Abstract
SR proteins are essential splicing factors whose phosphorylation by the SRPK family of protein kinases regulates nuclear localization and mRNA processing activity. In addition to an N-terminal extension with unknown function, SRPKs contain a large, non-homologous spacer insert domain that bifurcates the kinase domain and anchors the kinase in the cytoplasm through interactions with chaperones. While structures for the kinase domain are now available, constructs that include regions outside this domain have been resistant to crystallographic elucidation. To investigate the conformation of the full-length kinase and the functional role of noncatalytic regions, hydrogen-deuterium exchange and steady-state kinetic experiments were performed on SRPK1. Unlike the kinase core, the large spacer insert domain lacks stable, hydrogen-bonded structure and may provide an intrinsically disordered region for chaperone interactions. Conversely, the N-terminus, which positively regulates SR protein binding, adopts stable structure when the insert domain is present and stabilizes a docking groove in the large lobe of the kinase domain. The N-terminus and spacer insert domain equally enhance SR protein turnover by altering the stability of several catalytic loop segments. These studies reveal that SRPK1 uses an N-terminal extension and a large, intrinsically disordered region juxtaposed to stable structure to facilitate high affinity SR protein interactions and phosphorylation rates.
Keywords: kinetics, hydrogen-deuterium exchange, phosphorylation, splicing, SR protein
RNA splicing is an essential posttranscriptional modification that increases proteome diversity and regulates cell development and growth. The transesterification reactions involved in the removal of introns from precursor mRNA (pre-mRNA) occur at the spliceosome, a macromolecular assembly of several snRNPs and over 100 polypeptides.1 With regard to the latter group, the SR proteins¶ are recognized as an essential family of splicing factors that play a role in spliceosome development and many additional post-splicing events.2 The SR proteins are composed of one or two RNA recognition motifs (RRMs) that bind short stretches of pre-mRNA known as exonic splicing enhancers and help establish the 5′ and 3′ splice sites. SR proteins derive their name from a C-terminal domain that can vary in length from 50 to over 300 residues and is composed of numerous arginine-serine dipeptide repeats (RS domains). The RS domains are polyphosphorylated by two important protein kinase families- SRPKs and CLKs.3 The SRPKs provide a basal level of phosphorylation that permits interaction with transportin SR and localization of the SR proteins in nuclear speckles.4; 5 The CLK family then further phosphorylates the RS domains leading to a more widespread nuclear distribution of the SR proteins.6; 7 Beyond controlling the subcellular localization of the SR proteins and their proximity to the spliceosome, there is some evidence that RS domain phosphorylation could play a role in the selection of alternative splice sites. Splice variants of the caspase-9, Bcl-x and PKCβ genes have been shown to correlate with the phosphorylation status of SR proteins.8; 9
Given the essential role of RS domain phosphorylation for cytoplasmic-nuclear partitioning, investigations into the mechanism of SRPK-dependent phosphorylation are important for understanding how SR proteins ultimately engage the spliceosome and impact alternative gene splicing. Using protease footprinting techniques, we showed previously that SRPK1 rapidly phosphorylates about 10-12 serines in the N-terminal portion of the RS domain (RS1) of the prototype SR protein SRSF1.7; 10 By following the reaction progress, we showed that the phosphates are placed in a highly sequential manner with SRPK1 moving in a preferred C-to-N-terminal direction along the RS domain.11 Crystallographic studies indicate that this reaction is facilitated by a docking groove in the large lobe of the kinase domain that initially binds the N-terminal portion of RS1 allowing the C-terminal region access to the active site for initial phosphorylation.12 To process the lengthy RS1 segment, the enzyme-substrate complex undergoes a shift whereby the RS sequence, originally in the docking groove, slides along a channel into the active site and secondary structure from RRM2 (β4) unfolds and occupies the docking groove.12 While an electronegatively charged docking groove and channel bind the long Arg-Ser repeats, a small electropositive pocket (P+2 pocket) outside the active site stabilizes the growing phosphorylated RS domain as it is expelled from the active site.13 Although the RRMs in SRSF1 are not essential for initial binding affinity, they play an important role in later phosphorylation steps maintaining a processive interplay between enzyme and substrate.13
Our understanding of the structure-function relationship governing SRPK1 regulation is currently incomplete since approximately 40% of the primary structure of the kinase is not present in any of the X-ray models.3 Specifically, the current structure of SRPK1 lacks most of the N-terminus (70 aa), and a large spacer insert domain (SID, approx. 250 aa) that bifurcates the small, N-terminal and large, C-terminal lobes of the kinase (Fig. 1A). We showed previously that the SID interacts with co-chaperones (e.g., Aha1) sequestering the kinase in the cytoplasm. Removal of the SID or detachment of the chaperone complex through osmotic stress causes SRPK1 to increase its presence in the nucleus, hyper-phosphorylate SR proteins and change the alternative splicing pattern of the E1a splicing reporter.14; 15 At this time it is unclear whether the SID adopts any regular folded structure and how chaperones engage this large segment for cytoplasmic localization of the kinase. Less is known about the N-terminal extension, although recent studies show that it is essential for induced binding of one of the RRMs of SRSF1 (RRM2) upon engagement of the docking groove with an Arg-Ser peptide.16 While most protein kinases interact weakly with their substrates,17 SRPK1 forms a very stable, high affinity complex with SRSF1 (Kd ~20-50 nM).15; 18 The formation of the tight enzyme:substrate complex is likely important for sequential phosphorylation, which is possibly mediated by the presence of the N-terminus.
Figure 1. Effects of N-terminus & SID on Phosphorylation of SRSF1.
A) SRPK1 and SRSF1 Constructs. B) Steady-State Kinetics. Initial velocities are normalized to Vmax and plotted as a function of total SRSF1 for SRPK1 (●), SRPK1(ΔN) (■), and SRPK1(ΔS) (▲). Kinetic parameters derived from hyperbolic fitting are displayed in Table 1. C) Competition experiments. Relative initial velocities for the phosphorylation of 50 nM SR(ΔRRM2) is monitored as a function of increasing concentrations of SRSF1 for SRPK1, SRPK1(ΔN), and SRPK1(ΔS). Proteins are labeled as in panel B and KI values are displayed in Table 1. D) Single turnover experiments. SRSF1 (100 nM) is preequilbrated with 2 μM of SRPK1, SRPK1(ΔN), and SRPK1(ΔS) and the reaction is started with 32P-ATP. Proteins are labeled as in panel B. The progress curves for SRPK1 are fit to a double exponential with rate constants and amplitudes of 6 and 0.2 min−1 and 12 and 3 sites. The progress curves for SRPK1(ΔN) and SRPK1(ΔS) are fit to single exponential functions with a common rate constant of 0.5 min−1 and amplitudes of 14 and 13 sites. E) Directionality Experiments. Complexes of enzymes (2 mM) and clASF(214) (200 nM) are allowed to react with varying concentrations of 32P-ATP and then cleaved with LysC to obtain N- and C-terminal fragments on SDS-PAGE. Relative 32P in both bands (N/C) are plotted as a function of total phosphoryl content. Symbols are the same as in panels B-D.
