Abstract
Era, composed of a GTPase domain and a K homology domain, is essential for bacterial cell viability. It is required for the maturation of 16S rRNA and assembly of the 30S ribosomal subunit. We showed previously that the protein recognizes nine nucleotides (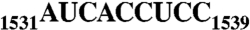 ) near the 3′ end of 16S rRNA, and that this recognition stimulates GTP-hydrolyzing activity of Era. In all three kingdoms of life, the
) near the 3′ end of 16S rRNA, and that this recognition stimulates GTP-hydrolyzing activity of Era. In all three kingdoms of life, the  sequence and helix 45 (h45) (nucleotides 1506–1529) are highly conserved. It has been shown that the
sequence and helix 45 (h45) (nucleotides 1506–1529) are highly conserved. It has been shown that the 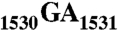 to
to  double mutation severely affects the viability of bacteria. However, whether Era interacts with G1530 and/or h45 and whether such interactions (if any) contribute to the stimulation of Era’s GTPase activity were not known. Here, we report two RNA structures that contain nucleotides 1506–1542 (RNA301), one in complex with Era and GDPNP (GNP), a nonhydrolysable GTP-analogue, and the other in complex with Era, GNP, and the KsgA methyltransferase. The structures show that Era recognizes 10 nucleotides, including G1530, and that Era also binds h45. Moreover, GTPase assay experiments show that G1530 does not stimulate Era’s GTPase activity. Rather, A1531 and A1534 are most important for stimulation and h45 further contributes to the stimulation. Although G1530 does not contribute to the intrinsic GTPase activity of Era, its interaction with Era is important for binding and is essential for the protein to function, leading to the discovery of a new cold-sensitive phenotype of Era.
double mutation severely affects the viability of bacteria. However, whether Era interacts with G1530 and/or h45 and whether such interactions (if any) contribute to the stimulation of Era’s GTPase activity were not known. Here, we report two RNA structures that contain nucleotides 1506–1542 (RNA301), one in complex with Era and GDPNP (GNP), a nonhydrolysable GTP-analogue, and the other in complex with Era, GNP, and the KsgA methyltransferase. The structures show that Era recognizes 10 nucleotides, including G1530, and that Era also binds h45. Moreover, GTPase assay experiments show that G1530 does not stimulate Era’s GTPase activity. Rather, A1531 and A1534 are most important for stimulation and h45 further contributes to the stimulation. Although G1530 does not contribute to the intrinsic GTPase activity of Era, its interaction with Era is important for binding and is essential for the protein to function, leading to the discovery of a new cold-sensitive phenotype of Era.
Keywords: ribosome biogenesis, cell growth regulation
Ribosome is the protein manufacturing machinery in all living cells. Ribosomal RNA is the RNA component of the ribosome. The function of rRNA is to provide the mechanism for decoding messenger RNA into amino acids by interacting with transfer RNA during translation and providing the peptidyl transferase activity. One direct involvement of rRNA in protein translation is the intermolecular interaction between the complementary Shine–Dalgarno (SD) sequence near the 5′ end of mRNA and the antiSD sequence (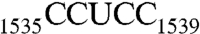 , Escherichia coli numbering, Fig. 1A) near the 3′ end of 16S rRNA (1, 2).
, Escherichia coli numbering, Fig. 1A) near the 3′ end of 16S rRNA (1, 2).
Fig. 1.
The 3′ end of 16S rRNA (nucleotides 1506–1542). (A) In the tetraloop of h45 (nucleotides 1506–1529), A1518, and A1519 (orange) are methylation targets of KsgA. Downstream from h45 are nucleotides 1530–1542, containing the conserved sequence GAUCA (red) and the antiSD sequence CCUCC (green). The SD helix is formed by CCUCC near the 3′ end of 16S rRNA and the SD sequence GGAGG (brown) near the 5′ end of mRNA during translational initiation. (B) A dimer of RNA301, which contains a U1506C mutation, is observed in the crystal structure as an RNA duplex with four A-G pairs in the middle and a 13-nt overhang on each end of the duplex.
The antiSD sequence is highly conserved in Archaea and bacteria (3); the adjacent 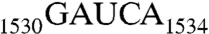 sequence is highly conserved in all three kingdoms of life (4, 5). Further upstream is located helix 45 (h45), a stem loop structure encompassing nucleotides 1506–1529 (Fig. 1A). It is one of the most conserved helices in 16S rRNA (6) and plays important roles in ribosome assembly and protein synthesis initiation (7–9). This helix has been crosslinked to initiation factor 3 (IF3) (10) and also to 23S rRNA (11). Located downstream from h45 and stacked with each other, G1530 and A1531 exhibit almost identical conformations in several crystal structures of the ribosome (12). G1530 has been crosslinked to the U of the mRNA start codon AUG (13). It has been shown that the 30S ribosomal subunit bearing the
sequence is highly conserved in all three kingdoms of life (4, 5). Further upstream is located helix 45 (h45), a stem loop structure encompassing nucleotides 1506–1529 (Fig. 1A). It is one of the most conserved helices in 16S rRNA (6) and plays important roles in ribosome assembly and protein synthesis initiation (7–9). This helix has been crosslinked to initiation factor 3 (IF3) (10) and also to 23S rRNA (11). Located downstream from h45 and stacked with each other, G1530 and A1531 exhibit almost identical conformations in several crystal structures of the ribosome (12). G1530 has been crosslinked to the U of the mRNA start codon AUG (13). It has been shown that the 30S ribosomal subunit bearing the 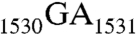 to
to 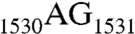 double mutation is unable to associate with the 50S subunit to form the 70S complex in vitro (14). Recently, these two nucleotides have been suggested to function as a hinge point for the movement of the SD helix during translation initiation (12).
double mutation is unable to associate with the 50S subunit to form the 70S complex in vitro (14). Recently, these two nucleotides have been suggested to function as a hinge point for the movement of the SD helix during translation initiation (12).
