Abstract
The ability of cancer cells to invade, metastasize, and form distant colonies, is one of the key characteristics that confers lethality to cancer. Metastatic cancer cells typically become refractory to treatment. The metastatic cascade is a multi-step process that is governed by events within the cancer cell, the tumor microenvironment, and the distant environments that are invaded and colonized by the cancer cells. Noninvasive imaging techniques are facilitating a close examination of the stepwise journey of the cancer cell from the primary tumor to the distant metastatic site. Here we have discussed the metastatic process, and how molecular and functional imaging of cancer are providing new insights into the metastatic cascade that can be exploited for treatment of metastatic disease.
Keywords: Invasion, metastasis, molecular and functional imaging, theranostics of metastasis
1. Introduction
The metastatic phenotype is probably the single most lethal phenotype exhibited by cancer cells and preventing or combating metastatic spread continues to be the challenge of our times. The initial escape of a cancer cell from its primary site requires the disruption of cell-to-cell attachment, followed by invasion of underlying connective tissue, initial invasion of the vessel, arrest of the circulating tumor cells and adhesion to the blood vessel, extravasation from the vessel and finally further invasion at the site of the metastatic lesion resulting in growth of the metastatic nodule [1–4], as outlined in the schematic in Fig. 1. Cancer metastasis can be lymphogenous, hematogenous or peritoneal, although the lymphatics and vasculature are recognized as the major routes for dissemination. There may be possible links between the three routes, particularly between lymphogenous and hematogenous metastasis due to lymphatico-venous communication.
Fig. 1.
Stages of Metastatic Progression. Metastasis proceeds through the progressive acquisition of traits that allow malignant cells originating in one organ to disseminate and colonize a secondary site. Although these traits are depicted as part of a contiguous biological sequence, their acquisition during metastatic progression need not follow this particular order. Although in some cases several factors may be necessary to implement a single step in this cascade, other mediators of metastasis may facilitate execution of multiple stages simultaneously. Similarly, the specific steps of this sequence that are rate limiting for metastatic progression may also vary from one tumor to the next. Reprinted with permission from [3].
Successful colonization of distant organs coupled with the inability of current treatments to arrest or eliminate these metastatic cells results in organ failure, which is the primary cause of mortality in cancer-related deaths. The long-held view that metastatic potential is a function of tumor growth is giving way to a paradigm shift that metastatic dissemination may be an early event, with several recent observations supporting the latter view [4,5]. First, successful metastatic cells maintain fewer genetic alterations than cancer cells from advanced primary tumors [6,7]. Second, genetic alterations found in metastases are significantly different from cells in the advanced primary tumor site [5]. Third, instances of rapid dissemination and successful metastasis irrespective of tumor size have been documented in certain forms of cancer including inflammatory breast cancer [8].
As early as 1889, Stephen Paget noticed the ‘dependence of the seed upon the soil’ while describing patterns of metastasis from breast cancer [9]. In his analysis of autopsy records of women with breast cancer, he observed that the pattern of metastases was not random, but that the cancer cell or ’seed’ had an affinity for certain sites or ’soil’. It is now well established that although the primary site of metastases occurs at the first capillary bed encountered, the microenvironment of the primary tumor, and the microenvironment at the secondary sites play a critical role in the metastatic cascade (schematic in Fig. 2) [10–12].
Fig. 2.
Cancer cells in primary tumors are surrounded by a complex microenvironment comprising numerous cells including endothelial cells of the blood and lymphatic circulation, stromal fibroblasts and a variety of bone marrow-derived cells (BMDCs) including macrophages, myeloid-derived suppressor cells (MDSCs), TIE2-expressing monocytes (TEMs) and mesenchymal stem cells (MSCs). Reprinted with permission from [11].
Metastasis-promoting changes have been described within cancer cells, in the primary tumor microenvironment and the distant site of metastasis. Within the epithelial cancer cell, changes associated with the assumption of a less differentiated mesenchymal phenotype known as the epithelial to mesenchymal transition or EMT have been observed [13]. EMT is generally characterized by loss of markers of differentiation such as E-cadherin and the acquisition of heavy cytokeratins, vimentin and smooth muscle actin [13] and has been associated with increased invasion and metastasis in mice [13]. However, significant debate has arisen from the lack of evidence of EMTs in humans [14,15].
In the primary tumor, physiological environments that are hypoxic with an acidic extracellular pH [16, 17] are frequently found and have been shown to increase invasion and metastasis [16,17]. Experimental results from several studies provide evidence that hypoxia initiates adaptation and dissemination in several well orchestrated steps that promote the expression of several oncogenes by the transcription factor hypoxia inducible factor-1 (HIF-1) [18–20]. Although not sufficient for metastasis, genetic inactivation of tumor suppressor genes such as p53, VHL and PTEN [21–23], activation of oncogenes such Src, HER2, KRAS and modulation of signaling involving the RAS, RAF MAPK and PI3K pathways [24–27], are a prerequisite for tumor cell adaptation to hypoxia, and transformation to a more invasive and metastatic phenotype.
Similarly, distant sites of metastasis also display phenotypes that provide an attractive site for circulating tumor cells, now termed the premetastatic niche [28,29]. Vascular endothelial growth factor receptor 1 (VEG-FR1) activation in the lung has been shown to prime the metastatic niche by inducing matrix metalloproteinase (MMP)-9 [30]. Ablation of VEGFR1 positive progenitor cells, using specific antibodies, inhibited metastasis [31]. Clinical trials using antibody-mediated VEGFR1 signaling blockade have shown no significant improvement of 5-year survival rates even though overall survival improved compared to chemoradiation alone [28]. Further trials need to focus on the effect of VEGFR1 blockade on lung-associated metastasis. Consistent with the involvement of progenitor cells in the lung metastatic niche [31], breast tumor initiating CD44+CD24− cancer cells have been isolated in the bone marrow of breast cancer patients [32]. It has thus been proposed that tumor-initiating pluripotent cells are involved in preparing the metastatic niche [33].
Some of the broad challenges for molecular and functional imaging in addressing the complexities of the metastatic cascade and reducing mortality from metastasis are: (i) prevention through clearer understanding of the invasive-metastatic processes, (ii) early detection of metastatic lesions and identification of primary lesions that are most likely to metastasize, and (iii) effective image-guided treatment that would target metastases and minimize damage to normal tissue. Imaging techniques have already made significant inroads into providing critical insights into the metastatic process. Microscopy and bioluminescence imaging permit the tracking of single cells in living organisms allowing for longitudinal studies within the same organism.
With its array of functional imaging capabilities, magnetic resonance imaging (MRI) and spectroscopy (MRS) techniques are identifying potential physiological and metabolic biomarkers that can be used to identify metastatic lesions and aggressive tumors with metastasis-permissive environments that are likely to metastasize [34]. With its exquisite sensitivity, positron emission tomography (PET) imaging can be used to detect metastatic cells using radiolabeled nuclei that report on glucose metabolism, receptors, and antigens. Both MR and PET are clinically translatable, and image-guided ‘theranostic’ agents in which diagnosis is combined with therapy, are being developed that provide realistic expectations for effective treatment of metastatic disease. Here we have provided examples of the applications of molecular and functional imaging in these three broad areas, with an emphasis on the mechanisms of invasion and metastasis.
2. Imaging-based insights into mechanisms of invasion and metastasis
2.1. Imaging changes within the cancer cell
Initiation of the metastatic process requires the acquisition of a motile phenotype by transformed epithelial cells. In concert with the tumor-permissive microenvironment, transformed epithelial cells undergo changes in the expression and function of the proteins regulating cytoskeletal movements [35]. Using fluorescence microscopy Holland et al., observed the induction of actin polymerization by the chemokine CXCL12/SDF-1 in metastatic MDA-MB-231 breast cancer cells, but not in non-metastatic MDA-MB-453 cells [36]. Functional activation through intracellular G protein signaling, and not just expression of the CXCL12 receptor CXCR4, correlated with actin polymerization and metastatic potential [36]. While fluorescence microscopy is useful for endpoint analysis, it does not provide three-dimensional information.
