Abstract
The role of Tumor necrosis factor-α (TNF-α) in contributing to allergen induced airway remodeling in asthma is unknown. In this study we have utilized a mouse model of chronic OVA allergen induced airway remodeling to determine whether TNF p55/p75 receptor deficient mice (abbreviated TNF-R KO) had reduced levels of airway remodeling. Chronic OVA challenged WT mice had significantly increased levels of lung eosinophilic inflammation as well as features of airway remodeling including increased peribronchial fibrosis, thickness of the peribronchial smooth muscle layer, mucus expression, and deposition of extracellular matrix proteins. In contrast, TNF-R KO mice had significantly reduced levels of major basic protein positive peribronchial eosinophils and significantly reduced peribronchial fibrosis assessed by quantitating the area of peribronchial trichrome staining and total lung collagen. In addition, TNF-R KO mice had significantly reduced thickness of the peribronchial smooth muscle layer, area of peribronchial α-smooth muscle actin immunostaining, and levels of the extracellular matrix protein fibronectin. There was a non-significant trend for reduced mucus expression in TNF-R KO mice. Levels of peribronchial cells immunostaining positive for TGF-β1 were significantly reduced in TNF-R KO mice suggesting that reduced levels of TGF-β1 expression in TNF-R KO mice may contribute to reduced airway remodeling. Overall, this study suggests an important role for TNF-α in contributing to many features of allergen induced airway remodeling including changes in levels of peribronchial smooth muscle, subepithelial fibrosis, and deposition of extracellular matrix.
Keywords: eosinophil, fibronectin, smooth muscle
1. Introduction
Tumor necrosis factor-α (TNF-α) is a pro-inflammatory cytokine that is expressed at increased levels in the airway in asthmatics [1]. Although TNF-α is expressed in the airway in asthma its role in the pathogenesis of asthma is uncertain based on conflicting results from studies of inhibiting TNF-α in asthma [2]. In four randomized placebo controlled studies which have examined the effect of inhibiting TNF-α in asthma, two studies have observed a benefit [3,4], while an additional two studies have not observed a benefit in asthma outcomes [5,6]. The end-points of these clinical studies have included asthma symptoms, asthma quality of life questionnaire, asthma exacerbations, FEV1, airway hyperreactivity, and biomarkers of inflammation [3-6], but not airway remodeling which is the focus of this pre-clinical study. Airway remodeling in asthma is characterized by subepithelial fibrosis, increased extracellular matrix deposition, smooth muscle hyperplasia/hypertrophy, and mucus metaplasia. The studies demonstrating a benefit of inhibiting TNF-α in asthma have demonstrated reductions in the number of acute asthma exacerbations [4], improvements in FEV1 [3], reductions in airway responsiveness [3], and improvements in asthma quality of life [3]. In contrast, other studies have not noted improvement in these same end-points [5,6]. At present no studies in humans or animal models have examined whether inhibiting TNF-α reduces levels of airway remodeling a structural end-point associated with asthma.
The potential relationship between asthma exacerbations, TNF-α, and airway remodeling is suggested from several studies [1,3,7,8]. For example, symptomatic asthma exacerbations are associated with both increased BAL levels of TNF-α [1], and increased levels of airway remodeling [7,8]. The demonstration that inhibiting TNF-α in asthma can reduce asthma exacerbations [3] provides support for studying whether inhibiting TNF-α reduces airway remodeling. In this study we have utilized TNF p55/75 Receptor deficient mice (TNF-R KO) which are deficient in both TNF-α receptors and thus unable to respond to TNF-α, to determine whether TNF-α plays a role in allergen induced airway remodeling in a mouse model of chronic OVA allergen induced airway remodeling.
The potential for TNF-α to contribute to airway remodeling is suggested from studies demonstrating that TNF-α contributes to remodeling in diseases other than asthma including proliferative retinopathy [9], cardiac remodeling [10], and remodeling of blood vessels and lymphatics in the lung [11]. For example, in an in vitro model of proliferative retinopathy, TNF-α is an important inducer of epithelial mesenchymal associated fibrotic focus formation [9]. In this proliferative retinopathy model, TNF-α triggers increased CD44 expression (the principal receptor for hyaluronic acid) and the subsequent formation of a membrane spanning complex interaction (i.e. hyaluronic acid-CD44-moesin) which is required for activation of TGF-β signaling [9]. As TGF-β1 has been implicated as contributing to airway remodeling in mouse models [12,13], as well as in human studies of asthmatics [14], the potential importance of TNF-α to airway remodeling in asthma through either activating TGF-β signaling and/or alternate mechanisms needs further study. In addition, in mouse models of cardiac remodeling, TNF-α induces expression of matrix metalloproteases, and TNF deficient mice have reduced collagenase activity [10]. Thus, in this study we have utilized TNF-R deficient mice to determine whether TNF-α contributes to features of allergen induced airway remodeling including peribronchial fibrosis and smooth muscle changes in a mouse model.