To investigate the structure of the full-length enzyme, we studied the catalytic and conformational roles of the N-terminus and SID of SRPK1. While both segments equally augment phosphorylation turnover rates, the N-terminus specifically enhances SRSF1 binding. Using hydrogen-deuterium (H-D) exchange methods, we demonstrate that while the SID is largely flexible and lacks significant protection, the N-terminus possesses regions with intermediate-to-high protection consistent with predicted areas of secondary structure. Although both segments stabilize regions important for the phosphoryl transfer reaction (the glycine-rich and activation loops), the N-terminus stabilizes the docking groove in the large lobe of the kinase domain. The latter may explain why deletion of the N-terminus reduces SR protein binding affinity. In contrast, the H-D exchange data indicate that the presence of the SID destabilizes helix αC in the N-terminal lobe and stabilizes the N-terminus. These studies demonstrate that SRPK1 uses a combination of structured and intrinsically disordered regions for maintaining efficient SR protein binding and phosphorylation.
Results
Regions Outside the Kinase Core Impact SRSF1 Phosphorylation Efficiency
The present X-ray structures consist of a truncated version of SRPK1 that contains only the kinase core and about 15 and 50 residues from the N-terminus and SID.12 To determine how regions outside the kinase core impact catalysis, we performed steady-state kinetic assays on two forms of SRPK1 lacking either the full-length N-terminus [SRPK1(ΔN)] or the SID [SRPK1(ΔS)] (Fig.1A). Removal of either region in SRPK1 lowers turnover (kcat) equivalently by about 5-fold (Table 1). These effects are not likely to be the result of greater instability upon deletion since we found that SRPK1, SRPK1(ΔN) and SRPK1(ΔS) show similar thermal stabilities (Supplementary Fig.S1). Whereas the insert had no effect on Km,SR (Km for SRSF1), SRPK1(ΔN) displays a 3-fold higher Km,SR suggesting that the N-terminus may play a role in SR protein recognition (Fig.1B & Table 1). To determine whether either region impacts nucleotide binding, Km,ATP (Km for ATP) was measured and found to be increased by about 2-5 fold by N-terminal and SID deletion (Table 1). Overall, we found that while the insert enhances catalysis by about 5-fold based on kcat/Km,SR, the N-terminus increases catalysis by about 20-fold making this polypeptide segment critical for SR protein recognition.
Table 1.
Kinetic Effects of SRPK1 Deletions on SRSF1 Phosphorylation
| Parameter | Substrate | SRPK1 | SRPK1(ΔN) | SRPK1(ΔS) |
|---|---|---|---|---|
| kcat (min−1) | SRSF1 | 30 ± 2 | 6.5 ± 0.9 | 4.4 ± 0.4 |
| Km,SR (nM) | SRSF1 | 120 ± 20 | 630 ± 70 | 120 ± 20 |
| Km,ATP (μM) | SRSF1 | 5.0 ± 0.5 | 9 ± 2 | 28 ± 7 |
| KI,SR (nM) | SRSF1 | 130 ± 10 | 320 ± 30 | 110 ± 20 |
| kcat (min−1) | RS Domain | 12 ± 2 | 5.2 ± 0.4 | 11 ± 1 |
| Km,RS (nM) | RS Domain | 25 ± 10 | 60 ± 20 | 21 ± 10 |
| KI,RS (nM) | RS Domain | 150 ± 70 | 830 ± 180 | 140 ± 30 |
Since SRPK1(ΔN) displays an elevated Km,SR, it is possible that the N-terminus of the kinase modulates SR protein binding affinity. To evaluate this possibility, we used a competition assay to measure the dissociation constant of SRSF1 to several forms of SRPK1.13 In this experiment, the initial velocity for the phosphorylation of a fixed concentration of a truncated form of SRSF1 [SR(ΔRRM2)] is monitored as a function of varying wt-SRSF1 concentrations using catalytic amounts of SRPK1, SRPK1(ΔN), and SRPK1(ΔS). Since SR(ΔRRM2) is smaller than wt-SRSF1, its phosphorylation can be measured independently using SDS-PAGE and 32P-autoradiography. Previous studies revealed that wt-SRSF1 and its truncated forms containing the RS domain bind in the same site on SRPK1 so that these substrates can be treated as competitive inhibitors of each other.13 Decreases in relative velocity for SR(ΔRRM2) as a function of wt-SRSF1 are observed using all three enzymes (Fig.1C) and the data are fit to equation (1) to obtain KI values (Table 1). While removal of the SID has no effect on the KI for wt-SRSF1, removal of the N-terminus decreases SR protein binding affinity by about 2-3-fold. Since the KI for a competitive inhibitor is the same as its dissociation constant (Kd), these data indicate that the observed changes in Km correspond to real changes in the affinity of SRSF1.
Kinase Core Is Sufficient to Guide Directional, Multi-Site Phosphorylation
Although the N-terminus and SID play a role in binding and catalysis based on steady-state kinetic analyses, these methods measure initial velocities and early phosphate incorporation whereas SRPK1 is capable of extensive, multi-site phosphorylation of SRSF1.10 To understand whether noncatalytic regions in SRPK1 support this reaction, we performed single turnover experiments and analyzed the full progress curves for SRPK1, SRPK1(ΔN) and SRPK1(ΔS) using a low, fixed concentration of SRSF1. For the wild-type kinase, the complete phosphorylation of SRSF1 occurs in two kinetic phases: a fast phase that is complete in about 1-2 minutes and a slower phase that is typically complete in 20-30 minutes. Both SRPK1(ΔN) and SRPK1(ΔS) lack this fast phase (Fig.1D). The lower initial phosphorylation rate constants for these deletion mutants are consistent with their lower turnovers in the steady-state kinetic assays (Table 1). Despite these changes in catalytic efficiency, SRPK1(ΔN) phosphorylates SRSF1 to the same extent as SRPK1 (approx. 15 sites) whereas SRPK1(ΔS) appears to achieve a slightly lower phosphoryl content (approx 13 sites). Overall, although removal of the N-terminus and SID negatively affects the efficiency of phosphorylation, neither segment significantly alters multi-site phosphorylation.
Since the presence of the N-terminus and SID enhance the rate of polyphosphorylation, we wished to determine whether either noncatalytic polypeptide also affects phosphorylation patterns. We showed previously that SRPK1 rapidly phosphorylates about 10-12 serines in RS1 in a strict C-to-N-terminal direction and then slowly modifies about 3 additional serines in RS2 (Fig.1E). To evaluate whether regions outside the kinase core can alter this directional mechanism we monitored the phosphoryl content of a cleavable form of SRSF1 [clASF(214)] as a function of phosphorylation progress using ATP limitation experiments.10; 11 This substrate contains several Lys-to-Arg mutations in RRM2 (RRM2*) and a critical Arg-to-Lys mutation in the middle of RS1 so that upon proteolysis with LysC, two fragments of different molecular weights can be separated by SDS-PAGE that correspond to the N- and C-terminal halves of RS1, the principal region for SRPK1 modifications (Fig.1E). By performing these experiments using excess enzyme and varying, limiting amounts of 32P-ATP, a broad range of phosphorylation levels can be attained at equilibrium.11 Upon treatment of the reactions with LysC, the relative incorporation of 32P into both N- and C-terminal fragments can be assessed by autoradiography. Using this procedure, we found that like the wild-type kinase, SRPK1(ΔN) and SRPK1(ΔS) preferred to phosphorylate C-terminal rather than N-terminal serines early in the reaction, in line with a directional phosphorylation mechanism (Fig.1E). Thus, single turnover kinetic and proteolysis experiments indicate that the propensity for directional, multi-site phosphorylation is not dependent on either the N-terminus or SID but rather is an innate property of the kinase core.