By using laser point illumination and a beam splitter that directs emitted photons through a pinhole of adjustable aperture before being collected by a digital camera, confocal microscopy provides information along the z-axis of the specimen that can be reconstructed to provide three dimensional information. Confocal microscopy was used to study the formation of invadopodia, structures formed at the edge of cancer cells that determine directionality and invasion, in response to extracellular signaling from the epidermal growth factor (EGF) [37]. Extensive work from the Condeelis lab has carefully characterized the molecular processes required for the formation of these structures and their role in cancer cell invasion of the extracellular matrix (ECM) [35]. Imaging of processes that regulate and promote invasiveness of cancer cells in vivo has also utilized the principle of Förster (or Fluorescence) Resonance Energy Transfer (FRET) which predicts that an activated (donor) chromophore may transfer its energy to a neighboring (acceptor) chromophore depending partly on their distance and spectral overlap. Prag et al., used FRET to examine the regulation of the binding of the Rho GTPase Cdc42 to the downstream p21-activated kinase 1 (PAK1) by the actin-binding ezrin/radixin/moesin (ERM)-protein [38]. A PAK1-green fluorescence protein (GFP) donor molecule that bound the Cdc42 protein fused to the myc epitope was used in MDA-MB-231 cells. The presence of ezrin reduced the lifetime of GFP fluorescence particularly in peripheral cell protrusions, when a Cy3-anti-myc antibody was used to stain the cells. This decreased lifetime of GFP fluorescence identified the proximity of PAK1-GFP to Cdc42, from the increased resonance energy transfer of the GFP donor molecule to Cy3-labeled anti-myc antibody [38]. These studies demonstrated the importance of protein-protein interactions in the regulation of the actin cytoskeleton at cell membrane protrusions, mediated through Rho GTPase family of proteins that are known to promote invasiveness and increased motility [38,39].
2.2. Imaging the invasion of cancer cells and the role of the tumor microenvironment in invasion
The initial escape of tumor cells from a primary tumor requires local tumor invasion [1,40]. Cells not immediately adjacent to a blood vessel are thought to migrate through the ECM while those near a capillary only have to penetrate the capillary basement membrane. Basement membranes are thin sheets of ECM that separate parenchymal cells from interstitial stroma and present a major barrier to metastatic spread [41]. The ECM comprises of collagens, glycoproteins and proteoglycans several of which are altered in tumors [42]. These alterations are a result of tumor- and tumor microenvironment-derived signaling. Tumors actively secrete a host of proteolytic enzymes that continuously remodel the ECM [43]. Lysosomes carry multiple proteases including the different types of cathepsins that play a key role in cancer invasion and metastasis. Lysosomal cathepsins participate in cleaving fibrillar collagen [44], laminin [45], and fibronectin [46]. Non-invasive near infrared (NIR) optical imaging of 6-O′-glucosamine-labeled NIR fluorescent probes was recently developed to image lysosomes in breast cancer cells in culture and following systemic administration in human breast cancer xenografts [47]. In culture, a distinct translocation of lysosomes from the perinuclear region to the periphery of the cell membrane was observed in response to acidic extracellular pH [48].
Imaging has been used to detect degradative enzyme activity. Optical imaging of protease activity in vivo was performed using a self-quenched NIR probe that displayed significantly enhanced fluorescence when cleaved by secreted lysosomal cathepsins [49]. To allow for the quantification of enzymatic activity by optical imaging in vivo, McIntyre et al., utilized a peptide substrate susceptible to selective cleavage by MMP-7, which was covalently linked to fluorescein and a polyamido-amino-tetramethylrhodamine complex [50]. Cells and tumors rich in MMP-7 emitted enhanced fluorescein signal following intravenous administration of the reporter complex. An improved MMP-7 sensitive probe that utilized NIR fluorescent probes has since been described [51].
Changes in hyaluronan synthesis and degradation by hyaluronan synthase 2 and hyaluronidase 2, affect the quantity and quality of hyaluronan. Shorter hyaluronan fragments may provide hydrated pathways for metastasizing cells [52]. Hyaluronidase activity has been detected with MRI using a contrast agent that combines hyaluronan with GdDTPA and non-toxic agarose beads. MRI contrast was generated from hyaluronidase activity by its action on hyaluronan, that resulted in an alteration of the relaxation rate of water molecules in contact with the contrast agent [53].
The invasive potential of tumor cells is commonly assayed by determining the penetration of cells into reconstituted basement membrane gel (Matrigel®). Invasion is quantitated by counting the number of cells that invade Matrigel® coated filters over a period of 5 to 72 h [54]. An MRI compatible invasion assay has been developed to dynamically track the invasion of cancer cells and simultaneously characterize oxygen tensions, and physiological and metabolic parameters (schematic shown in Fig. 3a) [55]. The assay can be used to understand the effects of physiological environments that exist in tumors, on the ability of cells to degrade and invade ECM. This assay was used to determine the effects of the anti-inflammatory agent, indomethacin, on breast cancer cell invasion and metabolism (Fig. 3b) [56]. In-domethacin is a non-specific inhibitor of cyclooxygenase (COX)-1 and 2. COX-2, the inducible form of cyclooxygenase, is the rate-limiting step in the conversion of arachidonic acid to the potent inflammatory mediator prostaglandin E2 (PGE2) [57]. PGE2 has a significant impact on cell motility, invasion, vascular characteristics and metastatic dissemination [58]. The decrease of invasion observed in Fig. 3b is consistent with the profound decrease of invasion and metastasis observed following COX-2 silencing in metastatic breast cancer cells [59,60].
Fig. 3.
(A) Schematic display of the sample structure (center) of a cell-perfusion assay, which can be identified clearly in the representative T1-weighted 1H MR image on the right.
(B) T1-weighted 1H MR images and Invasion Indices over time demonstrating the effect of indomethacin treatment on degradation and invasion of ECM gel by MDA-MB-435 breast cancer cells. Values of the Invasion Indices are presented as mean ± SE. Reprinted with permission [56].
MRI has also been used to study invasion by labeling cancer cells with iron-oxide particles that generate negative contrast or loss of signal within the image [61]. Such an approach has also been used to study the interaction between cancer cells and iron-oxide labeled vascular endothelial or lymphatic endothelial cells [62,63]. Both vascular and lymphatic endothelial cells demonstrated a significant increase of invasion into ECM in the presence, and direction, of cancer cells. The invasion of lymphatic endothelial cells in the presence of cancer cells was also determined under hypoxic conditions, and was found to increase significantly compared to normoxic conditions [62].
Intrinsic second harmonic generation (SHG) microscopy is a valuable tool for understanding the remodeling of the collagen matrix in intact tissue [64]. SHG is a nonlinear optical process that produces signal from a molecule without a center of symmetry. This contrast mechanism has been used to image endogenous structural proteins such as collagen, with the advantage that differences in fiber structure and volume can be determined in 3D with micrometer resolution. A major component of the ECM in tumors is fibrillar collagen I [65,66]. SHG microscopy investigations of the interactions of cancer cells with fibrillar collagen in normal mammary glands, mammary tumors and tumor explants revealed that invading cancer cells align along collagen fibers while migrating [67].
2.3. Imaging metastasis
The most frequent routes of dissemination to distant target tissue are through the blood stream and to a much lesser extent by the lymphatic circulatory system. The final stage involves extravasation and establishment of micrometastasis. Imaging of metastasis in vivo has become a reality with multi-photon microscopy. By using two or more pulsed laser excitation beams [68] the energies of the lasers are combined, resulting in deeper penetrance, less background scattering and less phototoxicity. Kienast et al. [69], imaged red fluorescence protein-labeled cell metastasis from tumors with FITC-dextran labeled blood vessels using a cranial window chamber in nude mice. Based on the data obtained, they proposed that the colonization of the brain by melanoma, lung carcinoma and breast cancer cells is a four-step process that included arrest at blood vessels by size restriction, active extravasation through the holes of the vascular wall with localization at a perivascular position, and macrometastasis either through continuous growth of cell clusters and vascular cooption, or by the formation of new tumors from single cells with characteristic tumor angiogenesis as shown in Fig. 4 [69]. Other groups have used a “mammary window” or a dorsal implantation chamber to study the invasion of tumor cells in the mammary fat pad of mice and the interactions between tumor-associated fibroblasts and the ECM respectively [70, 71].