2. Materials and Methods
2.1 Mouse Model of Chronic OVA-induced Eosinophilic Inflammation and Airway Remodeling
The mouse model of OVA induced airway remodeling has previously been described [15,16]. In brief, eight- to ten-wk-old TNF p55/75 receptor deficient mice (n=16/group)[17,18] and WT mice (n=16/group) on a background of C57/Black were immunized sc on days 0, 7, 14, and 21 with 25 μg of OVA (grade V, Sigma) adsorbed to 1 mg of alum (Aldrich) in 200 μl of normal saline. Intranasal OVA challenges (20 μg/50 μl in PBS) were administered on days 27, 29, and 31 under isoflurane (Vedco, St. Joseph, MO) anesthesia. Intranasal OVA challenges were then repeated twice a week for 4 weeks. Age- and sex-matched control mice were sensitized but not challenged with OVA during the study. Mice were sacrificed 24h after the final OVA challenge and bronchoalveolar lavage (BAL) fluid was collected by lavaging the lung with 1 mL PBS via a tracheal catheter [15,16]. Lungs from the different experimental groups were processed as a batch for either histological staining or immunostaining under identical conditions. Stained and immunostained slides were all quantified under identical light microscope conditions, including magnification (×20), gain, camera position, and background illumination. All animal experimental protocols were approved by the University of California, San Diego Animal Subjects Committee.
2.2 BAL and peribronchial eosinophils
BAL was collected by lavaging the lung with 1 mL PBS via a tracheal catheter as previously described [15,16]. BAL was centrifuged, the supernatant was collected and frozen at −80°C, and cells were re-suspended in 1 mL PBS. Total leukocytes were counted using a hemocytometer. To perform differential cell counts, 200 μL re-suspended BAL cells was cytospun onto microscope slides and air-dried. Slides were stained with Wright-Giemsa and differential cell counts were performed under a light microscope [15,16].
Lung sections were processed for major basic protein (MBP) immunohistochemistry as previously described [15,16], using an anti-mouse MBP Ab (kindly provided by James Lee PhD, Mayo Clinic, Scottsdale, Arizona). The number of individual cells staining positive for MBP in the peribronchial space were counted using a light microscope. Results are expressed as the number of peribronchial cells staining positive for MBP per bronchiole with 150-200 μm of internal diameter. At least ten bronchioles were counted in each slide.
2.3 Peribronchial trichrome staining
Lungs in the different groups of mice were equivalently inflated with an intratracheal injection of the same volume of 4% paraformaldehyde solution (Sigma Chemicals, St. Louis, MO) to preserve the pulmonary architecture. The area of peribronchial trichrome staining in paraffin-embedded lungs was outlined and quantified under a light microscope (Leica DMLS, Leica Microsystems) attached to an image analysis system (Image-Pro plus, Media Cybernetics) as previously described [15,16]. Results are expressed as the area of trichrome staining per μm length of basement membrane of bronchioles 150-200 μm of internal diameter.
2.4 Lung collagen assay
The amount of lung collagen was measured in lung homogenates as previously described in this laboratory [15,16] with a collagen assay kit that uses a dye reagent that selectively binds to the [Gly-X-Y]n tripeptide sequence of mammalian collagens (Accurate Chemical and Scientific Co, Westbury, NY). In all experiments, a collagen standard was used to calibrate the assay.
2.5 Peribronchial Smooth Muscle Layer
The thickness of the airway smooth muscle layer was measured both by image analysis as well as by α-smooth muscle actin immunohistochemistry as previously described [15,16]. In brief, the thickness of the smooth muscle layer (the transverse diameter) was measured from the innermost aspect to the outermost aspect of the smooth muscle layer. The smooth muscle layer thickness in at least 10 bronchioles of similar size (150-200 μm) was counted on each slide. Lung sections were also immunostained with an anti-α-smooth muscle actin primary antibody (Sigma-Aldrich) to detect peribronchial smooth muscle cells as previously described in this laboratory [15,16]. Species- and isotype-matched Abs were used as controls in place of the primary Ab. The area of peribronchial α-smooth muscle actin staining in paraffin-embedded lungs was outlined and quantified under a light microscope (Leica DMLS) attached to an image analysis system (Image-Pro plus) as previously described [15,16]. Results are expressed as the area of peribronchial α-smooth muscle actin staining per μm length of basement membrane of bronchioles 150-200 μm of internal diameter.
2.6 Airway mucus expression
To quantitate the level of mucus expression in the airway, the number of periodic acid Schiff (PAS) - positive and PAS-negative epithelial cells in individual bronchioles were counted as previously described in this laboratory [15, 16]. At least ten bronchioles were counted in each slide. Results are expressed as the percentage of PAS-positive cells per bronchiole, which is calculated from the number of PAS-positive epithelial cells per bronchus divided by the total number of epithelial cells of each bronchiole.
2.7 Detection of extracellular matrix protein fibronectin
Lung sections were processed for fibronectin immunohistochemistry using a rabbit anti-mouse fibronectin Ab (Abcam, Cambridge, MA). The area of peribronchial fibronectin staining was outlined and quantified under a light microscope (Leica DMLS) attached to an image analysis system (Image-Pro plus) as described for trichrome staining. Results are expressed as the area of fibronectin staining per μm length of basement membrane of bronchioles 150-200 μm of internal diameter.
2.8 Peribronchial TGF-β1 + cells
The number of peribronchial TGFβ-1+ cells were quantitated by immunohistochemistry using an anti-TGF-β1 Ab as previously described in this laboratory [15,16]. Co-expression of TGF-β1 and MBP+ eosinophils was detected with immunofluorescence microscopy as previously described [11] using the anti-TGF-β1 Ab and anti-MBP Ab. The anti-MBP Ab was detected with an HRP labeled secondary Ab (alexa 546, red color), while the anti-TGF-β1Ab was detected with a different HRP labeled secondary Ab (alexa 488, green color). Cells co-expressing bFGF and TGF-β1 have a merged yellow color.