Era is a highly conserved Ras-like small GTPase. It is essential in E. coli, playing important roles in cell cycle regulation (15) and ribosome assembly (16, 17). When Era is included in an in vitro 30S reconstitution experiment, several late-binding ribosomal proteins bind rRNA more rapidly, and Era remains bound to 16S rRNA (18, 19) and pre30S particles throughout several RNA folding and protein binding events (20). KsgA is a universally conserved RNA methyltransferase. In E. coli, it methylates two adjacent adenines (Ade) in the tetraloop of h45 (21, 22) (Fig. 1A). The overexpression of KsgA has been found to suppress the impaired function of a cold-sensitive Era phenotype (23), suggesting that the two proteins may be functionally related. Previously, we determined the crystal structure of Era in complex with the  sequence (designated as RNA100 in this study, Table 1) and a nonhydrolyzable GTP analogue, GDPNP (GNP), showing that Era recognizes nucleotides 1531–1539 (17). We have also shown that RNA recognition stimulates the intrinsic GTP-hydrolyzing activity of Era and that, for this stimulation to occur, the recognition of AUCA is essential but not sufficient whereas the recognition of CCUCC is important but not essential. To ascertain whether or not G1530 is also recognized by Era, and if it is, whether its recognition contributes to the stimulation of Era’s GTPase activity and/or binding interaction, we have determined two more cocrystal structures: one of Era in complex with GNP and a longer RNA that contains h45 and the
sequence (designated as RNA100 in this study, Table 1) and a nonhydrolyzable GTP analogue, GDPNP (GNP), showing that Era recognizes nucleotides 1531–1539 (17). We have also shown that RNA recognition stimulates the intrinsic GTP-hydrolyzing activity of Era and that, for this stimulation to occur, the recognition of AUCA is essential but not sufficient whereas the recognition of CCUCC is important but not essential. To ascertain whether or not G1530 is also recognized by Era, and if it is, whether its recognition contributes to the stimulation of Era’s GTPase activity and/or binding interaction, we have determined two more cocrystal structures: one of Era in complex with GNP and a longer RNA that contains h45 and the 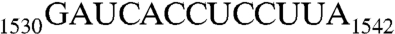 sequence (RNA301, Table 1), and the other in complex with GNP, RNA301, and KsgA. These structures reveal that Era does indeed recognize G1530 and also contacts h45. Furthermore, we have tested the role of each nucleotide in the
sequence (RNA301, Table 1), and the other in complex with GNP, RNA301, and KsgA. These structures reveal that Era does indeed recognize G1530 and also contacts h45. Furthermore, we have tested the role of each nucleotide in the 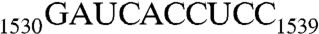 sequence for the stimulation of Era’s GTP-hydrolyzing activity.
sequence for the stimulation of Era’s GTP-hydrolyzing activity.
Table 1.
GTPase activity of Era in the absence or presence of RNA
| RNA | Sequence,* from 5′ to 3′ | GTPase activity,‡ mmol min-1 mol-1 |
| — | NA† | 20 ± 1 – 33 ± 1§ |
| RNA100 | AUCA CCUCC UUA | 110 ± 14 – 132 ± 14§ |
| RNA101 | UAGU CCUCC UUA | 17 ± 3§ |
| RNA102 | AUCA GGAGG UUA | 67 ± 3§ |
| RNA103 | UUCA CCUCC UUA | 113 ± 5 |
| RNA104 | AACA CCUCC UUA | 139 ± 4 |
| RNA105 | AUGA CCUCC UUA | 143 ± 6 |
| RNA106 | AUCU CCUCC UUA | 45 ± 1 |
| RNA107 | AUCA GCUCC UUA | 133 ± 3 |
| RNA108 | AUCA CGUCC UUA | 135 ± 8 |
| RNA109 | AUCA CCACC UUA | 150 ± 2 |
| RNA110 | AUCA CCUGC UUA | 132 ± 3 |
| RNA111 | AUCA CCUCG UUA | 130 ± 10 |
| RNA112 | GUCA CCUCC UUA | 79 ± 2 |
| RNA113 | UCA CCUCC UUA | 28 ± 2 |
| RNA200 | GAUCA CCUCC UUA | 136 ± 2 |
| RNA201 | AAUCA CCUCC UUA | 134 ± 2 |
| RNA202 | GGUCA CCUCC UUA | 86 ± 3 |
| RNA203 | AGUCA CCUCC UUA | 89 ± 3 |
| RNA300 | UAG CCG UAG GGG AAC CUG CGG CUG GAUCA CCUCC U | 176 ± 2 |
| RNA301 | CAA CCG UAG GGG AAC CUG CGG UUG GAUCA CCUCC UUA | 197 ± 3 |
| RNA302 | CAA CCG UAG GGG AAC CUG CGG UUG AAUCA CCUCC UUA | 201 ± 2 |
*Mutated nucleotides are shown in boldface and underlined.
†NA, not applicable.
‡Each value of GTPase activity is the average of three duplicates.
§Data taken from ref. 17.
Results
Structures of Era-GNP-RNA301 and Era-GNP-RNA301-KsgA.
The recombinant Aquifex aeolicus Era and KsgA (UniProt release no. O67800 and O67680, respectively) proteins are used. RNA301 is the 3' end of 16S rRNA containing nucleotides 1506–1542 in which U1506 has been replaced by a cytosine (Cyt) as C1506 (Fig. 1B). In the ribosome, h45 adopts a hairpin structure with a GGAA tetraloop (Fig. 1A). Previously, we found that the stem loop opens up and dimerizes in the KsgA-h45 structure (22). In this study, we have obtained the structure of RNA301 in two conformations. In the Era-GNP-RNA301 structure, two RNA301 molecules form a 24-base pair (BP) duplex with four mismatched G-A pairs in the middle and a 13-nt 3′ overhang (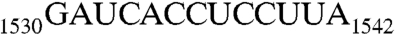 ) at each end of the duplex (Fig. 1B). Each overhang is recognized by an Era molecule and the two Era-GNP-RNA301 complexes are related by crystallographic twofold rotational symmetry (Fig. 2A). In contrast, RNA301 does not dimerize in the Era-GNP-RNA301-KsgA complex, but forms the stem loop structure of h45 in the ribosome with the 13-nt 3′ overhang (Fig. 1A). The 3′ overhang is recognized by Era whereas the hairpin interacts with both Era and KsgA (Fig. S1). Nonetheless, the active site of KsgA located in its N-terminal domain is distant from the methylation targets A1518 and A1519 in the tetraloop of h45. Therefore, the mode of protein–RNA interaction is not consistent with the methyltransferase activity of KsgA. Rather, the interaction mimics previously predicted KsgA-h44 interactions (24). Although the Era-GNP-RNA301-KsgA structure does not provide insight into the mechanism of the protein as an RNA methyltransferase, it is important in validating the Era-h45 interactions revealed by the Era-GNP-RNA301 structure (vide infra).
