 10.1172/JCI6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verkman AS, Song Y, Thiagarajah JR. Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am J Physiol Cell Physiol. 2003;284:C2–C15. doi: 10.1152/ajpcell.00417.2002. [DOI] [PubMed] [Google Scholar]
- 5.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 6.Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173:475–482. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 7.Boucher RC. Airway surface dehydration in cystic fibrosis: Pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 8.Knowles M, Gatzy JT, Boucher RCJ. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N Engl J Med. 1981;305:1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- 9.Knowles MR, et al. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983;221:1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- 10.Widdicombe JH, Welsh MJ, Finkbeiner WE. Cystic fibrosis decreases the apical membrane chloride permeability of monolayers cultured from cells of tracheal epithelium. Proc Natl Acad Sci USA. 1985;82:6167–6171. doi: 10.1073/pnas.82.18.6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boucher RCJ, Stutts MJ, Knowles MR, Cantley LC, Gatzy JT. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest. 1986;78:1245–1252. doi: 10.1172/JCI112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sachs LA, Finkbeiner WE, Widdicombe JH. Effects of media on differentiation of cultured human tracheal epithelium. In Vitro Cell Dev Biol Anim. 2003;39:56–62. doi: 10.1290/1543-706X(2003)039<0056:EOMODO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Smith JJ, Welsh MJ. cAMP stimulates bicarbonate secretion across normal, but not cystic fibrosis airway epithelia. J Clin Invest. 1992;89:1148–1153. doi: 10.1172/JCI115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Standaert TA, et al. Standardized procedure for measurement of nasal potential difference: An outcome measure in multicenter cystic fibrosis clinical trials. Pediatr Pulmonol. 2004;37:385–392. doi: 10.1002/ppul.10448. [DOI] [PubMed] [Google Scholar]
- 15.Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631–1636. doi: 10.1378/chest.07-0288. [DOI] [PubMed] [Google Scholar]
- 16.Stutts MJ, et al. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 17.Nagel G, et al. CFTR fails to inhibit the epithelial sodium channel ENaC expressed in Xenopus laevis oocytes. J Physiol. 2005;564:671–682. doi: 10.1113/jphysiol.2004.079046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers CS, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyerholz DK, Stoltz DA, Pezzulo AA, Welsh MJ. Pathology of gastrointestinal organs in a porcine model of cystic fibrosis. Am J Pathol. 2010;176:1377–1389. doi: 10.2353/ajpath.2010.090849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ostedgaard LS, et al. The ΔF508 mutation causes CFTR misprocessing and cystic fibrosis-like disease in pigs. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001868. 74ra24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoltz DA, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2(29) doi: 10.1126/scitranslmed.3000928. 29ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J-H, et al. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell. 2010;143:911–923. doi: 10.1016/j.cell.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horisberger JD. ENaC-CFTR interactions: The role of electrical coupling of ion fluxes explored in an epithelial cell model. Pflugers Arch. 2003;445:522–528. doi: 10.1007/s00424-002-0956-0. [DOI] [PubMed] [Google Scholar]
- 24.Duszyk M, French AS. An analytical model of ionic movements in airway epithelial cells. J Theor Biol. 1991;151:231–247. doi: 10.1016/s0022-5193(05)80362-5. [DOI] [PubMed] [Google Scholar]
- 25.Karp PH, et al. An in vitro model of differentiated human airway epithelia: Methods and evaluation of primary cultures. In: Wise C, editor. Epithelial Cell Culture Protocols. Vol 188. Totowa, NJ: Humana; 2002. pp. 115–137. [DOI] [PubMed] [Google Scholar]
- 26.Uyekubo SN, et al. cAMP-dependent absorption of chloride across airway epithelium. Am J Physiol. 1998;275:L1219–L1227. doi: 10.1152/ajplung.1998.275.6.L1219. [DOI] [PubMed] [Google Scholar]
- 27.Welsh MJ. Inhibition of chloride secretion by furosemide in canine tracheal epithelium. J Membr Biol. 1983;71:219–226. doi: 10.1007/BF01875463. [DOI] [PubMed] [Google Scholar]