2.9 OVA specific IgE
Levels of OVA specific IgE were measured in serum from WT and TNF-R KO mice on two occasions, the first on experiment day 0 and the second following completion of all chronic OVA challenges on the day of mouse sacrifice. OVA specific IgE was measured by ELISA (AbD Serotec, Oxford, UK).
2.10 BAL IL-5
BAL IL-5 was measured by ELISA (R&D, Minneapolis, MN).
2.11 Statistical Analysis
Results in the different groups of mice were compared by ANOVA using the non-parametric Kruskal-Wallis test followed by post-testing using Dunn's multiple comparison of means. All results are presented as mean ± SEM. A statistical software package (Graph Pad Prism, San Diego, CA) was used for the analysis. P values of < 0.05 were considered statistically significant.
3. Results
3.1 Chronic OVA challenged TNF-R deficient mice have reduced levels of BAL eosinophils and peribronchial eosinophils
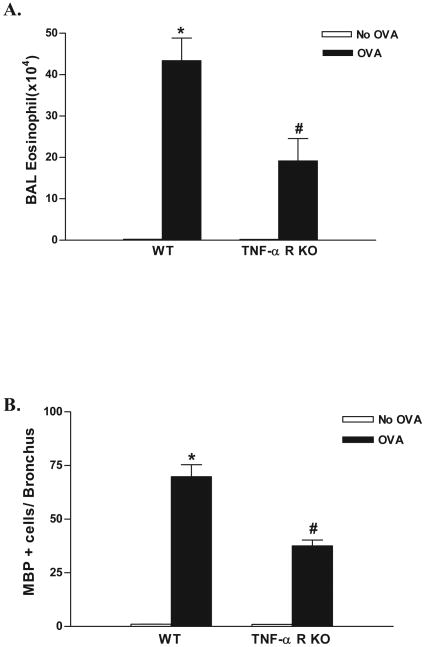
Chronic OVA challenge in WT mice induced a significant increase in the number of BAL eosinophils (p<0.0001)(WT OVA vs WT no OVA)(Fig 1A), as well as a significant increase in the number of peribronchial eosinophils (p<0.0001)(WT OVA vs WT no OVA)(Fig 1B) compared to non-OVA challenged mice. The number of BAL eosinophils in chronic OVA challenged TNF-R deficient mice were significantly lower than that in chronic OVA challenged WT mice (19.1 ± 5.4 vs 43.4 ± 5.4 BAL eosinophils × 104)(TNF-R deficient OVA vs WT OVA)(p=0.02)(Fig 1A). Similarly, the number of peribronchial eosinophils in chronic OVA challenged TNF-R deficient mice were significantly lower than that in chronic OVA challenged WT mice (37.5 ± 2.8 vs 69.8 ± 5.6 MBP+ eosinophils/bronchus)(TNF-R deficient OVA vs WT OVA)(p<0.0001)(Fig 1B).
Figure 1. Levels of BAL and lung eosinophils in TNF-R deficient vs WT mice.
TNF-R deficient or WT mice were subjected to chronic OVA challenge. Non-OVA challenged mice served as a control. Eosinophils in bronchoalveolar lavage (BAL) were quantitated in cytospin slides stained with Wright-Giemsa, whereas eosinophils in lung sections were quantitated by immunostaining with an anti-MBP Ab. Chronic OVA challenge in WT mice induced a significant increase in BAL eosinophils (p<0.0001*)(Fig 1A), and peribronchial eosinophils (p<0.0001*)(Fig 1B)(WT no OVA vs WT OVA). Levels of eosinophils in chronic OVA challenged TNF-R deficient mice were significantly reduced compared to WT mice challenged with OVA in the BAL (p= 0.02#)(Fig 1A) and lung (p<0.0001#)(Fig 1B)(n=16 mice/group).
3.2 Chronic OVA challenged TNF-R deficient mice have reduced levels of peribronchial fibrosis
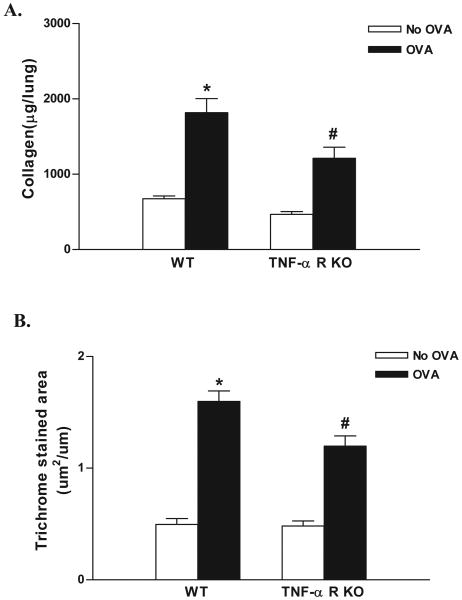
Chronic OVA challenge in WT mice induced a significant increase in levels of peribronchial fibrosis as assessed by either increases in lung collagen (p<0.0001)(WT OVA vs WT no OVA)(Fig 2A), or the area of peribronchial trichrome staining (p<0.0001)(WT OVA vs WT no OVA)(Fig 2B) compared to non-OVA challenged mice. The amount of lung collagen in chronic OVA challenged TNF-R deficient mice was significantly lower than that in chronic OVA challenged WT mice (1,212 ± 146 vs 1,817 ± 186 μg collagen/lung)(TNF-R deficient OVA vs WT OVA)(p<0.001)(Fig 2A).The area of peribronchial trichrome staining was also significantly lower in chronic OVA challenged TNF-R deficient mice compared to chronic OVA challenged WT mice (p<0.001)(Fig 2B).