Fig. 4.
Schematic overview of the four essential steps of brain metastasis formation. 1. Arrest at blood vessels by size restriction. 2. Active extravasation though the holes of the vascular wall. 3. Localization at a perivascular position. 4. Macrometastasis either through continuous growth of cell clusters and vascular cooption, or by the formation of new tumors from single cells. Reprinted with permission from [69].
An innovative approach that detected and quantified GFP-expressing circulating tumor cells was recently described [72]. A single fiber optic module bearing a two-photon optical fiber fluorescence probe was constructed and implanted in the liver of mice that were injected intravenously with GFP-expressing cells. Such approaches are designed to allow the visualization of circulating tumor cells that express receptors related to metastasis, such as CXCR4, folate or HER2 that can be examined by fluorescence-labeled antibodies in vivo. Wang et al. [73] utilized the polyoma middle T oncogene (PyMT)–derived mammary tumor model to examine the interaction of PyMT-induced primary tumors with blood vessels in vivo. A subpopulation of tumor cells associated with increased invasiveness was isolated, and the molecular changes in these cells were characterized ex vivo, resulting in the identification of a ‘cancer cell motility signature’.
Bioluminescence imaging (BLI) has also been used to study metastasis. This typically requires cloning the firefly enzyme of choice into DNA vectors that are expressed in stable or inducible forms in a variety of cells and organisms [74,75]. Cell-based applications of bioluminescence that use a variety of stable and inducible gene-specific promoters have been employed in the longitudinal tracking of metastatic cells in vivo. Using BLI, Sun et al., showed that a focal adhesion kinase inhibitor reduced the metastasis of a prostate cancer cell line following intracardiac injection [76]. The discovery of secreted and more sensitive luciferase, termed Gaussia luciferase or Gluc, was recently described [77]. In preclinical studies, Chung et al. [78], stably expressed Gluc in metastatic poorly differentiated breast cancer cells and found good correlation between orthotopic tumor growth and experimental metastasis in the brain with blood Gluc levels. Importantly, it was demonstrated that Gluc blood assay provided useful quantitative information for the evaluation of metastatic tumor burden in response to the dual tyrosine kinase inhibitor lapatinib. Bioluminescence imaging requires the administration of a substrate, and pharmacokinetics of substrate availability introduces some limitations [79]. Most luciferase enzymes emit light in the blue or green area of the spectrum, which is attenuated in living tissues. Despite these drawbacks, BLI is an easily configurable and quantitative platform that can be applied to study metastasis in vivo.
MRI has also been used in preclinical models to track iron oxide labeled breast cancer cells. A subset of cells injected in the left ventricle were able to establish metastatic colonies in the brain [80].
3. Identification of primary lesions that are most likely to metastasize, early detection of metastatic lesions, and therapy
Identifying noninvasive imaging-based parameters that can be used to identify primary tumors that are likely to metastasize is a major challenge in the field of molecular and functional imaging. Early detection of metastasis is particularly important for staging and selecting treatments. Effective methods of imaging metastatic tumors can also be combined with the delivery of therapeutic cargo as ‘theranostic’ agents for the treatment of metastatic disease.
3.1. Imaging predictors of metastasis
Evidence that tumors selectively colonize organs has led to the search for molecular signatures of cancer cells that predict the metastatic ability and site of metastasis. Gupta et al., used short hairpin RNA (shRNA) to stably silence four different genes (EREG, COX2, MMP-1 and MMP-2) in MDA-MB-231 breast cancer cells that promote breast cancer cell colonization of the lung [81]. Expression of these genes as well as that of other markers such as CXCR4 (discussed below) may be used to image the metastatic process. The same group has also demonstrated that late bone metastasis in breast cancer patients is associated with the tyrosine kinase activity of Src [82]. Early identification of tumors that are likely to metastasize would provide significant advantages in cancer management, with aggressive treatments and careful monitoring of patients with high-risk lesions. Multi-modality noninvasive imaging derived parameters of primary tumors may fill an important niche in providing such biomarkers. Unlike biopsy evaluation, the entire lesion area can be imaged with techniques such as MRI/S and PET, allowing a more thorough evaluation of the tumor.
Dietzel et al., have explored the potential of breast MRI to predict lymph node metastases [83]. The study included 350 patients with invasive breast cancer, with histological analyses performed to define the nodal status of each patient. A close association was found between selected breast MRI descriptors to nodal status. Seven of the 17 descriptors selected were associated with lymph node metastases, with skin thickening and internal enhancement after GdDTPA injection being the most accurate [83].
The ability to identify cancers that have a high risk of metastasizing from those that will not would also result in sparing those patients with indolent disease, such as in prostate cancer, the unnecessary side-effects associated with aggressive treatments. DeSouza and colleagues have identified diffusion-weighted MRI as a potential non-invasive marker of aggressiveness in localized prostate cancer [84]. Diffusion weighted imaging (DWI) reports on the degree of restriction of water in tissues. Apparent diffusion coefficients (ADC) of water derived from DWI were different between aggressive and indolent prostate cancers. Since water diffusion depends upon cellular and structural characteristics, these results imply that these were significantly different between indolent and aggressive lesions.
Elevation of total choline is one of the hallmarks of cancers and can be detected by 1H MRS [85]. Most cancers are characterized by increased phosphocholine, which is primarily a membrane phospholipid precursor that is formed by the phosphorylation of free choline by choline kinase [86]. Not surprisingly, increased expression of choline kinase is also observed in cancers [87]. A higher rate of choline transport and increased phospholipase C and D activity also play a role in the altered choline metabolism observed in tumors [88]. These enzymes are involved in the biosynthesis and breakdown pathways of the major membrane phospholipid, phosphatidylcholine. A trend towards increasing total choline to citrate, a compound that is found in normal prostate tissue was observed with increasing Gleason score [89].
In a preclinical study, imaging was used to compare vascular, metabolic and physiological characteristics of a human prostate cancer xenograft implanted ortho-topically in the prostate or subcutaneously in the flank; human malignant cell lines metastasize more readily from orthotopic sites than from heterotopic sites. Hypoxia was detected in these xenografts by placing an enhanced GFP optical reporter under the control of a hypoxia response element. A multiparametric analysis of hypoxia, extracellular pH, vascularization, and metabolism provided a characterization of environments that are permissive for metastasis to occur. Orthotopic tumors, which metastasized more easily, were characterized by higher vascular volume, permeability, and total choline and a more acidic extracellular pH [34]. Interestingly, metastatic deposits in the lymph nodes as well as cancer cells in ascites fluid were found to be hypoxic [34]. Since hypoxic cells are resistant to treatment [90], this may explain, in part, the refractory nature of metastatic disease.
3.2. Imaging distant metastasis and response to treatment
Several PET probes are currently being developed to detect primary tumors as well as metastases. Novel probes with high specificity and sensitivity are necessary to detect small metastatic lesions. Ren et al., have developed an 18F labeled probe, specifically targeting melanin that can be used to image melanoma metastasis [91]. Dialkylamino-alkyl-benzamides possess an affinity for melanin, and were used to specifically target primary tumors and metastatic melanoma lesions in a B16F10 mouse model of melanoma. 18F-FBZA PET imaging revealed the presence of lesions in the lungs [91].