) at each end of the duplex (Fig. 1B). Each overhang is recognized by an Era molecule and the two Era-GNP-RNA301 complexes are related by crystallographic twofold rotational symmetry (Fig. 2A). In contrast, RNA301 does not dimerize in the Era-GNP-RNA301-KsgA complex, but forms the stem loop structure of h45 in the ribosome with the 13-nt 3′ overhang (Fig. 1A). The 3′ overhang is recognized by Era whereas the hairpin interacts with both Era and KsgA (Fig. S1). Nonetheless, the active site of KsgA located in its N-terminal domain is distant from the methylation targets A1518 and A1519 in the tetraloop of h45. Therefore, the mode of protein–RNA interaction is not consistent with the methyltransferase activity of KsgA. Rather, the interaction mimics previously predicted KsgA-h44 interactions (24). Although the Era-GNP-RNA301-KsgA structure does not provide insight into the mechanism of the protein as an RNA methyltransferase, it is important in validating the Era-h45 interactions revealed by the Era-GNP-RNA301 structure (vide infra).
Fig. 2.
Crystal structure of Era-GNP-RNA301 and of Era-GNP-RNA301-KsgA. (A) The Era-GNP-RNA301 complex forms a dimer in the crystal lattice, in which the two subunits are shown in cyan and orange (darker color, GTPase domain and RNA; lighter color, KH domain), respectively. The protein is represented by spirals (helices), arrows (strands), and tubes (loops), and the RNA by tube-and-sticks. The Mg2+ is indicated with a gray sphere and the GNP is shown as a stick model in atomic color scheme (C, green; N, blue; O, red; and P, orange). The structure in the box represents an Era-GNP-RNA301 complex in which the RNA contains a pseudo-h45. (B) The Era-GNP-RNA structure enclosed in the box in panel A (Era, cyan; RNA, cyan and orange) is superimposed with the corresponding portion of the Era-GNP-RNA301-KsgA structure (magenta, Fig. S1). The protein is illustrated as a Cα trace, the Mg2+ ion as a sphere, and the GNP and RNA molecules as sticks. Side chains of Era which contact h45 are shown as sticks and highlighted in atomic colors (C, cyan or magenta; N, blue).
Era Interacts with h45 and Recognizes G1530.
h45 is formed by base pairing between nucleotides 1506–1515 and nucleotides 1520–1529 of 16S rRNA (Fig. 1A). The dimeric Era-GNP-RNA301 structure can be thought of as a domain-swapped dimer in which nucleotides 1506–1515 base pair with nucleotides 1520–1529 from a different RNA molecule (Fig. 1B). Analyzing the Era-GNP-RNA301 structure in the domain-swapped form is both convenient and informative (Fig. 2A).
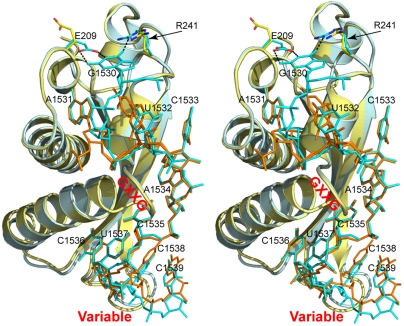
Fig. 2B depicts the superposition of the Era-RNA components in the two crystal structures, Era-GNP-RNA301 (Fig. 2A) and Era-GNP-RNA301-KsgA (Fig. S1). The alignment is based on Cα positions in Era. It shows that the two complexes superimpose well except for the variable loop in the K homology (KH) domain and the peptide linker between the GTPase and KH domains. The protein-RNA interface is conserved in the two structures, showing that Era interacts with h45 and recognizes 10 nucleotides (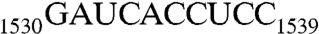 ). The conformations of nucleotides C1538 and C1539 differ slightly in the two structures, consistent with the notion that the variable loop is flexible.
). The conformations of nucleotides C1538 and C1539 differ slightly in the two structures, consistent with the notion that the variable loop is flexible.
A cold-sensitive Era mutant (E200K) can be rescued by the overexpression of KsgA in E. coli (23). We have placed the mutation to generate the E200K mutant on a multicopy plasmid and as described the wild-type strain carrying this mutant is cold sensitive. We examined a deletion of ksgA (ΔksgA) for effects on the E200K mutant. The deletion causes only those cells carrying era(E200K) to be exceptionally cold sensitive with a severe growth defect even at 37 °C. At the same time, we created a second mutant of ksgA, E66A, in which the active center for methylation was inactivated as described (25). This mutation, unlike ΔksgA, shows the same growth properties in combination with era(E200K) as wild-type ksgA. Thus, the presence even of a single copy of wild-type Era or this enzymatically inactive KsgA mutant protein enhances the activity of Era(E200K) relative to that of a strain deleted for the ksgA gene. As aforementioned, the KsgA-RNA interaction in the Era-GNP-RNA301-KsgA structure is not compatible with the methyltransferase activity of KsgA. Furthermore, the two proteins in the structure do not interact with each other (Fig. S1). Therefore, it remains to be determined how the inactive KsgA mutant enhances the activity of Era(E200K).
RNase III and Era are expressed from the same operon in E. coli, and we know from our structures and binding studies that Era attaches to the 16S rRNA region just upstream from the dsRNA-binding site in the 16S precursor that is cleaved by RNase III (17). In bacteria, genes with related or interacting functions are often found transcribed together in operons. In this regard, RNase III and Era bind adjacent to one another in the same 16S precursor segment. We also know that KsgA binds just upstream of Era to the h45 dsRNA; this binding by KsgA stimulates the binding or activity of Era from its h45 site in vivo. It is interesting that the analogous genes for ksgA and era in Caenorhabditis elegans are cotranscribed from a two-gene operon indicating this type of relationship holds even for some eukaryote functions. These observations suggest that Era and KsgA may be located in proximity to each other and that Era may interact with h45. In both structures, we see that Era indeed contacts h45 directly. The side chains of E209 and R241 anchor the helix by recognizing the G1530 base, whereas the side chains of K66, K67, and R289 interact with the phosphate backbone of h45 in an RNA sequence independent manner (Fig. 2B).