Figure 2. Levels of peribronchial fibrosis in TNF-R deficient vs WT mice.
TNF-R deficient or WT mice were subjected to chronic OVA challenge. Non-OVA challenged mice served as a control. Levels of peribronchial fibrosis were quantitated by assaying collagen levels in lungs (Fig 2A), as well as by quantitating the area of peribronchial trichrome staining by image analysis (Fig 2B). Chronic OVA challenge in WT mice induced a significant increase in lung collagen (p<0.0001*)(Fig 2A), and in the area of peribronchial trichrome staining (p<0.0001*)(Fig 2B)(WT no OVA vs WT OVA). Levels of lung collagen (p<0.001#)(Fig 2A) as well as the area of peribronchial trichrome staining (p<0.001#)(Fig 2B), were significantly reduced in chronic OVA challenged TNF-R deficient mice compared to WT mice challenged with OVA (n=16 mice/group).
3.3 Chronic OVA challenged TNF-R deficient mice have reduced levels of the extracellular matrix protein fibronectin
Chronic OVA challenge in WT mice induced a significant increase in the area of peribronchial immunostaining of the extracellular matrix protein fibronectin compared to non-OVA challenged mice (Fig 3 A-D). The area of peribronchial fibronectin immunostaining in chronic OVA challenged TNF-R deficient mice was significantly lower than that of chronic OVA challenged WT mice (p<0.0001)(Fig 3 E).
Figure 3. Levels of peribronchial fibronectin in TNF-R deficient vs WT mice.
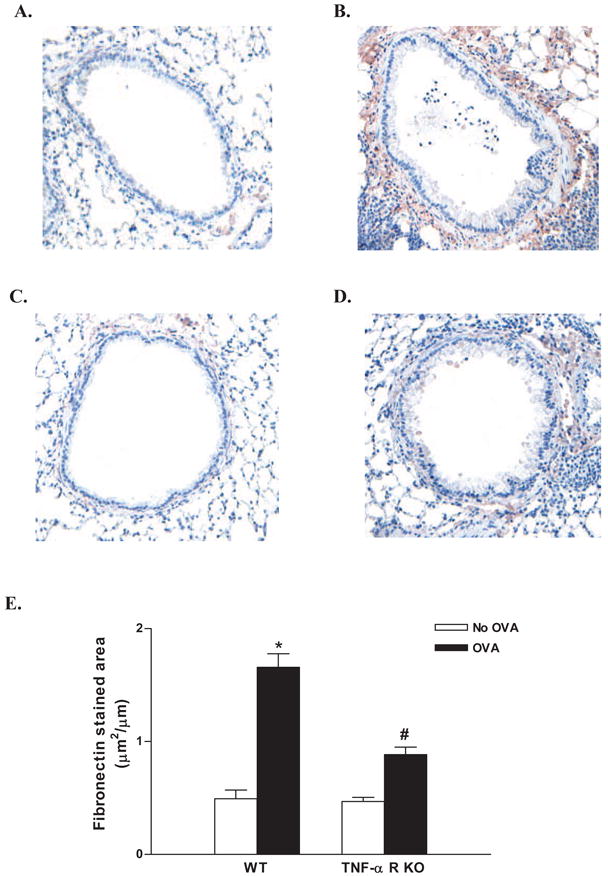
TNF-R deficient or WT mice were subjected to chronic OVA challenge. Non-OVA challenged mice served as a control. Lung sections were immunostained with an anti-fibronectin Ab and the area of peribronchial fibronectin immunostaining determined by image analysis in WT mice (Fig 3A non-OVA; Fig 3B OVA) and TNF-R KO mice (Fig 3C non-OVA; Fig 3D OVA). Chronic OVA challenge in WT mice induced a significant increase the area of peribronchial fibronectin immunostaining (p<0.0001*)(Fig 3 E)(WT no OVA vs WT OVA). The area of peribronchial fibronectin immunostaining was significantly reduced in chronic OVA challenged TNF-R deficient compared to chronic OVA challenged WT mice (p<0.0001#)(Fig 3E)(n=16 mice/group).
3.4 Chronic OVA challenged TNF-R deficient mice have reduced numbers of peribronchial TGF-β1+ cells
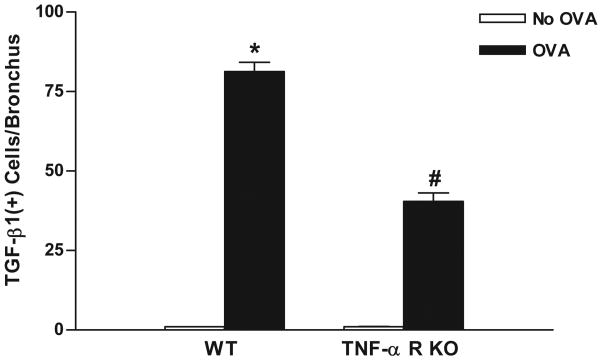
Chronic OVA challenge in WT mice induced a significant increase in the number of peribronchial cells immunostaining positive for TGF-β1 compared to non-OVA challenged WT mice (p<0.0001)(Fig 4). Immunofluorescence microscopy demonstrated that the vast majority of peribronchial TGF-β1+ cells were MBP+ (Figure 5). The number of peribronchial cells immunostaining positive for TGF-β1 in chronic OVA challenged TNF-R deficient mice was significantly lower than that of chronic OVA challenged WT mice (p<0.0001)(Fig 4).