As shown in Fig. 5, metastases detection in pre-clinical studies can be improved by combining several imaging techniques, such as PET, CT and BLI [92]. 18FDG-PET and CT were combined to image lung metastasis in SCID mice 45 days following an intravenous injection of melanoma cells. In another set of experiments, systemic metastases from a reporter gene-expressing melanoma cell line were imaged with BLI and 18F-FHBG (9-[4-18F-fluoro-3-(hydroxymethyl)butyl]guanine) PET/CT after intra-ventricular injection. 18F-FHBG, which is phosphorylated by the reporter gene product, was used as reporter probe to localize the metastases. BLI was acquired from the same mice; since cells expressed luciferase the same lesions were visible, but without information on depth [92].
Fig. 5.
Additional value of CT in 18F-FHBG PET images of metastasis. (A) Thirty-five days after intraventricular injection of 1.5 × 106 A375M-3F melanoma cells in nude mouse, 18F-FHBG PET/CT allows precise anatomic localization of metastasis in interscapular fat (a), right eye (b), right humeral head (c), and left mandibula (d) as shown by green arrows. Lack of anatomic landmarks on PET alone is illustrated by white arrows. (B) BLI shows same lesions as seen on 18F-FHBG PET/CT but does not provide information on depth of lesion. (C) Ex vivo thymidine kinase and luciferase assays of lesions (1) and contralateral controls (2) validate imaging observations. Reprinted with permission from [92].
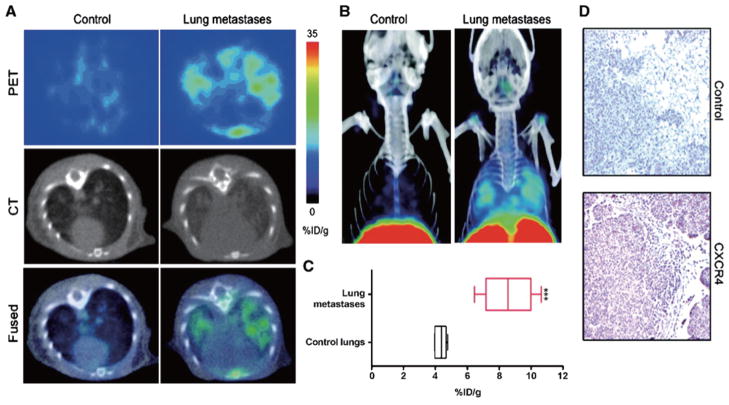
As mentioned earlier, the chemokine CXCL12 (also known as SDF-1) and its receptor CXCR4 play an important role in metastasis. CXCR4 receptor expression is regulated by hypoxia [93] and by COX-2 [59], and its expression is increased at the sites of metastasis. CXCR4 inhibitors have also been shown to reduce the incidence of metastasis [94]. CXCR4 receptor imaging in preclinical studies has been performed with 125I-labeled antibodies using SPECT imaging. More recently Nimmagadda et al., developed a PET imaging agent using [64Cu]AMD3100, a positron-emitting analog of the stem cell mobilizing agent plerixafor [95], to detect metastasis in a preclinical model of breast cancer (Fig. 6). The noninvasive detection of CXCR4 expression in the primary tumor may also provide an index of the metastatic potential of the lesion.
Fig. 6.
CXCR4 imaging of MDA-MB-231–derived lung metastases with [64Cu]AMD3100. NOD/SCID mice that received either 2 × 106 MDA-MB-231 cells or HBSS received 11 MBq (300 μCi) of [64Cu]AMD3100 at 35 d after inoculation. Whole body images were acquired at 90 min after injection. A. Transaxial PET, CT, and fused sections of lung metastasis and control mice. B. Volume-rendered whole body image showing clear accumulation of radioactivity in the lung metastases. Top slices of the volume-rendered images were cut for clear visualization of lung uptake. C. Box-and-whisker plot of the biodistribution analysis of lungs from mice injected with 740 kBq of [64Cu]AMD3100 at 90 min post-injection. All radioactivity values were converted into %ID/g of tissue and are means ± SEM of four to five animals. D. Representative microscopy images of 10-μm-thick CXCR4-stained and control antibody–stained sections of the lung metastases obtained at × 10 magnification. Significance is indicated by asterisks (*), and the comparative reference are lungs from mice injected with Hanks balanced salt solution. ***P < 0.001. Reprinted with permission from [95].
Clinically, lymph node metastases have been detected by MRI in patients with prostate cancer following an intravenous injection of highly lymphotropic super-paramagnetic particles, that localize in the lymph nodes through interstitial-lymphatic fluid transport [96]. The presence of cancer cells in the lymph nodes results in an abnormal pattern of superparamagnetic particle accumulation that can be detected by MRI [96]. Lymphotropic nanoparticle enhanced MRI (LNMRI) was applied on 26 patients with prostate cancer after radical prostatectomy and was able to identify occult lymph node metastases; positive lymph nodes identified were not enlarged based on size criteria [97]. Diffusion-weighted MRI has also been used to detect metastases. The detection of pelvic lymph nodes was improved, with a sensitivity of 83% and a specificity of 99% by combining size analysis with ADC values [98].
The efficiency of DWI, 11C-methionine PET and bone scintigraphy in detecting bone metastases was compared in a study involving 29 patients. The overall bone metastasis detection rates were 56.5% for PET, 23.1% for bone scintigraphy and 92.3% for DWI, indicating the accuracy of DWI in detecting bone metastases [99].
In another study involving 25 patients with colorectal cancer, 18FDG PET and DWI detection capabilities were compared. With a sensitivity of 80%, a specificity of 76.9% and an accuracy of 78.3%, DWI showed better detection of lymph nodes metastases, but 18FDG PET was superior for the detection of primary tumors [100].
A high level of total choline has been observed in an enlarged lymph node of a patient with recurrent prostate cancer by 1H MRSI at 3T using a matrix of external surface coils. Histopathologic analysis confirmed the presence of cancer cells in the lymph node. Noninvasive 1H MRSI may be used to detect prostate cancer cells in enlarged lymph nodes by quantifying the amount of total choline [101] (Fig. 7). PET imaging with 11C-choline is also being evaluated to detect metastatic lesions. Initial studies suggest that 11C-choline PET-CT can be used for early detection of metatases in radical prostatectomy patients [102].
Fig. 7.
A. Axial T2-weighted fast spin-echo image (TR (repetition time)/TE (echo time): 3100/135 milliseconds) at 3 T with combined body and spine matrix coils for localization of the enlarged lymph node (arrows). I, iliac bone; S, sigmoid; V, iliac vessels. B. The phase-corrected 1H-MR spectrum (without baseline correction) obtained from the TE-averaged and combined water and lipid-suppressed single voxel MR spectroscopy measurement. A peak at 4.7 ppm for residual water spins and a peak at about 3.2 ppm assigned to the methyl groups of choline-containing compounds (tCho) were observed. Note the absence of lipid signals from 1 to 2.5 ppm. The coronal reference image from the T1-weighted 3-dimensional gradient-echo sequence (TR/TE: 6.47/2.54 milliseconds) and the axial T2-weighted fast spin-echo image are shown to indicate the voxel placement. C. An axial spectral map of all voxels inside the volume-of-interest from the 1H-MR spectroscopic imaging measurement (TR/TE: 1500/100 milliseconds) in which the tCho resonances are underlined. Outside the lymph node, large lipid signals are present; the tCho signal is only present inside. D. Interpolated coronal and axial tCho color-coded maps constructed using the integral of a Gaussian fit to the tCho signal. Reprinted with permission from [101].
18FDG PET/CT imaging has been used to detect nodal involvement in the thorax of patients with lung cancer with a specificity of 65.6% and an accuracy of 81.7% [103]. When combined with short-τ-inversion-recovery MRI, the capability to detect nodal involvement increased significantly in specificity and accuracy, 96.9% and 90.3% respectively [103].
Ultrasonography has a diagnostic accuracy of 78.8% to detect axillary lymph node metastasis in breast cancer patients. The percentage improved to 91.6% when combined with FDG-PET scan [104]. FDG-PET applied alone presented an accuracy of 76.4%. Staging of axillary lymph node in breast cancer patients with combined ultrasonography and FDG-PET was a sensitive and accurate method.