SRPK1 N-terminus Enhances the Binding Affinity of the RS Domain
Based on crystallographic data, SRPK1 makes contact with RS1 from the RS domain and RRM2 (RS2 & RRM1 are not in co-crystal) in SRSF1.12 To determine whether the effects of the SRPK1 N-terminus on SR protein binding emanate from changes in interaction of the kinase with the RS domain, we studied the binding of this domain. Previous studies have shown that in single turnover experiments, SRPK1 can phosphorylate the RS domain in the absence of RRMs to the same extent (15 sites) as the full-length SRSF1.13 We now show that kcat and Km,RS (Km for the RS domain) are about 2- and 5-fold lower than those for SRSF1 using wild-type SRPK1 suggesting that the RRMs may play some role in the phosphorylation mechanism under steady-state control (Table 1). This observation is also supported by the effects of deletion upon the kinetic parameters for the RS domain. While both the N-terminus and SID are important for SRSF1 turnover, only the N-terminus appears to play a role in RS domain turnover (Table 1). Also, while the SID does not influence apparent RS domain affinity, the effects of N-terminal deletion on Km,RS is less significant compared to the effects on SRSF1. However, since the Km values may not reflect intrinsic binding affinities, we used the competition assay to measure directly the Kd’s for the RS domain to wild-type and mutant enzymes. Similar to the effects on SRSF1 binding, deletion of the SID has no effect on RS domain binding affinity while the deletion of the N-terminus reduces affinity by about 5-fold (Table 1). These findings indicate that the N-terminus regulates SRSF1 binding affinity largely by modulating interactions with the RS domain.
Since the RS domain is large (50 aa; Fig.1A) and may contact several regions in SRPK1 that lie outside the active site and docking groove, we investigated the direct binding of two Arg-Ser peptides that are labeled at their N-termini with fluorescein [fl-(RS)8 & fl-(RS)16] and represent shorter forms of the complete RS domain. The addition of SRPK1 to fixed amounts of both fl-(RS)8 and fl-(RS)16 lead to increases in the fluorescence emission spectra of fluorescein (Fig.2A,B). Similar spectral changes were also observed upon adding SRPK1(ΔN) and SRPK1(ΔS) to either peptide (data not shown). To ensure that fluoroscein labeling did not affect the ability of the peptide to bind in the same site as SRSF1, steady-state kinetic studies were performed. We found that fl-(RS)16 competitively inhibits SRSF1 phosphorylation indicating that the fluorescently-labeled peptide displaces the natural substrate from the active site (data not shown). The changes in observed fluorescence followed a hyperbolic relationship with total concentration of SRPK1, SRPK1(ΔN) and SRPK1(ΔS) (Fig.2C,D) and were fit to equation (2) to obtain Kd values (Table 2). In all cases, the 32-residue peptide binds with either equivalent or a 2-fold greater affinity compared to the 16-residue peptide. While removal of the SID had no effect on the binding of fl-(RS)16, the affinity of the longer fl-(RS)32 was reduced by 3-fold. In comparison to these modest effects, removal of the N-terminus reduces the binding affinities of both peptides by about 15-fold (Table 2). Overall, the binding studies indicate that the N-terminus in SRPK1 plays an important role in supporting interactions with Arg-Ser repeats and that the full RS domain is not necessary for this phenomenon.
Figure 2. Binding of Fluorescently-Labeled Arg-Ser Peptides to SRPK1 and Deletion Mutants.
(A-B) Fluorescence emission spectra of 10 nM fl(RS)8 (A) and fl(RS)16 (B) in the absence (black) and presence of 25 nM (red), 50 nM (green) and 75 nM (blue) SRPK1. (C-D) Plots of changes in fluorescence at 520 nM (normalized to total fluorescence change, ΔFmax) as a function varying SRPK1 (●), SRPK1(ΔN) (■), and SRPK1(ΔS) (▲). The Kd values are displayed in Table 2.
Table 2.
Dissociation Constants of Fluorescent Peptides to SRPK1 and Deletion Mutants
| Peptide | SRPK1 | SRPK1(ΔN) | SRPK1(ΔS) |
|---|---|---|---|
| Fl-(RS)8 | 25 ± 5 | 360 ± 80 | 22 ± 6 |
| Fl-(RS)16 | 11 ± 5 | 160 ± 25 | 34 ± 4 |
Stability of the SRPK1 N-terminus & SID
To investigate the solution conformation of full-length SRPK1, we used hydrogen-deuterium (H-D) exchange mass spectrometry (DXMS) to assess the stability/flexibility of amide backbone regions in and outside the kinase core. DXMS analysis provides structural and configurational information for systems not amenable for classical structure-determination techniques like X-ray crystallography. The deuterium-exchange reaction is monitored by analyzing the protein at different time points using LC/MS. High quality fragments (probes) that encompass the N-terminus, kinase domain and SID were reproducibly generated from multiple time-course experiments for each SRPK construct. The characteristic mass envelopes of the probes increase as a function of incubation in solvent deuterium as shown for one representative probe in Fig.3. By monitoring time-dependent incorporation of deuterium into SRPK1, we found that many probes exhibiting more protection to deuterium exchange map back to stable secondary structural elements in the crystal structure of the kinase core (Fig.4). The majority of the SID probes were exchanged immediately upon deuterium exposure (<10s), excluding the two regions bridging the SID from the small and large lobes of the kinase domain that show limited protection. A consideration of the primary structure using the program IUPred19 suggests that the SID, unlike the small and large lobes of the kinase domain, is likely to be unfolded in keeping with the observed H-D exchange profile (Supplementary Fig.S2). Although the X-ray structure for SRPK1 lacks the complete SID, two short segments from this domain are included in the crystals and have helical structure (helices αS1 & αS2), in line with the observed intermediate protection. The existing x-ray structures of SRPK also lack the full N-terminal sequence. Our exchange data show regions of protection (Fig.4) although IUPred19 calculations predict that much of the N-terminus is unstructured (Supplementary Fig.S2). The latter observations suggest that the N-terminus may be intrinsically unstructured but then may adopt stable structure upon binding to the kinase core. Overall, the H-D exchange data are consistent with a structural model for the full-length SRPK1 in which the N-terminus is stably folded, possibly interacting directly with the kinase core, whereas the backbone of the SID is solvent accessible and not significantly protected from exchange.