Figure 4. Levels of peribronchial TGF-β1+ cells in TNF-R deficient vs WT mice.
TNF-R deficient or WT mice were subjected to chronic OVA challenge. Non-OVA challenged mice served as a control. Lung sections were immunostained with an anti-TGF-β1 Ab and the number of peribronchial TGF-β1+ cells determined by image analysis. Chronic OVA challenge in WT mice induced a significant increase in the number of peribronchial TGF-β1+ cells (p<0.0001*)(WT no OVA vs WT OVA). The number of peribronchial TGF-β1+ cells were significantly reduced in chronic OVA challenged TNF-R deficient mice compared to WT mice challenged chronically with OVA (p<0.0001#)(n=16 mice/group).
Figure 5. Detection of peribronchial cells co-expressing MBP and TGF-β1.
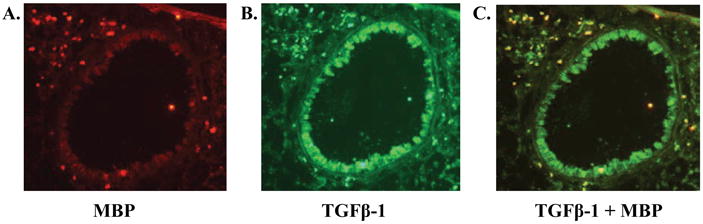
Lung sections from WT mice which had been subjected to chronic OVA challenge were immunostained with both an anti-MBP Ab and an anti-TGF-β1 Ab. Mice chronically challenged with OVA had peribronchial cells expressing MBP (immunofluoresce red in figure 5A) as well as peribronchial cells expressing TGF-β1 (immunofluoresce green in figure 5B). Peribronchial cells co-expressing both MBP and TGF-β1 immunofluoresce yellow (figure 5C).
3.5 Chronic OVA challenged TNF-R deficient mice have reduced smooth muscle thickness
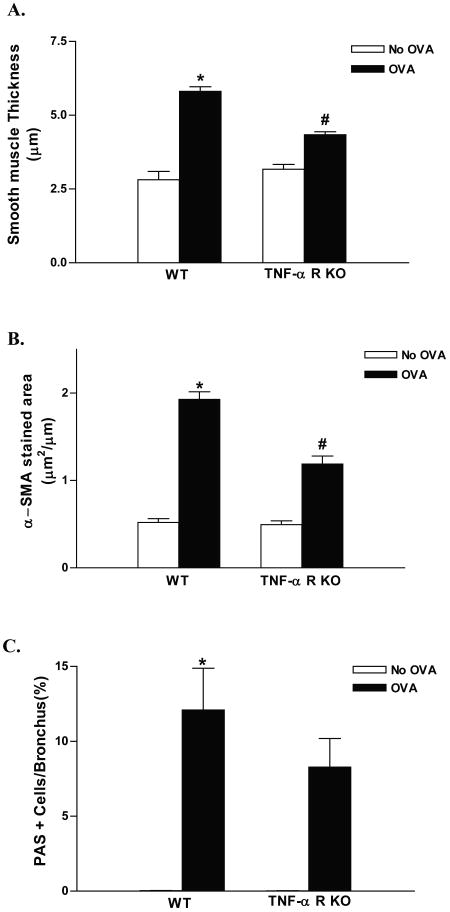
Chronic OVA challenge in WT mice induced a significant increase in the thickness of the peribronchial smooth muscle layer (p<0.0001)(WT OVA vs WT no OVA)(Fig 6A) compared to non-OVA challenged WT mice. TNF-R deficient mice challenged chronically with OVA had a significant reduction in the thickness of the peribronchial smooth muscle layer compared to OVA challenged WT mice (p<0.0001)(Fig 6A).
Figure 6. Peribronchial smooth muscle and mucus in TNF-R deficient vs WT mice.
TNF-R deficient or WT mice were subjected to chronic OVA challenge. Non-OVA challenged mice served as a control. The thickness of the peribronchial smooth muscle layer (Fig 6A), the area of α-smooth muscle actin immunostaining (Fig 6B), and the level of mucus expression (Fig 6C) was quantitated in lung sections. Chronic OVA challenge in WT mice induced a significant increase in the thickness of the peribronchial smooth muscle layer (p<0.0001*)(Fig 6A), the area of α-smooth muscle actin immunostaining (p<0.0001*)(Fig 6B), and the number of PAS+ mucus cells (p<0.0001*)(Fig 6C)(WT no OVA vs WT OVA). In chronic OVA challenged TNF-R deficient mice the thickness of the peribronchial smooth muscle layer (p<0.0001#)(Fig 6A) and the area of α-smooth muscle actin immunostaining (p<0.0001#)(Fig 6B) were significantly reduced compared to WT mice challenged chronically with OVA. There was no significant difference in mucus expression between chronic OVA challenged WT and chronic OVA challenged TNF-R KO mice (p=0.44) (n=16 mice/group).