The binding of Mg2+ and GNP in the Era-GNP-RNA301 structure is the same as we have previously reported in the structure of Era in complex with GNP and  (RNA100, Table 1) (17). However, the conformation of U1532 is significantly different in the presence and absence of G1530. In the Era-GNP-RNA100 structure [Protein Data Bank (PDB) ID code 3IEV], U1532 exhibits two conformations with equal probabilities, whereas in Era-GNP-RNA301, U1532 adopts only one of those conformations (Fig. 3). The conformations of nucleotides C1538 and C1539 are noticeably different in the two structures, adapting to the conformational changes of the variable loop in the KH domain. RNA301 has offered the opportunity to elucidate the Era-G1530 interactions and the structures with RNA301show that this universally conserved G1530 is specifically recognized by Era. Thus, the KH domain of Era is extensive and recognizes 10 nucleotides, which is in contrast to all other known KH domains that recognize 4–5 nucleotides (26).
(RNA100, Table 1) (17). However, the conformation of U1532 is significantly different in the presence and absence of G1530. In the Era-GNP-RNA100 structure [Protein Data Bank (PDB) ID code 3IEV], U1532 exhibits two conformations with equal probabilities, whereas in Era-GNP-RNA301, U1532 adopts only one of those conformations (Fig. 3). The conformations of nucleotides C1538 and C1539 are noticeably different in the two structures, adapting to the conformational changes of the variable loop in the KH domain. RNA301 has offered the opportunity to elucidate the Era-G1530 interactions and the structures with RNA301show that this universally conserved G1530 is specifically recognized by Era. Thus, the KH domain of Era is extensive and recognizes 10 nucleotides, which is in contrast to all other known KH domains that recognize 4–5 nucleotides (26).
Fig. 3.
Stereoview showing the superposition of two KH-RNA complexes, one from the Era-GNP-RNA301 structure (RNA in cyan, KH in pale cyan, this work) and the other from the Era-GNP-RNA100 structure (RNA in orange, KH in yellow, PDB ID code 3IEV). The protein is illustrated as cartoons (helices as spirals, strands as arrows, and loops as tubes) and the RNA as sticks. The E209 and R241 side chains are also shown as sticks, but in atomic color scheme (C, pale cyan or yellow; N, blue; and O, red). Hydrogen bonds are represented with dashed lines, and the GXXG motif and the variable loop are indicated.
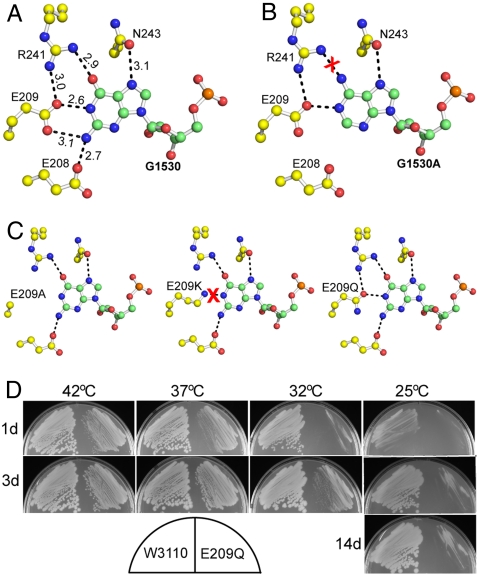
G1530 interacts extensively with Era (Fig. 4A). Five hydrogen bonds of 2.6–3.1 Å in length are formed between G1530 and the side chains of E208, E209, R241, and N243. Among these four amino acid residues, E209 is strictly conserved and R241 is highly conserved in all Era sequences from bacteria to humans. All of these hydrogen bonds are coplanar with the guanine (Gua) base except the one with E208, which is slightly off plane. A pseudobase pair is formed by the G1530 base and the side chains of E209 and R241.
Fig. 4.
The recognition of G1530 by Era is essential for function. (A) Details of Era-G1530 recognition. G1530 and the side chains of E208, E209, R241, and N243 are illustrated as ball-and-stick models in atomic colors (C, green or yellow; O, red; N, blue; and P in orange). Dashed lines in black indicate hydrogen bonds and their distances are in angstroms. (B) Model of the G1530A mutant, suggesting that the mutant is not favorable for the recognition by Era due to the loss of two hydrogen bonds (between E208/E209 and the Gua base, A) and the creation of a repulsive interaction (between R241 and the Ade base, indicated with a red cross). (C) Models of the E209A, E209K, and E209Q mutants. Indicated is the impact of each mutation on the pseudobase pairing interaction between the G1530 base and the side chains of amino acids 209 and 241. (D) Era(E209Q) mutant is cold sensitive. Compared with wild-type Era (W3110, Left), the Era(E209Q) mutant (Right) grows slower at all temperatures tested. Growth impairment is observed at 37 and 42 °C. At 32 °C, small colonies appear after 3 d. At 25 °C, however, colonies do not appear even after 14 d.
Role of Individual Nucleotide in Stimulation of Era’s GTPase Activity.
The intrinsic GTP-hydrolyzing activity of Era is low (27), but is stimulated 3- to 12-fold in the presence of 16S rRNA (18, 19). We previously showed that the recognition of RNA100 stimulates the GTPase activity of Era approximately sixfold (17). Using the transversion mutant of  (
(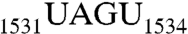 , RNA101) and of
, RNA101) and of 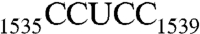 (
(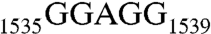 , RNA102, Table 1), we showed that the recognition of AUCA is essential, but the recognition of both AUCA and CCUCC is necessary for optimal stimulation. Having now shown that Era interacts with G1530 and with h45, we next sought to elucidate the contribution of h45 and every nucleotide in the
, RNA102, Table 1), we showed that the recognition of AUCA is essential, but the recognition of both AUCA and CCUCC is necessary for optimal stimulation. Having now shown that Era interacts with G1530 and with h45, we next sought to elucidate the contribution of h45 and every nucleotide in the 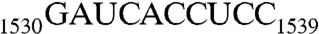 sequence to the stimulation.
sequence to the stimulation.