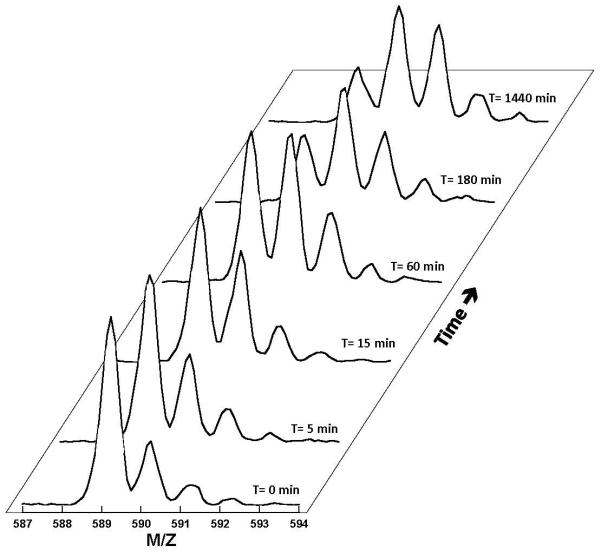
Figure 3. Mass Spectral Shift Upon Solvent Deuterium Incorporation.
Mass spectra of a peptide probe (residues 143-147) from SRPK1 are recorded as a function of time.
Figure 4. Time-Dependent Deuterium Incorporation Into SRPK1 Probes.

Deuteration levels of probes as a function of time are colored coded and assigned to the primary structure in SRPK1. Regions of secondary structure known from crystallographic data6 are assigned above the residues. Regions not defined (N-terminus & SID) are displayed as dotted brackets above the primary structure whereas those defined by X-ray crystallographic data (kinase 1 & 2) are displayed with solid brackets above the primary structure.
Regulation of Kinase Core Dynamics By The N-Terminus and SID
Since the N-terminus and SID impact SRSF1 phosphorylation, we wished to explore how these unique segments of SRPK1 influence the conformation of the kinase core. To accomplish this goal, we performed H-D exchange experiments on SRPK1(ΔN) and SRPK1(ΔS) for comparison with the full-length kinase. Many identical probes obtained for SRPK1 were also detected in either SRPK1(ΔN) or SRPK1(ΔS) with a subset common to all three proteins. The time-dependent incorporation of deuterium into a representative set of probes common in all three kinase forms is shown in Fig.5. There are groups of probes that have different levels of protection in both the short (0-5 min) and long (15-1440 min) time frames (Figs. 5A-F) as well as probes displaying the identical protection when all three SRPK constructs are compared (Fig. 5G). In general, we defined two probes to exchange differently if the incorporation levels varied by 2 or more deuterons at two or more points in either time frame. To better visualize how the N-terminus and SID affect deuterium exchange within the kinase core, we mapped the probes onto the X-ray structure of truncated SRPK1 (Fig.6). We found that removal of the N-terminus destabilizes the probes located in the N-terminal kinase lobe of SRPK1 while removal of the SID had more modest effects on exchange. Although the N-terminus stabilizes most of the small lobe, the SID stabilizes fewer regions and instead destabilizes part of helix αC in this lobe. For SRPK1(ΔN), regions encompassing the docking groove (helix αG and the MAP kinase insert) exchange faster in the absence of the N-terminus. In comparison, deletion of the SID has no effect on helix αG but reduced exchange in the MAP kinase insert compared to SRPK1. Thus, while removal of the N-terminus increases flexibility in the docking groove, removal of the SID does not. These differences correlate well with the reduced binding affinity of SRSF1 to SRPK1(ΔN) compared to SRPK1(ΔS).
Figure 5. Effects of N-Terminal and SID Deletion on Time-Dependent Deuterium Incorporation Into SRPK1 Probes.
Solvent deuterium incorporation as a function time for several probes are plotted for SRPK1 (red) SRPK1(ΔN) (blue) and SRPK1(ΔS) (green). The probes are defined by residues 76-91 (A), 109-122 (B), 151-164 (C), 202-220 (D), 512-523 (E), 569-590 (F), 591-608 (G), and 635-647 (H). These probes encompass portions of the following structural elements: glycine-rich loop (A), helix αC (B), β4-5 (C), catalytic loop (D), activation loop (E), helix αG (F), the MAP kinase insert (G) and helix αI (H).
Figure 6. Probes Affected by Deletion Mapped to the SRPK1 X-Ray Core Structure.

Probes showing faster H-D exchange in SRPK1(ΔN) or SRPK1(ΔS) relative to SRPK1 are colored red, probes showing slower exchange are colored blue and those that are unaffected by deletion are colored yellow. Regions lacking high quality overlapping probes are gray and regions present in the X-ray structure but not in SRPK1(ΔS) and SRPK1(ΔN) are black. Critical structural elements in the kinase core are labeled. The space-filling models are shown in the same orientation as the ribbon diagram and then rotated by 180° around the y-axis.
In addition to providing a means for understanding differences in binding affinities between SRPK1(ΔN) and SRPK1(ΔS), the H-D exchange data offer insights into how the phosphoryl transfer reaction is facilitated. Both N-terminal and SID deletions increase exchange rates (increase flexibility) in the glycine-rich and activation loops, two segments important for maintaining efficient phosphoryl transfer rates in protein kinases.17; 20 The similar effects on H-D exchange in these regions in SRPK1(ΔN) and SRPK1(ΔS) correlate well with the similar decreases in turnover rates (Table 1). However, the N-terminus and SID appear to differentially stabilize the catalytic loop and helix αC (Fig. 6), two poylpeptide regions important for stabilizing the phosphates of ATP and the hydroxyl serine of the substrate. Thus, facilitation of substrate turnover via several noncatalytic segments of SRPK1 may use both common and unique modes of communication across the kinase.
Dissection of the N-Terminus Using Charge-to-Alanine Mutagenesis
To better understand the role of the N-terminus, we made a series of charge-to-alanine mutations to see whether any might affect the binding and phosphorylation of SRSF1. We originally selected 4 short spans of 3-4 like-charged residues in two sections of the N-terminus that encompass H-D exchange probes (Fig.7A). Since some of the triple and quadruple mutants did not express, we selected more modest single and double mutations in two regions (see M2-5; Fig.7A). In general, while all mutants were not expressed to the same extent as wild type, sufficient quantities were attained for steady-state kinetic analyses. Although M1, M4 and M6 had minor or no effects on SRSF1 Km, M2, M3 and M5 had large effects that more closely mimic the effects of SRPK1(ΔN) (Fig.7A). Also, M2, M4 and M5 display low turnover numbers that are in line with SRPK1(ΔN). Given the separation of the charged residues in these affected regions, the data suggest that the N-terminus could adopt secondary structure and possibly make multiple contacts with the kinase core, supporting SRSF1 binding and phosphorylation.
Figure 7. Molecular Connectivities in the N-Terminus.