In addition to measuring the thickness of the smooth muscle layer we also determined the area of peribronchial α-smooth muscle actin immunostaining. Chronic OVA challenge induced a significant increase in the area of peribronchial α-smooth muscle actin immunostaining compared to non-OVA challenged mice (1.92 ± 0.09 vs 0.52 ± 0.04 μm2/μm circumference of bronchiole) (p<0.0001*)(figure 6B). TNF-R deficient mice challenged chronically with OVA had a significant reduction in the area of peribronchial α-smooth muscle actin immunostaining compared to OVA challenged WT mice (p<0.0001#)(Fig 6B).
3.6 Chronic OVA challenged TNF-R deficient mice and levels of airway mucus
Chronic OVA challenge in WT mice induced a significant increase in the number of PAS+ mucus cells (p<0.0001)(WT OVA vs WT no OVA)(Fig 6C) compared to non-OVA challenged mice. TNF-R deficient mice challenged with OVA had a non-significant trend for reduction in the percentage of PAS+ mucus cells compared to OVA challenged WT mice (8.3 ± 1.9 vs 12.1 ± 2.8 % PAS positive cells)(TNF-R deficient OVA vs WT OVA) (p= 0.44)(Fig 6C).
3.7 OVA specific IgE
Measurement of OVA specific IgE levels in serum in TNF-R KO and WT mice demonstrated that TNF-R KO mice had a 7.4 fold increase in OVA specific IgE (day 0 vs final day of chronic OVA protocol), whereas WT mice had a 11. 5 fold increase in OVA specific IgE during this same time period (p=ns WT vs TNF-R KO).
3.8 BAL IL-5
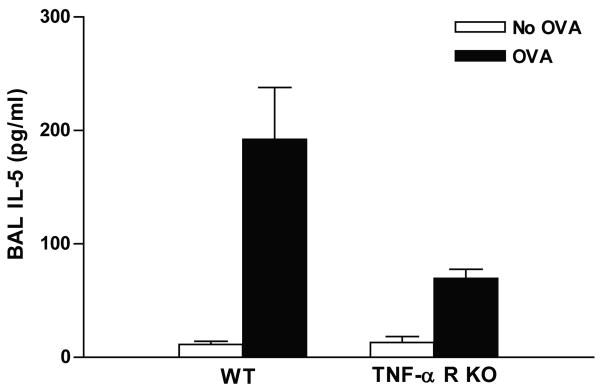
Chronic OVA challenge induced a significant increase in levels of BAL IL-5 in WT mice (192.0 ± 45.9 vs 11.5 ± 2.7 pg/ml) (OVA vs no OVA)(p=0.001) (Fig 7). TNF-R KO mice subjected to chronic OVA challenge had significantly reduced levels of BAL IL-5 (69.6 ± 8.0 vs 192.0 ± 45.9 pg/ml) (TNF-R KO OVA vs WT OVA)(p= 0.003) (Fig 7).
Figure 7. BAL IL-5 levels in TNF-R deficient vs WT mice.
BAL IL-5 levels were measured by ELISA. Chronic OVA challenge induced a significant increase in levels of BAL IL-5 in WT mice (OVA vs no OVA)(p=0.001). TNF-R KO mice subjected to chronic OVA challenge had significantly reduced levels of BAL IL-5 (TNF-R KO OVA vs WT OVA)(p= 0.003).
4. Discussion
In this study we have demonstrated that TNF plays an important role in allergen induced airway remodeling as TNF-R deficient mice had significantly reduced levels of structural remodeling changes (peribronchial fibrosis, smooth muscle changes), and deposition of extracellular matrix proteins compared to WT mice. In addition, we demonstrated that TNF-R deficient mice have significantly reduced levels of eosinophils and peribronchial cells expressing TGF-β1 suggesting that reduced inflammation and reduced expression of pro-fibrotic growth factors contributed to reduced airway remodeling in TNF-R deficient mice.
We have previously used intravital videomicroscopy to demonstrate that eosinophil tethering as well as firm adhesion to endothelium in TNF-R deficient mice is significantly reduced in acute allergen challenged TNF-R deficient mice and that this is associated with reduced tissue recruitment of eosinophils [18]. TNFα is an important stimulus for the expression of endothelial cell adhesion molecules, including intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, E-selectin, and in the mouse P-selectin [19-21] This effect of TNF-α on endothelial cell adhesion molecule expression is mediated through the TNF-R p55 as evidenced from in vivo studies demonstrating reduced expression of endothelial cell adhesion molecules including VCAM-1 and E-selectin in TNF challenged TNF-R p55 deficient mice [20]. As studies have demonstrated reduced levels of airway remodeling as well as reduced levels of TGF-β1 both in mice deficient in eosinophils [15], and in human asthmatics treated with an anti-IL-5 Ab [14], the reduced eosinophilic inflammation and TGF-β1 expression in TNF-R deficient mice could account for the reduced remodeling in these mutant mice. This study demonstrated that both airway inflammation and airway remodeling were reduced. However, the study design is unable to determine whether the reduced remodeling is a consequence of the reduced inflammation. In addition, this study is unable to determine the direct target of TNF in remodeling as TNF-R1 and TNF-R2 receptors are expressed on most cell types including inflammatory cells and structural cells such as smooth muscle and fibroblasts.