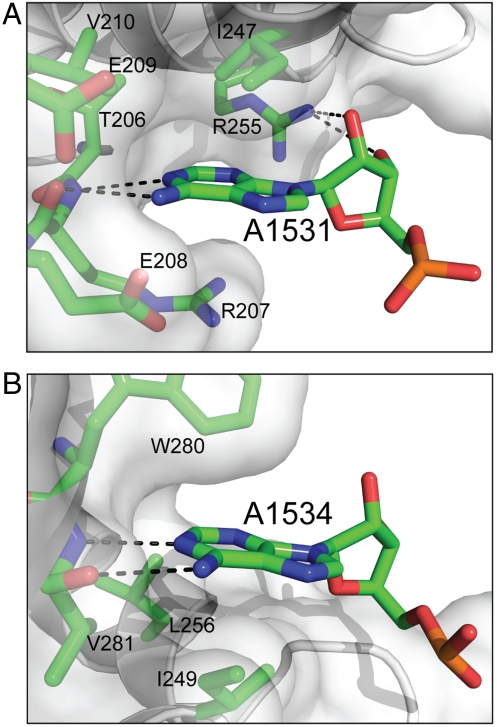
To this end, we have performed a total of 51 experiments (17 GTPase assays in triplicate) in the presence of each of the 17 RNAs (Table 1). As expected on the basis of previous results (17), the five individual mutations in the CCUCC sequence (RNA107-111) have no impact on the stimulation. In contrast, certain mutations in the GAUCA sequence have a large impact. Genetically, A1531 is universally conserved, whereas A1534 is conserved in RNAs that are recognized by KH domains (17). The A1531Δ (RNA113) and A1534U (RNA106) mutants exhibit low activities (28–45 mmol min-1 mol-1), whereas the U1532A (RNA104) and C1533G (RNA105) mutations do not have any impact on the stimulation (139–143 mmol min-1 mol-1). Thus, in the  sequence, the two Ade nucleotides are essential for stimulation. In contrast, each of the other seven nucleotides, individually, does not contribute to the function. Collectively, however, the CCUCC sequence is required for optimal stimulation (compare RNA100 with RNA102, Table 1). Although both A1531 and A1534 are important, the transversion mutation of A1531 (A1531U, RNA103, 113 mmol min-1 mol-1) does not have an impact as dramatic as that of A1534 (A1534U, RNA106, 45 mmol min-1 mol-1). Furthermore, the substitution of A1531 by a Gua (A1531G, RNA112, 79 mmol min-1 mol-1) also does not have a dramatic impact on stimulation. The difference between the two Ade nucleotides in their degree of substitution tolerance can be explained by the structure. As depicted in Fig. 5A, the A1531 base fits snugly in a pocket formed by the side chains of T206, R207, E208, E209, V210, I247, and R255. In addition, the base forms two hydrogen bonds with the backbone groups of R207 and the A1531 ribose forms two hydrogen bonds with the guanidinium group of R255. Thus, any mutant of A1531 would still have its base fit in the pocket and its ribose hydrogen bonded to R255. In contrast, although the base of A1534 also forms two hydrogen bonds with the backbone groups of V281, the A1534 ribose does not form hydrogen bonds with the protein (Fig. 5B). Moreover, Era does not provide a binding pocket for the A1534 base. Instead, one side of the A1534 base contacts the side chains of I249 and L256, but the other side is perpendicular to and barely contacts the W280 side chain (Fig. 5B). Hence, almost an entire side of the A1534 base is exposed to solvent. The impact of the A1534U mutation is therefore twofold: First, the uridine (Uri) base cannot form hydrogen bonds with the backbone of V281; second, the Uri base cannot interact with the W280 side chain, leading to the interrupted recognition of nucleotide 1534.
sequence, the two Ade nucleotides are essential for stimulation. In contrast, each of the other seven nucleotides, individually, does not contribute to the function. Collectively, however, the CCUCC sequence is required for optimal stimulation (compare RNA100 with RNA102, Table 1). Although both A1531 and A1534 are important, the transversion mutation of A1531 (A1531U, RNA103, 113 mmol min-1 mol-1) does not have an impact as dramatic as that of A1534 (A1534U, RNA106, 45 mmol min-1 mol-1). Furthermore, the substitution of A1531 by a Gua (A1531G, RNA112, 79 mmol min-1 mol-1) also does not have a dramatic impact on stimulation. The difference between the two Ade nucleotides in their degree of substitution tolerance can be explained by the structure. As depicted in Fig. 5A, the A1531 base fits snugly in a pocket formed by the side chains of T206, R207, E208, E209, V210, I247, and R255. In addition, the base forms two hydrogen bonds with the backbone groups of R207 and the A1531 ribose forms two hydrogen bonds with the guanidinium group of R255. Thus, any mutant of A1531 would still have its base fit in the pocket and its ribose hydrogen bonded to R255. In contrast, although the base of A1534 also forms two hydrogen bonds with the backbone groups of V281, the A1534 ribose does not form hydrogen bonds with the protein (Fig. 5B). Moreover, Era does not provide a binding pocket for the A1534 base. Instead, one side of the A1534 base contacts the side chains of I249 and L256, but the other side is perpendicular to and barely contacts the W280 side chain (Fig. 5B). Hence, almost an entire side of the A1534 base is exposed to solvent. The impact of the A1534U mutation is therefore twofold: First, the uridine (Uri) base cannot form hydrogen bonds with the backbone of V281; second, the Uri base cannot interact with the W280 side chain, leading to the interrupted recognition of nucleotide 1534.
Fig. 5.
The recognition of nucleotides A1531 and A1534 by Era. (A) Interactions between A1531 and Era. (B) Interactions between A1534 and Era. Protein and nucleotide residues are shown as stick models in atomic colors (C, green; N, blue; O, red; and P, orange). Protein backbone is shown as a cartoon (helices as spirals, strands as arrows, and loops as tubes), and protein surface is shown with 50% transparency.
Surprisingly, adding the universally conserved G1530 does not further stimulate the GTPase activity. The specific activities of Era in the presence of RNA100 (110–132 mmol min-1 mol-1) or RNA200 (136 mmol min-1 mol-1) are virtually identical. Furthermore, the G1530A mutation (RNA201, 134 mmol min-1 mol-1) also does not change the specific activity although the mutation would significantly weaken the interaction between the nucleotide and the protein (Fig. 4B). However, once A1531 is mutated to a Gua with either G1530 (RNA202) or A1530 (RNA203), the specific activity drops to 86–89 mmol min-1 mol-1, virtually the same as RNA112 (79 mmol min-1 mol-1).