A) Steady-State Kinetic Effects of Charge-to-Ala Mutations. Charged residues in two regions of the N-terminus defined by available H-D exchange probes (boxes) were mutated to alanine to generate 6 mutant kinases (M1-6). The steady-state kinetic parameters were measured and plotted as initial velocity (normalized to the total enzyme concentration) versus total SRSF1 concentrations. The steady-state kinetic parameters are displayed in the bar graph relative to the wild-type parameters for kcat and SRSF1 Km values. For comparison, the data for SRPK1(ΔN) are also displayed in the bar graph. B) Effects of the SID on H-D exchange in the N-Terminus. Deuteration levels of probes in the N-terminus of SRPK1(ΔS) are compared to the wild-type enzyme. Color coding and time parameters are the same as in Fig. 4.
SID Stabilizes the N-Terminus in SRPK1
Although it is unknown whether the N-terminus or SID interacts with the kinase core, the H-D exchange data can be used to determine if both segments are dynamically coupled. We compared the backbone exchange kinetics of the N-terminus and the SID in SRPK1(ΔS) and SRPK1(ΔN) relative to wild-type SRPK1. We found that the probes in the SID exchange completely within the first time point (10 sec) whether the N-terminus is present or not (data not shown) so that any potential effects of the N-terminus on the SID could not be evaluated using this method. However, we found that probes in the N-terminus show significantly different exchange rates depending on the presence of the SID. While the available probes in the N-terminus of SRPK1 exchange with intermediate rates indicative of stable structure, the available probes in the N-terminus of SRPK1(ΔS) exchange very quickly (Fig.7B). In fact, much of the N-terminus appears to adopt a highly flexible conformation that is more closely analogous to the SID. These findings suggest that the SID is essential for maintaining a stable form of the N-terminus of SRPK1.
Discussion
SRPK1 catalyzes extensive, multi-site phosphorylation using a mechanism that incorporates highly flexible, yet stable, contacts with its substrate target, SRSF1.3 We showed previously that an electronegative docking groove supports a unique feeding mechanism in which N-terminal arginine-serine dipeptide repeats are firmly held in queue while downstream residues in the RS domain are sequentially phosphorylated.3 Using kinetic analyses, we now show that the N-terminus, while not essential for sequential, multi-site phosphorylation, enhances high affinity binding and phosphorylation efficiency of an SR protein (Figs.1&2). Based on changes in H-D exchange profiles, we showed that reductions in SRSF1 affinity upon N-terminal deletion correlate with increased flexibility (decreased protection) in the docking groove (Figs.5&6). Thus, it appears that optimal stability within this critical groove is essential for efficient substrate processing and the N-terminus is an important modulator of this region of the kinase core. In support of this model, we found that deletion of the SID displays no destabilization within the docking groove (i.e.- helix αG and the MAP kinase insert) and also has no effect on SRSF1 affinity. While the molecular mechanism underlying this phenomenon is not clear at this time, mutagenesis data suggest that multiple residues within the N-terminus may contact parts of the small lobe (Fig.7). These local contacts could then transmit effects indirectly to the large lobe. In fact, a communication path from the small to the large lobe is apparent in the space-filling models that compare SRPK1 and SRPK1(ΔN) H-D exchange profiles (Fig.6). Overall, the data provide a firm connection between SR protein phosphorylation and the N-terminal extension in SRPK1 through the docking groove.
Although the N-terminus and SID vary considerably in size and stability (Fig.4) and only the former regulates SR protein binding, both play similar roles in controlling SRSF1 turnover rates (Table 1). A comparison of the affected regions in the H-D exchange kinetics of both SRPK1(ΔN) and SRPK1(ΔS) suggest that there are some common and unique structural elements that are impacted by deletion and could regulate phosphorylation rates (Fig.6). Removal of either the N-terminus or SID increases exchange rates (increases flexibility) in the glycine-rich and activation loops. Both of these loops are critical for protein substrate phosphorylation. Mutagenesis studies have shown that the glycine-rich loop enhances the rate of phosphoryl transfer from ATP to peptide substrates in several protein kinases.21; 22 The activation loops in protein kinases generally regulate phosphoryl transfer rates and, in some cases, control substrate access serving as a gate to the active site.20 In addition to these two critical loops, we found that the SID stabilizes the catalytic loop in SRPK1. This short loop contains a strictly conserved aspartic acid (Asp-213) that is expected to orient the serine hydroxyl of the substrate and abstract the proton in the transition state.23; 24; 25 Replacement of this conserved aspartate with alanine in yeast PKA lowers kcat by about 300-fold.26 In contrast, the presence of the N-terminus does not appear to alter exchange rates in the catalytic loop. Differential stabilization is also observed for helix αC and a segment containing a conserved lysine in β strand 3 (Lys-109). This lysine directly interacts with the phosphates of ATP and is stabilized by a conserved glutamate in helix αC in active protein kinases.27 While the N-terminus reduces flexibility and possibly secures the Lys-Glu dyad, the SID increases flexibility in this region. Overall, the H-D exchange analyses suggest that both shared (glycine-rich and activation loops) and unique (helix αC and catalytic loop) mechanisms serve to optimize SR protein phosphorylation through regions outside the kinase core.
How the N-terminus and SID in SRPK1 control catalytically important residues is not yet clear but the H-D exchange results hint to the capacity of SRPK1 to recruit multiple, communication pathways for the regulation of SR protein phosphorylation reaction. We showed in previous studies how long-range effects control the activity of a protein kinase involved in T cell signaling.28; 29; 30; 31 For the tyrosine kinase Csk, nucleotide binding in the active site affects exchange rates in a region of the large kinase lobe important for regulation through PKA phosphorylation and a distal SH2 domain that controls both subcellular localization and catalytic activity.28 We showed that binding of a phosphotyrosine peptide mimicking a physiological adaptor protein (Cbp) to the neighboring SH2 domain controls exchange rates in active-site loops, thereby, delineating a bidirectional communication pathway in Csk.31 In the present study H-D exchange data for two deletion mutants now suggest that multiple routes in SRPK1 can equally impact SR protein phosphorylation through active-site residues. Overall, the emerging view from these studies is one of catalytic regulation through distal contacts.29 While the kinase core possesses the rudimentary structural elements for SR protein activation, auxiliary polypeptides (N-terminus & SID) fine-tune this mechanism for maximum efficiency.
SRPK1 localization is the result of interactions of the SID with chaperone complexes that tether the kinase to the cytoplasm.14 We now show that although the SID in SRPK1 is large (approx 250 aa), it appears to lack stable structure (Fig.4). Our current X-ray structures for SRPK1 lack most of the SID with the exception of small regions from the N- and C-terminal ends that adopt helical conformations.12 Interestingly, two probes are available in these regions that show intermediate resistance to H-D exchange consistent with stable secondary structure. Nonetheless, the data suggest that the majority of the SID represents a large, intrinsically disordered region in SRPK1. These findings now raise interesting questions regarding how this domain functions as a cytoplasmic anchor for SRPK1. Given the propensity for chaperones to bind to unfolded proteins, the SID may provide a large, unstructured surface for chaperone complex assembly. We showed previously that SRPK1 increases its footprint in the nucleus under certain conditions such as osmotic stress where the large chaperone complex is disassembled.14 Akt is also capable of phosphorylating the C-terminal end of the SID in SRPK2 promoting its entry into the nucleus, phosphorylation of the SR protein SC35 and up-regulation of cyclin D.32 It will be interesting to see whether such phosphorylation events or protein-protein interactions that modulate nuclear entry could also induce structure in the SID. It is noteworthy that despite the increased flexibility, the SID curiously stabilizes the N-terminus (Fig.7) suggesting that the kinase strikes a careful balance between factors that promote and limit motion. In conclusion, these studies paint a broader picture of how this important SR-protein regulator functions, and how regions distal to the active site in yet another kinase impacts its primary function beyond their own regulatory duties.