Previous murine studies investigating the role of TNF-α in asthma have demonstrated an important role for TNF-α in contributing to acute allergen [22-24] or acute toluene diisocyanate [25] induced eosinophilic lung inflammation, Th2 cytokine production, and airway responsiveness The potential sources of TNF-α in asthma include macrophages [26], mast cells [23,24], and eosinophils [27]. TNF-α may contribute to allergen induced airway inflammation in asthma as IgE dependent stimulation of lung tissue [26], or alveolar macrophages [26] induces expression of TNF-α. Studies of mice deficient in the ability of mast cells to express TNF-α have demonstrated an essential role for mast cell derived TNF-α to eosinophilic lung inflammation, Th2 cytokine production, and airway responsiveness [23,24]. In this study, we extend these observations to demonstrate that following chronic allergen challenge TNF-R deficient mice not only have reduced recruitment of eosinophils as demonstrated in studies of acute allergen challenge in TNF-R deficient mice [18], but also have reduced levels of structural remodeling changes (peribronchial fibrosis, smooth muscle changes), deposition of extracellular matrix proteins, and numbers of peribronchial TGF-β1+ cells compared to WT mice.
In summary, this study has utilized TNF-R deficient mice to demonstrate an important role for TNF-α in airway remodeling in a mouse model of chronic allergic inflammation. The reduced eosinophilic airway inflammation and reduced number of peribronchial TGF-β1+ cells in TNF-R deficient mice suggest that reduced recruitment of eosinophils expressing TGF-β1 may be one mechanism contributing to reduced remodeling in TNF-R deficient mice. However, studies in mouse models may not necessarily predict inflammation and remodeling outcomes in human allergic asthmatics with established sensitization to allergens, Human studies have not yet investigated whether inhibiting TNF-α influences levels of airway remodeling. Further studies are thus needed in human asthmatic subjects with airway remodeling to determine whether targeting TNF-α will influence the progression of airway remodeling.
Acknowledgments
This study was supported by NIH grants AI 38425, AI 70535, and AI 72115.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jae Youn Cho, Email: jacho@ucsd.edu.
Alexa Pham, Email: akpham@ucsd.edu.
Peter Rosenthal, Email: prosenthal@ucsd.edu.
Marina Miller, Email: mamiller@ucsd.edu.
Taylor Doherty, Email: tdoherty@ucsd.edu.
References
- 1.Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol. 1992;89:958–967. doi: 10.1016/0091-6749(92)90218-q. [DOI] [PubMed] [Google Scholar]
- 2.Matera MG, Calzetta K, Cazzola M. TNF-α inhibitors in asthma and COPD: We must not throw the baby out with the bath water. Pulm Pharma Therap. 2010;23:121–128. doi: 10.1016/j.pupt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Berry MA, Hargadon B, Shelley M, Parker S, Shaw DE, Gree RH, Bradding P, Brightling CE, Wardlaw AJ, Pavord ID. Evidence of a role of tumor necrosis factor α in refractory asthma. N Engl J Med. 2006;354:697–708. doi: 10.1056/NEJMoa050580. [DOI] [PubMed] [Google Scholar]
- 4.Erin EM, Leaker BR, Nicholson GC, Tan AJ, Green LM, Neighbour H, Zacharasiewicz AS, Turner J, Barnathan ES, Kon OM, Barnes PJ, Hansel TT. The effects of a monoclonal antibody directed against tumor necrosis factor-α in asthma. Am J Respir Crit Care Med. 2006;174:753–762. doi: 10.1164/rccm.200601-072OC. [DOI] [PubMed] [Google Scholar]
- 5.Morjaria JB, Chauhan AJ, Babu KS, Polosa R, Davies DE, Holgate ST. The role of a soluble TNFα receptor fusion protein (etanercept) in corticosteroid refractory asthma: a double blind, randomised, placebo controlled trial. Thorax. 2008;63:584–591. doi: 10.1136/thx.2007.086314. [DOI] [PubMed] [Google Scholar]
- 6.Wenzel SE, Barnes PJ, Bleecker ER, Bousquet J, Busse W, Dahlén SE, Holgate ST, Meyers DA, Rabe KF, Antczak A, Baker J, Horvath I, Mark Z, Bernstein D, Kerwin E, Schlenker-Herceg R, Lo KH, Watt R, Barnathan ES, Chanez P. A randomized, double-blind, placebo-controlled study of tumor necrosis factor α blockade in severe persistent asthma. Am J Respir Crit Care Med. 2009;179:549–558. doi: 10.1164/rccm.200809-1512OC. [DOI] [PubMed] [Google Scholar]
- 7.O'Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;197:19–24. doi: 10.1164/rccm.200807-1126OC. [DOI] [PubMed] [Google Scholar]