- 28.Helman SI, Miller DA. Edge damage effect on electrical measurements of frog skin. Am J Physiol. 1973;225:972–977. doi: 10.1152/ajplegacy.1973.225.4.972. [DOI] [PubMed] [Google Scholar]
- 29.Quinton PM. Role of epithelial HCO3⁻ transport in mucin secretion: Lessons from cystic fibrosis. Am J Physiol Cell Physiol. 2010;299:C1222–C1233. doi: 10.1152/ajpcell.00362.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zabner J, Smith JJ, Karp PH, Widdicombe JH, Welsh MJ. Loss of CFTR chloride channels alters salt absorption by cystic fibrosis airway epithelia in vitro. Mol Cell. 1998;2:397–403. doi: 10.1016/s1097-2765(00)80284-1. [DOI] [PubMed] [Google Scholar]
- 31.Thomas CP, Campbell JR, Wright PJ, Husted RF. cAMP-stimulated Na+ transport in H441 distal lung epithelial cells: Role of PKA, phosphatidylinositol 3-kinase, and sgk1. Am J Physiol Lung Cell Mol Physiol. 2004;287:L843–L851. doi: 10.1152/ajplung.00340.2003. [DOI] [PubMed] [Google Scholar]
- 32.Ma T, et al. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang XF, Reddy MM, Quinton PM. Effects of a new cystic fibrosis transmembrane conductance regulator inhibitor on Cl− conductance in human sweat ducts. Exp Physiol. 2004;89:417–425. doi: 10.1113/expphysiol.2003.027003. [DOI] [PubMed] [Google Scholar]
- 34.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79(1, Suppl):S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- 35.Sun X, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120:3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79(1, Suppl):S23–S45. doi: 10.1152/physrev.1999.79.1.S23. [DOI] [PubMed] [Google Scholar]
- 37.Willumsen NJ, Boucher RCJ. Sodium transport and intracellular sodium activity in cultured human nasal epithelium. Am J Physiol. 1991;261:C319–C331. doi: 10.1152/ajpcell.1991.261.2.C319. [DOI] [PubMed] [Google Scholar]
- 38.Willumsen NJ, Boucher RCJ. Transcellular sodium transport in cultured cystic fibrosis human nasal epithelium. Am J Physiol. 1991;261:C332–C341. doi: 10.1152/ajpcell.1991.261.2.C332. [DOI] [PubMed] [Google Scholar]
- 39.Matsui H, et al. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 40.Jiang C, Finkbeiner WE, Widdicombe JH, McCray PB, Jr, Miller SS. Altered fluid transport across airway epithelium in cystic fibrosis. Science. 1993;262:424–427. doi: 10.1126/science.8211164. [DOI] [PubMed] [Google Scholar]
- 41.Van Goor F, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Derichs N, Jin BJ, Song Y, Finkbeiner WE, Verkman AS. Hyperviscous airway periciliary and mucous liquid layers in cystic fibrosis measured by confocal fluorescence photobleaching. FASEB J. 2011 doi: 10.1096/fj.10-179549. 10.1096/fj.10-179549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Griesenbach U, et al. Quantification of periciliary fluid (PCL) height in human airway biopsies is feasible, but not suitable as a biomarker. Am J Respir Cell Mol Biol. 2011;44:309–315. doi: 10.1165/rcmb.2009-0265OC. [DOI] [PubMed] [Google Scholar]
- 44.Hughey RP, et al. Epithelial sodium channels are activated by furin-dependent proteolysis. J Biol Chem. 2004;279:18111–18114. doi: 10.1074/jbc.C400080200. [DOI] [PubMed] [Google Scholar]
- 45.Snyder PM. Minireview: Regulation of epithelial Na+ channel trafficking. Endocrinology. 2005;146:5079–5085. doi: 10.1210/en.2005-0894. [DOI] [PubMed] [Google Scholar]
- 46.Myerburg MM, Harvey PR, Heidrich EM, Pilewski JM, Butterworth MB. Acute regulation of the epithelial sodium channel in airway epithelia by proteases and trafficking. Am J Respir Cell Mol Biol. 2010;43:712–719. doi: 10.1165/rcmb.2009-0348OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Widdicombe JH, Sachs LA, Finkbeiner WE. Effects of growth surface on differentiation of cultures of human tracheal epithelium. In Vitro Cell Dev Biol Anim. 2003;39:51–55. doi: 10.1290/1543-706X(2003)039<0051:EOGSOD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 48.Quinton PM. Cystic fibrosis: Lessons from the sweat gland. Physiology (Bethesda) 2007;22:212–225. doi: 10.1152/physiol.00041.2006. [DOI] [PubMed] [Google Scholar]
- 49.Joo NS, Irokawa T, Robbins RC, Wine JJ. Hyposecretion, not hyperabsorption, is the basic defect of cystic fibrosis airway glands. J Biol Chem. 2006;281:7392–7398. doi: 10.1074/jbc.M512766200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.