DWI has also been used to predict effects of selective internal radiotherapy on patients with colorectal hepatic metastases [105]. ADC values and lesion sizes were measured before the treatment, then 2 days and 6 weeks post-treatment. Responding and non-responding status were concluded depending on the decrease of the lesion size at 6 weeks. In the responding lesions, a significant decrease of ADC was observed 2 days post-treatment, which was not observed in non-responding patients [105].
Van Laarhoven et al., applied DCE-MRI and 19F MRS in patients with liver metastasis of colorectal cancer to investigate the predictive values of these MR techniques for tumor response to first-line chemotherapy [106]. The dynamic parameters of dynamic contrast enhanced MRI (DCE-MRI) measured before the treatment did not predict tumor response. 19F MRS was applied to measure the uptake of 5-fluorouracil (5-FU), retention and catabolism. These factors were not limiting for tumor response. A negative correlation was found between 5-FU half-life in liver metastases and the transfer constant of the contrast agent Ktrans measured by DCE-MRI, suggesting that in lesions with high blood flow or permeability, 5-FU was rapidly washed out [106].
Using the criteria that a biomarker is ‘a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacologic responses to a therapeutic “intervention” [107], quantitative imaging biomarkers that could potentially be used in a clinical setting to predict for metastatic risk and the detection of metastases are available, as discussed earlier. For MRI/MRS the potential candidates are DWI parameters, DCE-MRI parameters, total choline, contrast from lymphotropic superparamagnetic particles, and contrast from enzyme activity reporting contrast agents. While optical imaging is not easily translated to the clinic, advances in biopsy-needle compatible fiber optic probes may allow the detection of potential candidates such as proteolytic enzyme activity and SHG detected collagen fiber density. 18Fluorodeoxyglucose and 11C-choline uptake, and CXCR4 receptor expression with radiolabeled agents are potential candidates for PET imaging.
3.3. Theranostic agents for treatment of metastases
Looking towards the future, one key area where molecular and functional imaging will play an important role is in developing ‘theranostic agents’ for metastatic disease. Theranostic imaging, where diagnosis is combined with therapy, is particularly suitable for a disease that is as complex as cancer. Genomic and proteomic profiling can provide an extensive ‘finger-print’ of each tumor that can be used to treat metastases under image-guidance. These theranostic agents can also be used to target single or multiple pathways or networks, by incorporating single or multiple small interfering RNA (siRNA) within a single agent together with a prodrug enzyme that would generate a cytotoxic chemotherapy agent at the site of metastasis [108].
Recent preclinical studies are already exploring theranostic imaging of metastatic cancer. One such strategy using heparin was recently described by Lee et al. [109]. Heparin is a major component of the ECM, and plays a role in anti-coagulation, anti-inflammation, anti-angiogenesis, and anti-tumor cell proliferation [110]. Metastatic cancer cells are characterized by increased expression of heparinase and heparanase that facilitate migration through heparin-degraded ECM [109]. Heparin also induces apoptosis in cells by its interaction with transcription factors. Lee et al., developed heparin-immobilized gold nanoparticles (AuNP-HHep) as a theranostic agent for metastatic cancer cells. These nanoparticles exhibited fluorescence quenching, but once heparin was cleaved fluorescent signals were detected from dyes in the heparin chains. The released heparin induced apoptosis in the cells. Targeted cellular delivery of these nanoparticles was achieved by attaching an RGD peptide that resulted in receptor mediated endocytosis through the ανβ3 integrin receptor, in cells overexpressing this receptor. The delivery of a therapeutic cargo under image-guidance that can be personalized for each patient to minimize normal tissue damage provides substantial promise for the successful treatment of metastatic disease.
4. Conclusion
Several imaging modalities are complementing biochemical and systems biology approaches in defining the metastatic cascade, the leading cause of cancer-related mortality. Despite improvements in the understanding of metastasis several challenges and controversies remain, particularly with respect to the molecular changes that make cancer cells metastasize, as well as with respect to the molecular changes that occur in the premetastatic niche. While advances in optical technologies permit real-time imaging of metastatic cells in the act of colonization of distant organs with great resolution, these studies remain focused on animal models of cancer. Translation of these findings to improve the survival outcome of metastatic disease using clinically translatable imaging techniques such as MRI and nuclear imaging, and their use in theranostic imaging of metastatic disease provides both the vision and hope for solving this challenging problem.
Acknowledgments
Support from P50 CA103175, P30 CA006973, R01 CA73850, R01 CA82337, R01 CA136576, R01 CA138515, R01 CA138264, R21 CA140904 and R21 CA133600 is gratefully acknowledged.
Abbreviations
- ADC
Apparent diffusion coefficient
- BLI
Bioluminescence
- BMDC
Bone marrow derived cells
- CD
Cluster of differentiation
- COX
Cyclooxygenase
- CT
Computer tomography
- CXCR4
cysteine-x-cysteine receptor 4
- DCE-MRI
Dynamic contrast enhanced MRI
- DWI
Diffusion-weighted imaging
- ECM
Extracellular matrix
- EGF
Epidermal growth factor
- EMT
Epithelial–mesenchymal transition
- FDG
Fluorodeoxyglucose
- FRET
Forster resonance energy transfer
- GdDTPA
Gadolinium diethylenetriamine pentaacetic acid
- GLuc
Gaussia luciferase
- HIF
Hypoxia-inducible factor
- HER2
Human epidermal growth factor receptor 2
- LNMRI
Lymphotropic nanoparticles enhanced MRI
- MSCs
Mesenchymal stem cells
- MMP
Matrix metalloproteinase
- MRI
Magnetic resonance imaging
- MRS
Magnetic resonance spectroscopy
- NIR
Near infrared
- PAK1
p21-activated kinase 1
- PET
Positron emission tomography
- PGE2
Prostaglandin E2
- PyMT
Polyoma middle T
- SDF-1
Stromal derived factor-1
- SHG
Second harmonic generation
- Sh/siRNA
Short/small interfering RNA
- tCho
Total choline
- VEGF
Vascular endothelial growth factor
- VEGFR1
Vascular endothelial growth factor receptor1
- 5-FU
5-Fluorouracil
References
- 1.Dorudi S, Hart IR. Mechanisms underlying invasion and metastasis. Curr Opin Oncol. 1993;5:130–135. [PubMed] [Google Scholar]
- 2.Meyer T, Hart IR. Mechanisms of tumour metastasis. Eur J Cancer. 1998;34:214–221. doi: 10.1016/s0959-8049(97)10129-0. [DOI] [PubMed] [Google Scholar]
- 3.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Coghlin C, Murray GI. Current and emerging concepts in tumour metastasis. J Pathol. 2010;222:1–15. doi: 10.1002/path.2727. [DOI] [PubMed] [Google Scholar]