Materials & Methods
Materials
Acetic acid, acetonitrile, ATP, bovine serum albumin (BSA), ethylenediaminetetraacetic acid (EDTA), formic acid, glycerol, guanidine HCl, KCl, liquid scintillant, Lysobacter enzymogenes endoproteinase LysC (LysC), 2-(N-morpholino) ethanesulphonic acid (MES), MgCl2, 3-(N-morpholino) propanesulphonic acid (MOPS), NaCl, phenylmethanesulfonylfluoride (PMSF) sodium lauryl sulfate, trifluoroacetic acid, Tris (hydroxymethyl) aminomethane (Tris), Triton X-100 surfactant were obtained from Fisher Scientific. [γ-32P] ATP was obtained from Perkin Elmer. Blue Devil autoradiography film was acquired from Genesee Scientific. Deuterium oxide was acquired from Cambridge Isotope Laboratories, Inc. Peptides were synthesized from L-amino acids using solid-phase N-(9 fluorenylmethoxycarbonyl) chemistry on an Applied Biosystems 433A synthesizer, purified on a C18 HPLC column, and analyzed using electrospray mass spectrometry at 215 and 225 nM absorbance for concentration determination.
Expression & Purification of Recombinant Proteins
SRPK1(ΔN) and SRPK1(ΔS) were generated by polymerase chain reaction amplification and subcloning in the pET15b vector. SRPK1(ΔS) contains a three-residue linker, RRQ, that connects the small and large kinase lobes. All single- and multi-site mutations in SRPK1 and SRSF1 were generated by polymerase change reactions using the QuikChange™ mutagenesis kit (Stratagene) and relevant primers. Unless noted, all SRPK1 and SRSF1 mutants contain the N-terminal His6 tag. The expression and purification of His-tagged SRPK1 and SRSF1 constructs using Ni-Agarose affinity chromatography have been described previously.7; 11; 33 Briefly, plasmids were transformed into the BL21(DE3) E. coli cells (Novagen) and grown in LB broth with 100 μg/mL ampicillin at 37°C. SRSF1 mutants were induced with 0.4 mM IPTG for 4-6 hours at room temperature and purified using a refolding protocol.7 SRPK1 induction also used 0.4 mM IPTG and lasted for 12 hours at room temperature followed by purification using a Ni-resin.
Phosphorylation Reactions and LysC proteolysis
Previously published protocols were followed when performing the phosphorylation reactions involving both wild-type and mutant forms of SRPK1 and SRSF1.11; 13 Reaction conditions consisted of 50 mM MOPS (pH 7.4), 10 mM Mg2+, 1 mg/mL BSA, and 32P-ATP (600-1000 cpm/pmol) at 23° C. Reactions were initiated by the addition of 100 μM 32P-ATP and then a total reaction volume of 10 μL was quenched with 10 μL of SDS-PAGE loading buffer for each time point. The quenched reactions were loaded onto a 13% SDS-PAGE gel and the dried gels were exposed with Blue Devil Autoradiography Film (Genesee Scientific). The protein bands corresponding to phosphorylated substrates of interest were excised and counted on the 32P channel in liquid scintillant. Control experiments, specific activity determination, and time-dependant product concentrations were determined as described previously.33 Directionality experiments were initiated with 32P-ATP (1 or 100 μM) and then cold-chased at 8 s with 100 mM ATP. Proteolysis with LysC (100 ng) was then carried out at 37 °C for 4 h before quenching and running on SDS-PAGE.
Deuterium Exchange Optimization
The instrument setup for the deuterium exchange studies has been previously described.28; 34; 35 Determining the initial conditions for sample digestion is an essential step in the DXMS analysis and was performed prior to the exchange time course studies. The stock samples of SRPK mutants were buffer exchanged into 25 mM MOPS, 300 mM NaCl, 1 mM EDTA and 1 mM DTT to a concentration of 2 mg/mL. 80 μL of protein solution was then diluted with 120 μL of 0 °C quench solution containing 0.8 % formic acid, 16.6% glycerol with guanidine HCl at 0.05, 0.5, 1.0, 2.0, or 4.0 M final concentration. After quenching, the samples were immediately placed on dry ice and then stored at −80 °C until analysis. The reduction in pH during this quenching step induces a decrease in the hydrogen-deuterium exchange and denatures the protein prior to pepsin proteolysis. The samples were later thawed at 0 °C and run through a pepsin-66 column (Sigma, 66ul bed volume) for peptide generation. Peptide separation was obtained using a C18 HPLC column (Vydac) with a linear gradient of 0.046% trifluoroacetic acid, 6.4% (v/v) acetonitrile to 0.03% trifluoroacetic acid, 38.4% acetonitrile for 30 min and analysis was performed using LCQ classic (Thermo Finnigan, Inc) electrospray ion trap mass spectrometer. MS1 and MS2 data was used to identify peptide sequences and the quench conditions that generated the optimum fragmentation coverage and quality over the full-length of the protein was selected for the on-exchange experiments.
Deuterium On-exchange Experiments
The exchange course protocol for the DXMS studies was adapted from previously published experiments.28; 36; 37; 38 Protein aliquots were buffer exchanged into 25 mM MOPS, 300 mM NaCl, 1 mM EDTA and 1 mM DTT to a concentration of 2 mg/mL. Deuterated 25mM MOPS was prepared using D2O (Cambridge Isotope Laboratories, Inc.) and adjusted to a pH* of 6.6 with DCl. The non-deuterated controls were prepared as described in the previous section using the 0.05 M guanidine HCl quench conditions. Equilibrium deuterated controls were prepared by combining 20 μL of protein with 60 μL of 0.8% formic acid in D2O for 24 hrs prior to quenching on ice and then freezing on dry ice. For the exchange time course, 280 μL of protein was diluted in 420 μL of the deuterated MOPS buffer and the exchange was monitored at 4 °C over the course of 24 hrs with time points at 0.17, 0.5, 1, 5, 15, 60, 180, and 1440 min. At each interval, 80 μL aliquots of deuterated protein was quenched with 120 μL of the 0.05M guanidine HCl quench. After quenching, the samples were immediately placed on dry ice and stored at −80 °C until analysis. MS1 data was acquired for the samples obtained during the on-exchange time course.