Taken together, among the three purines in 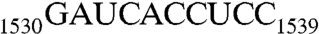 , G1530 is not involved in the stimulation of GTPase activity, whereas A1531 and A1534 are determinants for the stimulation; A1531 tolerates mutational substitutions, but A1534 does not. In addition, the h45 sequence upstream from G1530, further stimulates the GTPase activity of Era. In the presence of RNA300 or RNA301, the specific activities increased to 176 or 197 mmol min-1 mol-1, respectively. This increase, however, has nothing to do with G1530 as evidenced by the specific activity (201 mmol min-1 mol-1) for RNA302, in which G1530 is replaced with an Ade (Table 1).
, G1530 is not involved in the stimulation of GTPase activity, whereas A1531 and A1534 are determinants for the stimulation; A1531 tolerates mutational substitutions, but A1534 does not. In addition, the h45 sequence upstream from G1530, further stimulates the GTPase activity of Era. In the presence of RNA300 or RNA301, the specific activities increased to 176 or 197 mmol min-1 mol-1, respectively. This increase, however, has nothing to do with G1530 as evidenced by the specific activity (201 mmol min-1 mol-1) for RNA302, in which G1530 is replaced with an Ade (Table 1).
The G1530A Mutation Weakens Era-RNA Interaction.
We show that a pseudobase pair is formed by the G1530 base and the side chains of E209 and R241 (Fig. 4A), which stacks with the first BP of h45 and thus plays an important role in Era-RNA interaction (Fig. 2B). We also show that the G1530A mutation eliminates such pseudobase pairing (Fig. 4B): Two hydrogen bonds between Era and G1530 are lost; the mutant Ade base creates an unfavorable stereo clash with the side chain of R241. Thus, the Era-RNA interaction must be weakened. We have validated this structure-based prediction by isothermal titration calorimetry (ITC) experiments. The Kd value is 12.6 ± 3.2 nM for RNA301 (Fig. S2A), but is 26.4 ± 4.6 nM for RNA302 (Fig. S2B), showing a twofold decrease in Era-RNA affinity due to the G1530A mutation. It is concluded, therefore, that the G1530A mutation affects RNA binding rather than the intrinsic GTPase activity of Era.
The Recognition of G1530 by Era is Required for the Function of the Protein.
G1530 interacts with four Era side chains (Fig. 4A), among which E209 is universally conserved. We have tested the impact of three mutations in this residue: E209A, E209K, and E209Q (Fig. 4C). The E209A and E209K mutants cannot be made at any growth temperature and are presumed to be lethal, whereas the E209Q mutant can be made but is cold sensitive (Fig. 4D). The E209Q mutant severely impacts bacterial growth at 32 °C. At 25 °C, growth is totally arrested even after 14 d. At higher temperatures (37 or 42 °C), the mutation moderately retards cell growth. These data demonstrate that the Era-G1530 interaction is required for the function of Era.
Discussion
The functional cycle of Era indicates that the protein functions as an RNA chaperone for processing and maturation of 16S rRNA and a checkpoint for assembly of the 30S subunit (17, 20). We show that Era recognizes the  sequence near the 3′ end of 16S rRNA, the significance of which is fourfold. First, Era binding protects the GAUCACCUCC sequence from accidental damage during 16S rRNA processing and maturation. Second, the binding prevents base pairing between the antiSD and SD sequences, thereby preventing mRNA recruitment. Third, the ribosomal protein S1-binding site significantly overlaps with that of Era (28); hence, the binding of Era occludes the binding of S1 that directly affects the association of the SD and antiSD sequences. Fourth, conformational changes of the 16S rRNA precursor induced by the binding of Era may facilitate the activity of other ribosome biogenesis factors, including RNases that remove extra nucleotides from both ends of rRNA (28) and KsgA that methylates A1518 and A1519 in the tetraloop of h45 (23).
sequence near the 3′ end of 16S rRNA, the significance of which is fourfold. First, Era binding protects the GAUCACCUCC sequence from accidental damage during 16S rRNA processing and maturation. Second, the binding prevents base pairing between the antiSD and SD sequences, thereby preventing mRNA recruitment. Third, the ribosomal protein S1-binding site significantly overlaps with that of Era (28); hence, the binding of Era occludes the binding of S1 that directly affects the association of the SD and antiSD sequences. Fourth, conformational changes of the 16S rRNA precursor induced by the binding of Era may facilitate the activity of other ribosome biogenesis factors, including RNases that remove extra nucleotides from both ends of rRNA (28) and KsgA that methylates A1518 and A1519 in the tetraloop of h45 (23).
We also show that Era associates with h45 via two pivotal sites: at the first BP of h45, which stacks with the pseudobase pair formed by the G1530 base and the E209 and R241 side chains from the KH domain, and at the stem of h45, which is anchored to Era by the K66, K67, and R289 side chains (Fig. 2B). Obviously, these interactions stabilize the conformation of U1532, which is otherwise flexible as observed in the Era-GNP-RNA100 structure (Fig. 3), and the conformation of h45, which may be optimal for the activity of KsgA.
In the  sequence, nucleotide G1530 and A1531 are universally conserved. It has been shown that the
sequence, nucleotide G1530 and A1531 are universally conserved. It has been shown that the  to
to  mutation affects the binding of IF3 to the ribosome (14). However, IF3 does not interact with nucleotides 1530–1531; instead, it contacts nucleotides 1532–1534 as revealed by the crystal structure of IF3 in complex with the 30S ribosomal subunit (Fig. S3A) (29). Therefore, it is not clear how the double mutation affects IF3 binding although it is evident that the conformation of the 3′ end of 16S rRNA starting from G1530 is dramatically different in the Era-bound and IF3-bound forms (Fig. S3B). Contacting nucleotides 1532–1534, IF3 binds to the 30S ribosomal subunit at the upper end of the platform on the solvent side of 30S, close to the antiSD sequence of 16S rRNA (29), which is in agreement with the EM reconstruction of rat liver 40S ribosomal subunit in complex with eukaryotic IF3 (30). In contrast, the cryoEM reconstruction of the 30S ribosomal subunit positions the C-terminal domain of IF3 at the interface side of the subunit, in close proximity to h45 (31), for which strong biochemical support is provided by hydroxyl radical footprinting and directed probing studies (32). The discrepancy in the location of the protein on the small ribosomal subunit suggests different mechanisms of IF3 action.