- 5.Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9:302–312. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- 6.Schardt JA, Meyer M, Hartmann CH, Schubert F, Schmidt-Kittler O, Fuhrmann C, Polzer B, Petronio M, Eils R, Klein CA. Genomic analysis of single cytokeratin-positive cells from bone marrow reveals early mutational events in breast cancer. Cancer Cell. 2005;8:227–239. doi: 10.1016/j.ccr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt-Kittler O, Ragg T, Daskalakis A, Granzow M, Ahr A, Blankenstein TJ, Kaufmann M, Diebold J, Arnholdt H, Muller P, Bischoff J, Harich D, Schlimok G, Riethmuller G, Eils R, Klein CA. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci U S A. 2003;100:7737–7742. doi: 10.1073/pnas.1331931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 9.Paget S. Distribution of secondary growths in cancer of the breast. The Lancet. 1989:571–573. [PubMed] [Google Scholar]
- 10.Mendoza M, Khanna C. Revisiting the seed and soil in cancer metastasis. Int J Biochem Cell Biol. 2009;41:1452–1462. doi: 10.1016/j.biocel.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardiff RD. The pathology of EMT in mouse mammary tumorigenesis. J Mammary Gland Biol Neoplasia. 2010;15:225–233. doi: 10.1007/s10911-010-9184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarin D, Thompson EW, Newgreen DF. The fallacy of epithelial mesenchymal transition in neoplasia. Cancer Res. 2005;65:5996–6000. doi: 10.1158/0008-5472.CAN-05-0699. discussion 6000–1. [DOI] [PubMed] [Google Scholar]
- 15.Thompson EW, Newgreen DF, Tarin D. Carcinoma invasion and metastasis: a role for epithelial-mesenchymal transition? Cancer Res. 2005;65:5991–5995. doi: 10.1158/0008-5472.CAN-05-0616. discussion 5995. [DOI] [PubMed] [Google Scholar]
- 16.Fang JS, Gillies RD, Gatenby RA. Adaptation to hypoxia and acidosis in carcinogenesis and tumor progression. Semin Cancer Biol. 2008;18:330–337. doi: 10.1016/j.semcancer.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 2010;29:285–293. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P, Semenza GL. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138–1143. [PubMed] [Google Scholar]
- 19.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 20.Young SD, Marshall RS, Hill RP. Hypoxia induces DNA overreplication and enhances metastatic potential of murine tumor cells. Proc Natl Acad Sci U S A. 1988;85:9533–9537. doi: 10.1073/pnas.85.24.9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14:34–44. [PMC free article] [PubMed] [Google Scholar]
- 22.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 23.Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, Gottschalk AR, Ryan HE, Johnson RS, Jefferson AB, Stokoe D, Giaccia AJ. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14:391–396. [PMC free article] [PubMed] [Google Scholar]
- 24.Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem. 2001;276:9519–9525. doi: 10.1074/jbc.M010144200. [DOI] [PubMed] [Google Scholar]
- 25.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richard DE, Berra E, Gothie E, Roux D, Pouyssegur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J Biol Chem. 1999;274:32631–32637. doi: 10.1074/jbc.274.46.32631. [DOI] [PubMed] [Google Scholar]
- 27.Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997;90:3322–3331. [PubMed] [Google Scholar]
- 28.Duda DG, Jain RK. Premetastatic lung niche: is vascular endothelial growth factor receptor 1 activation required? Cancer Res. 2010;70:5670–5673. doi: 10.1158/0008-5472.CAN-10-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan RN, Rafii S, Lyden D. Preparing the soil: the premetastatic niche. Cancer Res. 2006;66:11089–11093. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, Shipley JM, Senior RM, Shibuya M. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 31.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balic M, Lin H, Young L, Hawes D, Giuliano A, Mc-Namara G, Datar RH, Cote RJ. Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clin Cancer Res. 2006;12:5615–5621. doi: 10.1158/1078-0432.CCR-06-0169. [DOI] [PubMed] [Google Scholar]
- 33.Wicha MS. Cancer stem cells and metastasis: lethal seeds. Clin Cancer Res. 2006;12:5606–5607. doi: 10.1158/1078-0432.CCR-06-1537. [DOI] [PubMed] [Google Scholar]
- 34.Penet MF, Pathak AP, Raman V, Ballesteros P, Artemov D, Bhujwalla ZM. Noninvasive multiparametric imaging of metastasis-permissive microenvironments in a human prostate cancer xenograft. Cancer Res. 2009;69:8822–8829. doi: 10.1158/0008-5472.CAN-09-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oser M, Condeelis J. The cofilin activity cycle in lamellipodia and invadopodia. J Cell Biochem. 2009;108:1252–1262. doi: 10.1002/jcb.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holland JD, Kochetkova M, Akekawatchai C, Dottore M, Lopez A, McColl SR. Differential functional activation of chemokine receptor CXCR4 is mediated by G proteins in breast cancer cells. Cancer Res. 2006;66:4117–4124. doi: 10.1158/0008-5472.CAN-05-1631. [DOI] [PubMed] [Google Scholar]
- 37.Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, Condeelis J. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prag S, Parsons M, Keppler MD, Ameer-Beg SM, Barber P, Hunt J, Beavil AJ, Calvert R, Arpin M, Vojnovic B, Ng T. Activated ezrin promotes cell migration through recruitment of the GEF Dbl to lipid rafts and preferential downstream activation of Cdc42. Mol Biol Cell. 2007;18:2935–2948. doi: 10.1091/mbc.E06-11-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vadlamudi RK, Adam L, Wang RA, Mandal M, Nguyen D, Sahin A, Chernoff J, Hung MC, Kumar R. Regulatable expression of p21-activated kinase-1 promotes anchorage-independent growth and abnormal organization of mitotic spindles in human epithelial breast cancer cells. J Biol Chem. 2000;275:36238–36244. doi: 10.1074/jbc.M002138200. [DOI] [PubMed] [Google Scholar]
- 40.Carr I, Orr FW. Invasion and metastasis. Can Med Assoc J. 1983;128:1164–1167. [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss L, Ward PM. Cell detachment and metastasis. Cancer Metastasis Rev. 1983;2:111–127. doi: 10.1007/BF00048965. [DOI] [PubMed] [Google Scholar]
- 42.Lochter A, Bissell MJ. Involvement of extracellular matrix constituents in breast cancer. Semin Cancer Biol. 1995;6:165–173. doi: 10.1006/scbi.1995.0017. [DOI] [PubMed] [Google Scholar]
- 43.Skrzydlewska E, Sulkowska M, Koda M, Sulkowski S. Proteolytic-antiproteolytic balance and its regulation in carcinogenesis. World J Gastroenterol. 2005;11:1251–1266. doi: 10.3748/wjg.v11.i9.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song F, Wisithphrom K, Zhou J, Windsor LJ. Matrix metalloproteinase dependent and independent collagen degradation. Front Biosci. 2006;11:3100–3120. doi: 10.2741/2036. [DOI] [PubMed] [Google Scholar]
- 45.Zheng WQ, Looi LM, Cheah PL. Correlation between laminin and cathepsin D expressions in breast carcinoma. Tumori. 2002;88:296–299. doi: 10.1177/030089160208800411. [DOI] [PubMed] [Google Scholar]
- 46.Guinec N, Dalet-Fumeron V, Pagano M. In vitro study of basement membrane degradation by the cysteine proteinases, cathepsins B, B-like and L. Digestion of collagen IV, laminin, fibronectin, and release of gelatinase activities from basement membrane fibronectin. Biol Chem Hoppe Seyler. 1993;374:1135–1146. doi: 10.1515/bchm3.1993.374.7-12.1135. [DOI] [PubMed] [Google Scholar]