Peptide Fragment Identification
Incorporating MS1 and MS2 data with the SEQUEST software program (Thermo Finnigan, Inc), the most likely identity of the parent peptide ions was determined. Peptide quality was evaluated through individual examination of each peptide fragment that was identified to appear in every sample during the time course. The amount of deuterium incorporation was calculated for each peptide during the time course by using a previously described specialized software package.34; 35
Data Analysis
Initial phosphorylation velocities were measured as a function of either total SRSF1 or ATP and fit to the Michaelis-Menten equation to obtain Vmax and Km. Maximum turnover (kcat) was obtained by dividing Vmax by the total enzyme concentration. Single turnover data were fit to either single or double exponential functions. Inhibition constants were measured by fitting the initial velocities for the phosphorylation of SR(ΔRRM2)] as function of competitor according to equation (1):
| (1) |
where vi/vo is the relative initial velocity (ratio of vo in the presence and absence of inhibitor), Km is the Michaelis constant for SR(ΔRRM2), [S] is the total, fixed concentration of SR(ΔRRM2), [I] is the total concentration of the competitive inhibitor and KI is the dissociation constant for the inhibitor. Km values of 200, 200 and 1100 nM for SRPK1, SRPK1(ΔS) and SRPK1(ΔN) for SR(ΔRRM2) respectively, were measured in plots of v versus substrate. The Kd values for the fluorescent peptides were determined by fitting the change in fluorescence as a function of total peptide concentration according to equation (2):
| (2) |
where ΔF is the fluorescence at each Et minus the fluorescence in the absence of Et, ΔFmax is the maximal change in fluorescence at infinite Et minus the fluorescence in the absence of Et, ΔF/ΔFmax is the fractional increase in fluorescence at each Et, Lt is the fixed concentration of peptide, and Et is the total concentration of enzyme
Supplementary Material
Acknowledgements
This work was supported by an NIH grant (GM67969) to J.A.A and NIH Grants (CA099835, CA118595, AI076961, AI081982, AI2008031, GM020501, GM066170, NS070899 GM093325 and RR029388) to V.L.W. R.P. was supported by NIH under the Ruth L. Kirschstein National Research Service Award (GM090484). J.W. was supported by the SURF program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
- SRSF1
- human alternative splicing factor or ASF/SF2
- H-D
- hydrogen-deuterium
- RS domain
- domain rich in arginine-serine repeats
- RRM
- RNA recognition motif
- SID
- spacer insert domain
- SR protein
- splicing factor containing arginine-serine repeats
- SRPK
- SR-specific protein kinase
References
- 1.Jurica MS, Moore MJ. Pre-mRNA splicing: awash in a sea of proteins. Mol Cell. 2003;12:5–14. doi: 10.1016/s1097-2765(03)00270-3. [DOI] [PubMed] [Google Scholar]
- 2.Long JC, Caceres JF. The SR protein family of splicing factors: master regulators of gene expression. Biochem J. 2009;417:15–27. doi: 10.1042/BJ20081501. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh G, Adams JA. Phosphorylation mechanism and structure of serine-arginine protein kinases. FEBS J. 278:587–97. doi: 10.1111/j.1742-4658.2010.07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai MC, Lin RI, Tarn WY. Transportin-SR2 mediates nuclear import of phosphorylated SR proteins. Proc Natl Acad Sci U S A. 2001;98:10154–9. doi: 10.1073/pnas.181354098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamelberg D, Shen T, McCammon JA. A proposed signaling motif for nuclear import in mRNA processing via the formation of arginine claw. Proc Natl Acad Sci U S A. 2007;104:14947–51. doi: 10.1073/pnas.0703151104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ngo JC, Chakrabarti S, Ding JH, Velazquez-Dones A, Nolen B, Aubol BE, Adams JA, Fu XD, Ghosh G. Interplay between SRPK and Clk/Sty Kinases in Phosphorylation of the Splicing Factor ASF/SF2 Is Regulated by a Docking Motif in ASF/SF2. Mol Cell. 2005;20:77–89. doi: 10.1016/j.molcel.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Velazquez-Dones A, Hagopian JC, Ma CT, Zhong XY, Zhou H, Ghosh G, Fu XD, Adams JA. Mass spectrometric and kinetic analysis of ASF/SF2 phosphorylation by SRPK1 and Clk/Sty. J Biol Chem. 2005;280:41761–8. doi: 10.1074/jbc.M504156200. [DOI] [PubMed] [Google Scholar]
- 8.Chalfant CE, Rathman K, Pinkerman RL, Wood RE, Obeid LM, Ogretmen B, Hannun YA. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J Biol Chem. 2002;277:12587–95. doi: 10.1074/jbc.M112010200. [DOI] [PubMed] [Google Scholar]
- 9.Massiello A, Chalfant CE. SRp30a (ASF/SF2) regulates the alternative splicing of caspase-9 pre-mRNA and is required for ceramide-responsiveness. J Lipid Res. 2006;47:892–7. doi: 10.1194/jlr.C600003-JLR200. [DOI] [PubMed] [Google Scholar]
- 10.Ma CT, Hagopian JC, Ghosh G, Fu XD, Adams JA. Regiospecific phosphorylation control of the SR protein ASF/SF2 by SRPK1. J Mol Biol. 2009;390:618–34. doi: 10.1016/j.jmb.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma CT, Velazquez-Dones A, Hagopian JC, Ghosh G, Fu XD, Adams JA. Ordered multi-site phosphorylation of the splicing factor ASF/SF2 by SRPK1. J Mol Biol. 2008;376:55–68. doi: 10.1016/j.jmb.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 12.Ngo JC, Giang K, Chakrabarti S, Ma CT, Huynh N, Hagopian JC, Dorrestein PC, Fu XD, Adams JA, Ghosh G. A sliding docking interaction is essential for sequential and processive phosphorylation of an SR protein by SRPK1. Mol Cell. 2008;29:563–76. doi: 10.1016/j.molcel.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagopian JC, Ma CT, Meade BR, Albuquerque CP, Ngo JC, Ghosh G, Jennings PA, Fu XD, Adams JA. Adaptable Molecular Interactions Guide Phosphorylation of the SR Protein ASF/SF2 by SRPK1. J Mol Biol. 2008;382:894–909. doi: 10.1016/j.jmb.2008.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong XY, Ding JH, Adams JA, Ghosh G, Fu XD. Regulation of SR protein phosphorylation and alternative splicing by modulating kinetic interactions of SRPK1 with molecular chaperones. Genes Dev. 2009;23:482–95. doi: 10.1101/gad.1752109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding JH, Zhong XY, Hagopian JC, Cruz MM, Ghosh G, Feramisco J, Adams JA, Fu XD. Regulated cellular partitioning of SR protein-specific kinases in mammalian cells. Mol Biol Cell. 2006;17:876–85. doi: 10.1091/mbc.E05-10-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huynh N, Ma CT, Giang N, Hagopian J, Ngo J, Adams J, Ghosh G. Allosteric interactions direct binding and phosphorylation of ASF/SF2 by SRPK1. Biochemistry. 2009;48:11432–40. doi: 10.1021/bi901107q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams JA. Kinetic and Catalytic Mechanisms of Protein Kinases. Chemical Reviews. 2001;101:2271–2290. doi: 10.1021/cr000230w. [DOI] [PubMed] [Google Scholar]