- 8.Kariyawasam HH, Aizen M, Barkans H, Robinson DS, Barry Kay A. Remodeling and airway hyperresponsiveness but not cellular inflammation persist after allergen challenge in asthma. Am J Respir Crit Care Med. 2007;175:896–904. doi: 10.1164/rccm.200609-1260OC. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi E, Nagano O, Ishimoto T, Yae T, Suzuki Y, Shinoda T, Nakamura S, Niwa S, Ikeda S, Koga H, Tanihara H, Saya H. TNF-α refulates TGF-β-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction. J Biol Chem. 2010;285:4060–4073. doi: 10.1074/jbc.M109.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Awad AE, Kandalam V, Chakrabarti S, Wang X, Penninger JM, Davidge ST, Oudit GY, Kassiri Z. Tumor necrosis factor induces matrix metalloproteinases in cardiomyocytes and cardiofibroblasts differentially via superoxide production in a PI3Kγ-depndent manner. Am J Physiol Cell Physiol. 2010;298:C679–692. doi: 10.1152/ajpcell.00351.2009. [DOI] [PubMed] [Google Scholar]
- 11.Baluk P, Yao LC, Feng J, Romano T, Jung SS, Schreiter JL, Yan L, Shealy DJ, McDonald DM. TNF-α drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest. 2009;119:2954–2964. doi: 10.1172/JCI37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le AV, Cho JY, Miller M, McElwain S, Golgotiu K, Broide DH. Inhibition of allergen-induced airway remodeling in Smad 3-deficient mice. J Immunol. 2007;178:7310–7316. doi: 10.4049/jimmunol.178.11.7310. [DOI] [PubMed] [Google Scholar]
- 13.McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-beta antibody: effect on the Smad signaling pathway. J Immunol. 2005;174:5774–5780. doi: 10.4049/jimmunol.174.9.5774. [DOI] [PubMed] [Google Scholar]
- 14.Flood-Page P, Menzies-Gow A, Phipps S, Ying S, Wangoo A, Ludwig MS, Barnes N, Robinson D, Kay AB. Anti-IL-5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest. 2003;112:1029–1036. doi: 10.1172/JCI17974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho JY, Miller M, Baek KJ, Han JW, Nayar J, Lee SY, McElwain K, McElwain S, Friedman S, Broide DH. Inhibition of airway remodeling in IL-5-deficient mice. J Clin Invest. 2004;113:551–560. doi: 10.1172/JCI19133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song DJ, Cho JY, Lee SY, Miller M, Rosenthal P, Soroosh P, Croft M, Zhang M, Varki A, Broide DH. Anti-Siglec-F antibody reduces allergen induced eosinophilic inflammation and airway remodeling. J Immunol. 2009;183:5333–5341. doi: 10.4049/jimmunol.0801421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peschon JJ, Torrance DS, Stocking KL, Glaccum MB, Otten C, Willis CR, Charrier K, Morrissey PJ, Ware CB, Mohler KM. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- 18.Broide DH, Stachnick G, Castaneda D, Nayar J, Sriramarao P. Inhibition of eosinophilic inflammation in allergen-challenged TNF receptor p55/p75- and TNF receptor p55-deficient mice. Am J Respir Cell Mol Biol. 2001;24:304–311. doi: 10.1165/ajrcmb.24.3.4071. [DOI] [PubMed] [Google Scholar]
- 19.Walsh LJ, Trincieri G, Waldorf HA, Whitaker D, Murphy GF. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci USA. 1991;88:4220–4224. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neumann B, Machleidt T, Lifka A, Pfeffer K, Vestweber D, Mak TW, Holzmann B, Kronke M. Crucial role of 55-kilodalton TNF receptor in TNF-induced adhesion molecule expression and leukocyte organ infiltration. J Immunol. 1996;156:1587–1593. [PubMed] [Google Scholar]
- 21.Mackay F, Loetscher H, Stueber D, Gehr G, Lesslauer W. Tumor necrosis factor α (TNF-α)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-r55. J Exp Med. 1993;177:1277–1286. doi: 10.1084/jem.177.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakae S, Lunderius C, Ho LH, Schäfer B, Tsai M, Galli SJ. TNF can contribute to multiple features of ovalbumin-induced allergic inflammation of the airways in mice. J Allergy CLin Immunol. 2007;119:680–686. doi: 10.1016/j.jaci.2006.11.701. [DOI] [PubMed] [Google Scholar]
- 23.Reuter S, Heinz A, Sieren M, Wiewrodt R, Gelfand EW, Stassen M, Buhl R, Taube C. Mast cell-derived tumour necrosis factor is essential for allergic airway disease. Eur Respir J. 2008;31:773–782. doi: 10.1183/09031936.00058907. [DOI] [PubMed] [Google Scholar]
- 24.Nakae S, Ho LH, Yu M, Monteforte R, Iikura M, Suto H, Galli SJ. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. 2007;120:48–55. doi: 10.1016/j.jaci.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 25.Ohkawara Y, Yamauchi K, Tanno Y, Tamura G, Ohtani H, Nagura H, Ohkuda K, Takishima T. Human lung mast cells and pulmonary macrophages produce tumor necrosis factor-alpha in sensitized lung tissue after IgE receptor triggering. Am J Respir Cell Mol Biol. 1992;7:385–392. doi: 10.1165/ajrcmb/7.4.385. [DOI] [PubMed] [Google Scholar]
- 26.Costa JJ, Matossian K, Resnick MB, Beil WJ, Wong DTW, Gordon JR, Dvork AM, Weller PF, Galli SJ. Human eosinophils can express the cytokines tumor necrosis factor-α and macrophage inflammatory protein-1 α. J Clin Invest. 1993;91:2673–2684. doi: 10.1172/JCI116506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matheson JM, Lemus R, Lange RW, Karol MH, Luster MI. Role of tumor necrosis factor in toluene diisocyanate asthma. Am J Respir Cell Mol Biol. 2002;27:396–405. doi: 10.1165/rcmb.4614. [DOI] [PubMed] [Google Scholar]