- 47.Li C, Greenwood TR, Glunde K. Glucosamine-bound near-infrared fluorescent probes with lysosomal specificity for breast tumor imaging. Neoplasia. 2008;10:389–398. doi: 10.1593/neo.07856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glunde K, Guggino SE, Solaiyappan M, Pathak AP, Ichikawa Y, Bhujwalla ZM. Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia. 2003;5:533–545. doi: 10.1016/s1476-5586(03)80037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 50.McIntyre JO, Fingleton B, Wells KS, Piston DW, Lynch CC, Gautam S, Matrisian LM. Development of a novel fluorogenic proteolytic beacon for in vivo detection and imaging of tumour-associated matrix metalloproteinase-7 activity. Biochem J. 2004;377:617–628. doi: 10.1042/BJ20030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scherer RL, McIntyre JO, Matrisian LM. Imaging matrix metalloproteinases in cancer. Cancer Metastasis Rev. 2008;27:679–690. doi: 10.1007/s10555-008-9152-9. [DOI] [PubMed] [Google Scholar]
- 52.Udabage L, Brownlee GR, Waltham M, Blick T, Walker EC, Heldin P, Nilsson SK, Thompson EW, Brown TJ. Antisense-mediated suppression of hyaluronan synthase 2 inhibits the tumorigenesis and progression of breast cancer. Cancer Res. 2005;65:6139–6150. doi: 10.1158/0008-5472.CAN-04-1622. [DOI] [PubMed] [Google Scholar]
- 53.Shiftan L, Israely T, Cohen M, Frydman V, Dafni H, Stern R, Neeman M. Magnetic resonance imaging visualization of hyaluronidase in ovarian carcinoma. Cancer Res. 2005;65:10316–10323. doi: 10.1158/0008-5472.CAN-04-3947. [DOI] [PubMed] [Google Scholar]
- 54.Gehlsen KR, Wagner HN, Jr, Hendrix MJ. Membrane invasion culture system (MICS) Med Instrum. 1984;18:268–271. [PubMed] [Google Scholar]
- 55.Pilatus U, Ackerstaff E, Artemov D, Mori N, Gillies RJ, Bhujwalla ZM. Imaging prostate cancer invasion with multi-nuclear magnetic resonance methods: the Metabolic Boyden Chamber. Neoplasia. 2000;2:273–279. doi: 10.1038/sj.neo.7900089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ackerstaff E, Gimi B, Artemov D, Bhujwalla ZM. Anti-inflammatory agent indomethacin reduces invasion and alters metabolism in a human breast cancer cell line. Neoplasia. 2007;9:222–235. doi: 10.1593/neo.06673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–182. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 58.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, Kaidi A. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis. 2009;30:377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 59.Stasinopoulos I, Mori N, Bhujwalla ZM. The malignant phenotype of breast cancer cells is reduced by COX-2 silencing. Neoplasia. 2008;10:1163–1169. doi: 10.1593/neo.08568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stasinopoulos I, O’Brien DR, Wildes F, Glunde K, Bhujwalla ZM. Silencing of cyclooxygenase-2 inhibits metastasis and delays tumor onset of poorly differentiated metastatic breast cancer cells. Mol Cancer Res. 2007;5:435–442. doi: 10.1158/1541-7786.MCR-07-0010. [DOI] [PubMed] [Google Scholar]
- 61.Bernas LM, Foster PJ, Rutt BK. Magnetic resonance imaging of in vitro glioma cell invasion. J Neurosurg. 2007;106:306–313. doi: 10.3171/jns.2007.106.2.306. [DOI] [PubMed] [Google Scholar]
- 62.Mikhaylova M, Mori N, Wildes FB, Walczak P, Gimi B, Bhujwalla ZM. Hypoxia increases breast cancer cell-induced lymphatic endothelial cell migration. Neoplasia. 2008;10:380–389. doi: 10.1593/neo.07854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gimi B, Mori N, Ackerstaff E, Frost EE, Bulte JW, Bhujwalla ZM. Noninvasive MRI of endothelial cell response to human breast cancer cells. Neoplasia. 2006;8:207–213. doi: 10.1593/neo.05547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol. 2003;21:1369–1377. doi: 10.1038/nbt899. [DOI] [PubMed] [Google Scholar]
- 65.Brown E, McKee T, diTomaso E, Pluen A, Seed B, Boucher Y, Jain RK. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 66.McKee TD, Grandi P, Mok W, Alexandrakis G, Insin N, Zimmer JP, Bawendi MG, Boucher Y, Breakefield XO, Jain RK. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66:2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 67.Provenzano PP, Eliceiri KW, Campbell JM, Inman DR, White JG, Keely PJ. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006;4(38) doi: 10.1186/1741-7015-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 69.Kienast Y, von Baumgarten L, Fuhrmann M, Klinkert WE, Goldbrunner R, Herms J, Winkler F. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16:116–22. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 70.Perentes JY, McKee TD, Ley CD, Mathiew H, Dawson M, Padera TP, Munn LL, Jain RK, Boucher Y. In vivo imaging of extracellular matrix remodeling by tumor-associated fibroblasts. Nat Methods. 2009;6:143–145. doi: 10.1038/nmeth.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kedrin D, Gligorijevic B, Wyckoff J, Verkhusha VV, Condeelis J, Segall JE, van Rheenen J. Intravital imaging of metastatic behavior through a mammary imaging window. Nat Methods. 2008;5:1019–1021. doi: 10.1038/nmeth.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang YC, Ye JY, Thomas TP, Cao Z, Kotlyar A, Tkaczyk ER, Baker JR, Jr, Norris TB. Fiber-optic multi-photon flow cytometry in whole blood and in vivo. J Biomed Opt. 2010;15:047004. doi: 10.1117/1.3463481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang W, Wyckoff JB, Goswami S, Wang Y, Sidani M, Segall JE, Condeelis JS. Coordinated regulation of pathways for enhanced cell motility and chemotaxis is conserved in rat and mouse mammary tumors. Cancer Res. 2007;67:3505–3511. doi: 10.1158/0008-5472.CAN-06-3714. [DOI] [PubMed] [Google Scholar]
- 74.Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng. 2002;4:235–260. doi: 10.1146/annurev.bioeng.4.111901.093336. [DOI] [PubMed] [Google Scholar]
- 75.Luker GD, Luker KE. Optical imaging: current applications and future directions. J Nucl Med. 2008;49:1–4. doi: 10.2967/jnumed.107.045799. [DOI] [PubMed] [Google Scholar]
- 76.Sun H, Pisle S, Gardner ER, Figg WD. Bioluminescent imaging study: FAK inhibitor, PF-562,271, preclinical study in PC3M-luc-C6 local implant and metastasis xenograft models. Cancer Biol Ther. 2010;10:38–43. doi: 10.4161/cbt.10.1.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wurdinger T, Badr C, Pike L, de Kleine R, Weissleder R, Breakefield XO, Tannous BA. A secreted luciferase for ex vivo monitoring of in vivo processes. Nat Methods. 2008;5:171–173. doi: 10.1038/nmeth.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chung E, Yamashita H, Au P, Tannous BA, Fukumura D, Jain RK. Secreted Gaussia luciferase as a biomarker for monitoring tumor progression and treatment response of systemic metastases. PLoS One. 2009;4:e8316. doi: 10.1371/journal.pone.0008316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dothager RS, Flentie K, Moss B, Pan MH, Kesarwala A, Piwnica-Worms D. Advances in bioluminescence imaging of live animal models. Curr Opin Biotechnol. 2009;20:45–53. doi: 10.1016/j.copbio.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heyn C, Ronald JA, Ramadan SS, Snir JA, Barry AM, MacKenzie LT, Mikulis DJ, Palmieri D, Bronder JL, Steeg PS, Yoneda T, MacDonald IC, Chambers AF, Rutt BK, Foster PJ. In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med. 2006;56:1001–1010. doi: 10.1002/mrm.21029. [DOI] [PubMed] [Google Scholar]
- 81.Gupta GP, Nguyen DX, Chiang AC, Bos PD, Kim JY, Nadal C, Gomis RR, Manova-Todorova K, Massague J. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446:765–770. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 82.Zhang XH, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, Foekens JA, Massague J. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dietzel M, Baltzer PA, Vag T, Groschel T, Gajda M, Camara O, Kaiser WA. Application of breast MRI for prediction of lymph node metastases-systematic approach using 17 individual descriptors and a dedicated decision tree. Acta Radiol. 2010;51:885–894. doi: 10.3109/02841851.2010.504232. [DOI] [PubMed] [Google Scholar]