mutation affects the binding of IF3 to the ribosome (14). However, IF3 does not interact with nucleotides 1530–1531; instead, it contacts nucleotides 1532–1534 as revealed by the crystal structure of IF3 in complex with the 30S ribosomal subunit (Fig. S3A) (29). Therefore, it is not clear how the double mutation affects IF3 binding although it is evident that the conformation of the 3′ end of 16S rRNA starting from G1530 is dramatically different in the Era-bound and IF3-bound forms (Fig. S3B). Contacting nucleotides 1532–1534, IF3 binds to the 30S ribosomal subunit at the upper end of the platform on the solvent side of 30S, close to the antiSD sequence of 16S rRNA (29), which is in agreement with the EM reconstruction of rat liver 40S ribosomal subunit in complex with eukaryotic IF3 (30). In contrast, the cryoEM reconstruction of the 30S ribosomal subunit positions the C-terminal domain of IF3 at the interface side of the subunit, in close proximity to h45 (31), for which strong biochemical support is provided by hydroxyl radical footprinting and directed probing studies (32). The discrepancy in the location of the protein on the small ribosomal subunit suggests different mechanisms of IF3 action.
Among Era sequences, amino acid E209 is universally conserved. We show that the G1530A mutation eliminates the pseudobase pairing between the 1530 base and the E209 and R241 side chains (Fig. 4B). Consequently, one of the two pivotal interactions between Era and h45 is disrupted, and hence h45 is most likely destabilized. We also show that the E209A and E209K mutations of Era are lethal, whereas the E209Q mutation is cold sensitive (Fig. 4D). Obviously, E209A and E209K eliminate two of the three pseudobase pairing hydrogen bonds between nucleotide 1530 and amino acids 209 and 241, whereas E209Q eliminates only one of the three (Fig. 4C). We also demonstrate that the G1530A mutation affects RNA binding by Era rather than the intrinsic GTPase activity of the protein. Taken together, these observations indicate that the recognition of G1530 by Era is required for Era-RNA interactions during the processing and maturation of 16S rRNA. G1530 may also play important roles during translation initiation. Considering that the nucleotide can be crosslinked to the mRNA near or within the AUG start codon (13, 33), it may function as a hinge point for the movement of the SD helix (12).
Materials and Methods
Protein and RNA.
A. aeolicus Era and KsgA were prepared as described (17, 22). The RNA301 sequence, corresponding to the RNA300 sequence near the 3′ end of E. coli 16S rRNA, was taken from A. aeolicus 16S rRNA with original U1506 replaced with a Cyt (Table 1). All of the RNAs used in this study were purchased (Integrated DNA Technologies) and used without further purification.
GTP-Hydrolyzing Activity Assay and Isothermal Titration Calorimetry.
The GTPase assay was performed as described (17). The Era-RNA interactions were studied using an isothermal titration microcalorimeter iTC200 (GE Healthcare/MicroCal) at 25 °C. The integrated interaction heat values were fit using the Origin 7.0-based ITC data analysis software provided by GE/MicroCal. Details of the ITC experiments are provided in SI Text.
Mutagenesis of Era by Single-Strand (ss) DNA Recombineering.
Bacterial strains and plasmid (Table S1) were constructed by either recombineering technology (34, 35) or P1 transduction (36). Detailed experimental procedures are described in SI Text.
Crystal Structure Determination.
Crystals were grown at 19 ± 1 °C using sitting-drop vapor diffusion. Diffraction data were collected at the Advanced Photon Source, Argonne National Laboratory. The structures were solved by molecular replacement. X-ray diffraction data statistics and structure parameters are summarized in Table S2. Detailed procedures of crystallization, X-ray diffraction data acquisition, and structure solution and refinement are described in SI Text.
Supplementary Material
Acknowledgments.
Data were collected at the Southeast Regional Collaborative Access Team (SER-CAT) of the Advanced Photon Source, Argonne National Laboratory. This research was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Crystallography, atomic coordinates, and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 3R9W and 3R9X).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017679108/-/DCSupplemental.
References
- 1.Gold L, et al. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- 2.Shine J, Dalgarno L. The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: Complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci USA. 1974;71:1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neefs JM, Van de Peer Y, Hendriks L, De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1990;18(Suppl):2237–2317. doi: 10.1093/nar/18.suppl.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raué HA, Klootwijk J, Musters W. Evolutionary conservation of structure and function of high molecular weight ribosomal RNA. Prog Biophys Mol Biol. 1988;51:77–129. doi: 10.1016/0079-6107(88)90011-9. [DOI] [PubMed] [Google Scholar]
- 5.Noller HF. In: The RNA World. Gesteland RF, Atkins JF, editors. Plainview, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 137–156. [Google Scholar]
- 6.Van Knippenberg PH, Van Kimmenade JM, Heus HA. Phylogeny of the conserved 3′ terminal structure of the RNA of small ribosomal subunits. Nucleic Acids Res. 1984;12:2595–2604. doi: 10.1093/nar/12.6.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poldermans B, Bakker H, Van Knippenberg PH. Studies on the function of two adjacent N6,N6-dimethyladenosines near the 3′ end of 16S ribosomal RNA of Escherichia coli. IV. The effect of the methylgroups on ribosomal subunit interaction. Nucleic Acids Res. 1980;8:143–151. doi: 10.1093/nar/8.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poldermans B, Van Buul CP, Van Knippenberg PH. Studies on the function of two adjacent N6,N6-dimethyladenosines near the 3′ end of 16 S ribosomal RNA of Escherichia coli. II. The effect of the absence of the methyl groups on initiation of protein biosynthesis. J Biol Chem. 1979;254:9090–9093. [PubMed] [Google Scholar]