- 18.Aubol BE, Chakrabarti S, Ngo J, Shaffer J, Nolen B, Fu XD, Ghosh G, Adams JA. Processive phosphorylation of alternative splicing factor/splicing factor 2. Proc Natl Acad Sci U S A. 2003;100:12601–12606. doi: 10.1073/pnas.1635129100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dosztanyi Z, Csizmok V, Tompa P, Simon I. IUPred: web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics. 2005;21:3433–4. doi: 10.1093/bioinformatics/bti541. [DOI] [PubMed] [Google Scholar]
- 20.Adams JA. Activation loop phosphorylation and catalysis in protein kinases: is there functional evidence for the autoinhibitor model? Biochemistry. 2003;42:601–7. doi: 10.1021/bi020617o. [DOI] [PubMed] [Google Scholar]
- 21.Hirai TJ, Tsigelny I, Adams JA. Catalytic assessment of the glycine-rich loop of the v-Fps oncoprotein using site-directed mutagenesis [In Process Citation] Biochemistry. 2000;39:13276–84. doi: 10.1021/bi001216g. [DOI] [PubMed] [Google Scholar]
- 22.Grant BD, Hemmer W, Tsigelny I, Adams JA, Taylor SS. Kinetic analyses of mutations in the glycine-rich loop of cAMP- dependent protein kinase. Biochemistry. 1998;37:7708–15. doi: 10.1021/bi972987w. [DOI] [PubMed] [Google Scholar]
- 23.Zhou J, Adams JA. Is there a catalytic base in the active site of cAMP-dependent protein kinase? Biochemistry. 1997;36:2977–84. doi: 10.1021/bi9619132. [DOI] [PubMed] [Google Scholar]
- 24.Valiev M, Kawai R, Adams JA, Weare JH. The Role of the Putative Catalytic Base in the Phosphoryl Transfer Reaction in a Protein Kinase: First-Principles Calculations. J Am Chem Soc. 2003;125:9926–9927. doi: 10.1021/ja029618u. [DOI] [PubMed] [Google Scholar]
- 25.Madhusudan, Trafny EA, Xuong NH, Adams JA, Ten Eyck LF, Taylor SS, Sowadski JM. cAMP-dependent protein kinase: crystallographic insights into substrate recognition and phosphotransfer. Protein Sci. 1994;3:176–87. doi: 10.1002/pro.5560030203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibbs CS, Zoller MJ. Rational scanning mutagenesis of a protein kinase identifies functional regions involved in catalysis and substrate interactions. J. Biol. Chem. 1991;266:8923–31. [PubMed] [Google Scholar]
- 27.Knighton DR, Zheng JH, Ten Eyck LF, Ashford VA, Xuong NH, Taylor SS, Sowadski JM. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991;253:407–14. doi: 10.1126/science.1862342. [see comments] [DOI] [PubMed] [Google Scholar]
- 28.Hamuro Y, Wong L, Shaffer J, Kim JS, Stranz DD, Jennings PA, Woods VL, Adams JA. Phosphorylation Driven Motions in the COOH-terminal Src Kinase, Csk, Revealed Through Enhanced Hydrogen-Deuterium Exchange and Mass Spectrometry (DXMS) J Mol Biol. 2002;323:871–81. doi: 10.1016/s0022-2836(02)01003-3. [DOI] [PubMed] [Google Scholar]
- 29.Wong L, Jennings PA, Adams JA. Communication pathways between the nucleotide pocket and distal regulatory sites in protein kinases. Acc Chem Res. 2004;37:304–11. doi: 10.1021/ar020128g. [DOI] [PubMed] [Google Scholar]
- 30.Wong L, Lieser S, Chie-Leon B, Miyashita O, Aubol B, Shaffer J, Onuchic JN, Jennings PA, Woods VL, Jr., Adams JA. Dynamic coupling between the SH2 domain and active site of the COOH terminal Src kinase, Csk. J Mol Biol. 2004;341:93–106. doi: 10.1016/j.jmb.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 31.Wong L, Lieser SA, Miyashita O, Miller M, Tasken K, Onuchic JN, Adams JA, Woods VL, Jr., Jennings PA. Coupled motions in the SH2 and kinase domains of Csk control Src phosphorylation. J Mol Biol. 2005;351:131–43. doi: 10.1016/j.jmb.2005.05.042. [DOI] [PubMed] [Google Scholar]
- 32.Jang SW, Yang SJ, Ehlen A, Dong S, Khoury H, Chen J, Persson JL, Ye K. Serine/arginine protein-specific kinase 2 promotes leukemia cell proliferation by phosphorylating acinus and regulating cyclin A1. Cancer Res. 2008;68:4559–70. doi: 10.1158/0008-5472.CAN-08-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aubol BE, Nolen B, Shaffer J, Ghosh G, Adams JA. Novel destabilization of nucleotide binding by the gamma phosphate of ATP in the yeast SR protein kinase Sky1p. Biochemistry. 2003;42:12813–20. doi: 10.1021/bi035200c. [DOI] [PubMed] [Google Scholar]
- 34.Golynskiy M, Li S, Woods VL, Jr., Cohen SM. Conformational studies of the manganese transport regulator (MntR) from Bacillus subtilis using deuterium exchange mass spectrometry. J Biol Inorg Chem. 2007;12:699–709. doi: 10.1007/s00775-007-0216-z. [DOI] [PubMed] [Google Scholar]
- 35.Burke JE, Hsu YH, Deems RA, Li S, Woods VL, Jr., Dennis EA. A phospholipid substrate molecule residing in the membrane surface mediates opening of the lid region in group IVA cytosolic phospholipase A2. J Biol Chem. 2008;283:31227–36. doi: 10.1074/jbc.M804492200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burns-Hamuro LL, Hamuro Y, Kim JS, Sigala P, Fayos R, Stranz DD, Jennings PA, Taylor SS, Woods VL., Jr. Distinct interaction modes of an AKAP bound to two regulatory subunit isoforms of protein kinase A revealed by amide hydrogen/deuterium exchange. Protein Sci. 2005;14:2982–92. doi: 10.1110/ps.051687305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu S, Kim Y, Li S, Durrant ES, Pace RM, Woods VL, Jr., Gentry MS. Structural insights into glucan phosphatase dynamics using amide hydrogen-deuterium exchange mass spectrometry. Biochemistry. 2009;48:9891–902. doi: 10.1021/bi9008853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hailey KL, Li S, Andersen MD, Roy M, Woods VL, Jr., Jennings PA. Pro-interleukin (IL)-1beta shares a core region of stability as compared with mature IL-1beta while maintaining a distinctly different configurational landscape: a comparative hydrogen/deuterium exchange mass spectrometry study. J Biol Chem. 2009;284:26137–48. doi: 10.1074/jbc.M109.027375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.