- 84.deSouza NM, Riches SF, Vanas NJ, Morgan VA, Ashley SA, Fisher C, Payne GS, Parker C. Diffusion-weighted magnetic resonance imaging: a potential non-invasive marker of tumour aggressiveness in localized prostate cancer. Clin Radiol. 2008;63:774–782. doi: 10.1016/j.crad.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 85.Tse GM, Yeung DK, King AD, Cheung HS, Yang WT. In vivo proton magnetic resonance spectroscopy of breast lesions: an update. Breast Cancer Res Treat. 2007;104:249–255. doi: 10.1007/s10549-006-9412-8. [DOI] [PubMed] [Google Scholar]
- 86.Katz-Brull R, Degani H. Kinetics of choline transport and phosphorylation in human breast cancer cells; NMR application of the zero trans method. Anticancer Res. 1996;16:1375–1380. [PubMed] [Google Scholar]
- 87.Ramirez de Molina A, Gutierrez R, Ramos MA, Silva JM, Silva J, Bonilla F, Sanchez JJ, Lacal JC. Increased choline kinase activity in human breast carcinomas: clinical evidence for a potential novel antitumor strategy. Oncogene. 2002;21:4317–4322. doi: 10.1038/sj.onc.1205556. [DOI] [PubMed] [Google Scholar]
- 88.Iorio E, Ricci A, Bagnoli M, Pisanu ME, Castellano G, Di Vito M, Venturini E, Glunde K, Bhujwalla ZM, Mezzanzanica D, Canevari S, Podo F. Activation of phosphatidylcholine cycle enzymes in human epithelial ovarian cancer cells. Cancer Res. 2010;70:2126–2135. doi: 10.1158/0008-5472.CAN-09-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nagarajan R, Gomez AM, Raman SS, Margolis DJ, McClure T, Thomas MA. Correlation of endorectal 2D JPRESS findings with pathological Gleason scores in prostate cancer patients. NMR Biomed. 2010;23:257–261. doi: 10.1002/nbm.1446. [DOI] [PubMed] [Google Scholar]
- 90.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8:425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ren G, Miao Z, Liu H, Jiang L, Limpa-Amara N, Mahmood A, Gambhir SS, Cheng Z. Melanin-targeted preclinical PET imaging of melanoma metastasis. J Nucl Med. 2009;50:1692–1699. doi: 10.2967/jnumed.109.066175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deroose CM, De A, Loening AM, Chow PL, Ray P, Chatziioannou AF, Gambhir SS. Multimodality imaging of tumor xenografts and metastases in mice with combined small-animal PET, small-animal CT, and bioluminescence imaging. J Nucl Med. 2007;48:295–303. [PMC free article] [PubMed] [Google Scholar]
- 93.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rose AA, Siegel PM. Emerging therapeutic targets in breast cancer bone metastasis. Future Oncol. 2010;6:55–74. doi: 10.2217/fon.09.138. [DOI] [PubMed] [Google Scholar]
- 95.Nimmagadda S, Pullambhatla M, Stone K, Green G, Bhujwalla ZM, Pomper MG. Molecular imaging of CX-CR4 receptor expression in human cancer xenografts with [64Cu]AMD3100 positron emission tomography. Cancer Res. 2010;70:3935–3944. doi: 10.1158/0008-5472.CAN-09-4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348:2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- 97.Ross RW, Zietman AL, Xie W, Coen JJ, Dahl DM, Shipley WU, Kaufman DS, Islam T, Guimaraes AR, Weissleder R, Harisinghani M. Lymphotropic nanoparticle-enhanced magnetic resonance imaging (LNMRI) identifies occult lymph node metastases in prostate cancer patients prior to salvage radiation therapy. Clin Imaging. 2009;33:301–305. doi: 10.1016/j.clinimag.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 98.Lin G, Ho KC, Wang JJ, Ng KK, Wai YY, Chen YT, Chang CJ, Ng SH, Lai CH, Yen TC. Detection of lymph node metastasis in cervical and uterine cancers by diffusion-weighted magnetic resonance imaging at 3T. J Magn Reson Imaging. 2008;28:128–135. doi: 10.1002/jmri.21412. [DOI] [PubMed] [Google Scholar]
- 99.Goudarzi B, Kishimoto R, Komatsu S, Ishikawa H, Yoshikawa K, Kandatsu S, Obata T. Detection of bone metastases using diffusion weighted magnetic resonance imaging: comparison with (11)C-methionine PET and bone scintigraphy. Magn Reson Imaging. 2010;28:372–379. doi: 10.1016/j.mri.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 100.Ono K, Ochiai R, Yoshida T, Kitagawa M, Omagari J, Kobayashi H, Yamashita Y. Comparison of diffusion-weighted MRI and 2-[fluorine-18]-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) for detecting primary colorectal cancer and regional lymph node metastases. J Magn Reson Imaging. 2009;29:336–340. doi: 10.1002/jmri.21638. [DOI] [PubMed] [Google Scholar]
- 101.Heijmink SW, Scheenen TW, Futterer JJ, Klomp DW, Heesakkers RA, Hulsbergenvan de Kaa CA, van Lin EN, Heerschap A, Barentsz JO. Prostate and lymph node proton magnetic resonance (MR) spectroscopic imaging with external array coils at 3 T to detect recurrent prostate cancer after radiation therapy. Invest Radiol. 2007;42:420–427. doi: 10.1097/01.rli.0000262759.46364.50. [DOI] [PubMed] [Google Scholar]
- 102.Castellucci P, Fuccio C, Rubello D, Schiavina R, Santi I, Nanni C, Allegri V, Montini GC, Ambrosini V, Boschi S, Martorana G, Marzola MC, Fanti S. Is there a role for (11)C-choline PET/CT in the early detection of metastatic disease in surgically treated prostate cancer patients with a mild PSA increase < 1.5 ng/ml? Eur J Nucl Med Mol Imaging. 2011;38:55–63. doi: 10.1007/s00259-010-1604-0. [DOI] [PubMed] [Google Scholar]
- 103.Morikawa M, Demura Y, Ishizaki T, Ameshima S, Miyamori I, Sasaki M, Tsuchida T, Kimura H, Fujibayashi Y, Okazawa H. The effectiveness of 18F-FDG PET/CT combined with STIR MRI for diagnosing nodal involvement in the thorax. J Nucl Med. 2009;50:81–87. doi: 10.2967/jnumed.108.056408. [DOI] [PubMed] [Google Scholar]
- 104.Ahn JH, Son EJ, Kim JA, Youk JH, Kim EK, Kwak JY, Ryu YH, Jeong J. The role of ultrasonography and FDG-PET in axillary lymph node staging of breast cancer. Acta Radiol. 2010;51:859–865. doi: 10.3109/02841851.2010.501342. [DOI] [PubMed] [Google Scholar]
- 105.Dudeck O, Zeile M, Wybranski C, Schulmeister A, Fischbach F, Pech M, Wieners G, Ruhl R, Grosser O, Amthauer H, Ricke J. Early prediction of anticancer effects with diffusion-weighted MR imaging in patients with colorectal liver metastases following selective internal radio-therapy. Eur Radiol. 2010;20:2699–2706. doi: 10.1007/s00330-010-1846-z. [DOI] [PubMed] [Google Scholar]
- 106.van Laarhoven HW, Klomp DW, Rijpkema M, Kamm YL, Wagener DJ, Barentsz JO, Punt CJ, Heerschap A. Prediction of chemotherapeutic response of colorectal liver metastases with dynamic gadolinium-DTPA-enhanced MRI and localized 19F MRS pharmacokinetic studies of 5-fluorouracil. NMR Biomed. 2007;20:128–140. doi: 10.1002/nbm.1098. [DOI] [PubMed] [Google Scholar]
- 107.Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89–95. doi: 10.1067/mcp.2001.113989. [DOI] [PubMed] [Google Scholar]
- 108.Li C, Penet MF, Wildes F, Takagi T, Chen Z, Winnard PT, Artemov D, Bhujwalla ZM. Nanoplex Delivery of siRNA and Prodrug Enzyme for Multimodality Image-Guided Molecular Pathway Targeted Cancer Therapy. ACS Nano. 2010;4:6707–6716. doi: 10.1021/nn102187v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee K, Lee H, Bae KH, Park TG. Heparin immobilized gold nanoparticles for targeted detection and apoptotic death of metastatic cancer cells. Biomaterials. 2010;31:6530–6536. doi: 10.1016/j.biomaterials.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 110.Robert F. The potential benefits of low-molecular-weight heparins in cancer patients. J Hematol Oncol. 2010;3:3. doi: 10.1186/1756-8722-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]