- 9.Connolly K, Rife JP, Culver G. Mechanistic insight into the ribosome biogenesis functions of the ancient protein KsgA. Mol Microbiol. 2008;70:1062–1075. doi: 10.1111/j.1365-2958.2008.06485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehresmann C, et al. Cross-linking of initiation factor IF3 to Escherichia coli 30S ribosomal subunit by trans-diamminedichloroplatinum(II): Characterization of two cross-linking sites in 16S rRNA; a possible way of functioning for IF3. Nucleic Acids Res. 1986;14:4803–4821. doi: 10.1093/nar/14.12.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell P, Osswald M, Brimacombe R. Identification of intermolecular RNA cross-links at the subunit interface of the Escherichia coli ribosome. Biochemistry. 1992;31:3004–3011. doi: 10.1021/bi00126a023. [DOI] [PubMed] [Google Scholar]
- 12.Kaminishi T, et al. A snapshot of the 30S ribosomal subunit capturing mRNA via the Shine–Dalgarno interaction. Structure. 2007;15:289–297. doi: 10.1016/j.str.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 13.La Teana A, Gualerzi CO, Brimacombe R. From stand-by to decoding site. Adjustment of the mRNA on the 30S ribosomal subunit under the influence of the initiation factors. RNA. 1995;1:772–782. [PMC free article] [PubMed] [Google Scholar]
- 14.Firpo MA, Connelly MB, Goss DJ, Dahlberg AE. Mutations at two invariant nucleotides in the 3′-minor domain of Escherichia coli 16S rRNA affecting translational initiation and initiation factor 3 function. J Biol Chem. 1996;271:4693–4698. doi: 10.1074/jbc.271.9.4693. [DOI] [PubMed] [Google Scholar]
- 15.Britton RA, et al. Cell cycle arrest in Era GTPase mutants: A potential growth rate-regulated checkpoint in Escherichia coli. Mol Microbiol. 1998;27:739–750. doi: 10.1046/j.1365-2958.1998.00719.x. [DOI] [PubMed] [Google Scholar]
- 16.Britton RA. Role of GTPases in bacterial ribosome assembly. Annu Rev Microbiol. 2009;63:155–176. doi: 10.1146/annurev.micro.091208.073225. [DOI] [PubMed] [Google Scholar]
- 17.Tu C, et al. Structure of ERA in complex with the 3′ end of 16S rRNA: Implications for ribosome biogenesis. Proc Natl Acad Sci USA. 2009;106:14843–14848. doi: 10.1073/pnas.0904032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier TI, Peery RB, Jaskunas SR, Zhao G. 16S rRNA is bound to era of Streptococcus pneumoniae. J Bacteriol. 1999;181:5242–5249. doi: 10.1128/jb.181.17.5242-5249.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meier TI, Peery RB, McAllister KA, Zhao G. Era GTPase of Escherichia coli: Binding to 16S rRNA and modulation of GTPase activity by RNA and carbohydrates. Microbiology+ 2000;146:1071–1083. doi: 10.1099/00221287-146-5-1071. [DOI] [PubMed] [Google Scholar]
- 20.Bunner AE, Nord S, Wikstrom PM, Williamson JR. The effect of ribosome assembly cofactors on in vitro 30S subunit reconstitution. J Mol Biol. 2010;398:1–7. doi: 10.1016/j.jmb.2010.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thammana P, Held WA. Methylation of 16S RNA during ribosome assembly in vitro. Nature. 1974;251:682–686. doi: 10.1038/251682a0. [DOI] [PubMed] [Google Scholar]
- 22.Tu C, et al. Structural basis for binding of RNA and cofactor by a KsgA methyltransferase. Structure. 2009;17:374–385. doi: 10.1016/j.str.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu Q, Inouye M. The gene for 16S rRNA methyltransferase (ksgA) functions as a multicopy suppressor for a cold-sensitive mutant of era, an essential RAS-like GTP-binding protein in Escherichia coli. J Bacteriol. 1998;180:5243–5246. doi: 10.1128/jb.180.19.5243-5246.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Farrell HC, Xu Z, Culver GM, Rife JP. Sequence and structural evolution of the KsgA/Dim1 methyltransferase family. BMC Res Notes. 2008;1:108. doi: 10.1186/1756-0500-1-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inoue K, Basu S, Inouye M. Dissection of 16S rRNA methyltransferase (KsgA) function in Escherichia coli. J Bacteriol. 2007;189:8510–8518. doi: 10.1128/JB.01259-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valverde R, Edwards L, Regan L. Structure and function of KH domains. FEBS J. 2008;275:2712–2726. doi: 10.1111/j.1742-4658.2008.06411.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen SM, et al. Expression and characterization of RNase III and Era proteins products of the rnc operon of Escherichia coli. J Biol Chem. 1990;265:2888–2895. [PubMed] [Google Scholar]
- 28.Sharma MR, et al. Interaction of Era with the 30S ribosomal subunit implications for 30S subunit assembly. Mol Cell. 2005;18:319–329. doi: 10.1016/j.molcel.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 29.Pioletti M, et al. Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine, and IF3. EMBO J. 2001;20:1829–1839. doi: 10.1093/emboj/20.8.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srivastava S, Verschoor A, Frank J. Eukaryotic initiation factor 3 does not prevent association through physical blockage of the ribosomal subunit-subunit interface. J Mol Biol. 1992;226:301–304. doi: 10.1016/0022-2836(92)90946-h. [DOI] [PubMed] [Google Scholar]
- 31.McCutcheon JP, et al. Location of translational initiation factor IF3 on the small ribosomal subunit. Proc Natl Acad Sci USA. 1999;96:4301–4306. doi: 10.1073/pnas.96.8.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dallas A, Noller HF. Interaction of translation initiation factor 3 with the 30S ribosomal subunit. Mol Cell. 2001;8:855–864. doi: 10.1016/s1097-2765(01)00356-2. [DOI] [PubMed] [Google Scholar]
- 33.Rinke-Appel J, et al. Contacts between 16S ribosomal RNA and mRNA, within the spacer region separating the AUG initiator codon and the Shine–Dalgarno sequence; a site-directed cross-linking study. Nucleic Acids Res. 1994;22:3018–3025. doi: 10.1093/nar/22.15.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu D, et al. An efficient recombination system for chromosome engineering in Escherichia coli. Proc Natl Acad Sci USA. 2000;97:5978–5983. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharan SK, Thomason LC, Kuznetsov SG, Court DL. Recombineering: A homologous recombination-based method of genetic engineering. Nat Protoc. 2009;4:206–223. doi: 10.1038/nprot.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller JH. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.