Abstract
Objective
To evaluate the psychometric properties of the 4-factor low literacy Decisional Conflict Scale (DCS-LL) with men eligible for prostate cancer screening (PCS).
Methods
We used baseline (T0; n = 149) and post-intervention (T2; n = 89) data from a randomized, controlled trial of a PCS decision aid to assess internal consistency reliability and construct, discriminant, and factor validity.
Results
There was evidence of excellent internal consistency reliability (α’s ≥ .80) and fair construct validity (most r’s ≥ .40) for the DCS-LL except for the Supported subscale. The DCS-LL was able to discriminate between men who had decided and those who had not. There was evidence for the original 4-factor model at T0 but exploratory analysis suggested a 3-factor solution at T0 and T2 with Informed and Value Clarity as one factor.
Conclusion
For men eligible for PCS, feeling informed and feeling clear about values may not reflect distinct cognitive processes. Feeling supported may not be a factor contributing to uncertainty. Research should address whether current DCS subscales best represent the factors that contribute to uncertainty for PCS and for other screening decisions. Research should also explore the influence of health literacy on the factor structure of the DCS-LL.
Keywords: decisional conflict, decision making, prostate cancer screening, measurement, validity, psychometrics
1. Introduction
Patient decision aids reduce decisional conflict, the perceived uncertainty about which course of action should be taken, by providing information about options and by clarifying personal values [1–5]. For prostate cancer screening (PCS), there is uncertainty about the benefits of early screening in reducing prostate cancer mortality [6] and the decision requires trade-off of the potential physical and psychological harm from treatment options [6–10]. A recent systematic review of PCS decision aids studies indicated that these aids helped resolve decisional conflict [11].
The original, 16-item version of the Decisional Conflict Scale (DCS) is widely used for decision aid evaluations [12–14]. O’Connor and colleagues offer an alternative, 10-item version of the DCS, appropriate for individuals with low literacy skills (DCS-LL). While the DCS uses item statements and a 5-point agreement scale, the DCS-LL expresses items as questions with ordered category responses (yes, no, unsure) [1]. Additionally, the DCS-LL does not include the Effective Decision subscale and has 1 item less for the Uncertainty and Values Clarity subscales. Published reports of the use and psychometric properties of the DCS-LL are lacking [1]. The overall purpose of this analysis was to provide evidence for the reliability and validity of the DCS-LL before and after the use of a PCS decision aid.
2. Method
2.1. DCS-LL Adaptation, Sample, and Procedures
The items of the DCS-LL were adapted for PCS, as recommended by O’Connor [1] and has been done in other studies [15, 16]. Cognitive interviews with ten men were conducted to ensure comprehension of the items. Following the DCS user manual [1], we calculated total and subscale scores by summing the number of items, dividing by the number of items, and multiplying by 25 (range, 0 – no decisional conflict to 100 – extremely high decisional conflict).
The sample came from a randomized, controlled trial that evaluated a computerized PCS decision aid and an audio information booklet in two different patient populations, one a general medicine clinic in a publicly-funded hospital [17]. Men at the general medicine clinic who were found eligible for PCS completed the DCS-LL before viewing the aid (T0, n = 149) and two weeks after their visit (T2, n = 89). The average age for the sample was 54 years old (see Table 1 for other characteristics). This study was approved by the University of Texas, School of Public Health Research Service Center.
Table 1.
Sample characteristics (n = 149)
| Characteristic | % (n) |
|---|---|
| Education | |
| Did not graduate from high school | 28.2 (42) |
| High school graduate/GEDa | 43.0 (64) |
| Some college/vocational training | 20.1 (30) |
| College graduate | 4.7 (7) |
| Postgraduate degree | 3.4 (5) |
| No response | .7 (1) |
| Ethnicity/Race | |
| Black or African American | 73.6 (109) |
| White | 16.2 (25) |
| Mexican-American, Hispanic/Latino | 8.8 (13) |
| Other | 1.4 (2) |
| Health Status | |
| Excellent | 6.1 (9) |
| Very Good | 14.1 (21) |
| Good | 27.5 (41) |
| Fair | 34.2 (51) |
| Poor | 17.0 (26) |
| No response | .7 (1) |
| Insurance Status | |
| No Insurance | 67.8 (101) |
| Medicaid only | 10.7 (16) |
| Medicare only | 13.4 (20) |
| Medicaid + Medicare | 0 (0) |
| Private | 8.1 (12) |
| No response | 0 (0) |
| Family History of Prostate Cancer | |
| Yes | 10.7 (16) |
| No | 79.7 (119) |
| I’m not sure | 9.5 (14) |
| Previous Prostate-Specific Antigen Test | |
| Yes | 30.9 (46) |
| No | 51.7 (77) |
| Not sure | 17.4 (26) |
| Missing | 0 (0) |
GED = general educational development
2.3. Analysis Strategy
Internal consistency reliability was assessed by examining the intraclass correlation coefficients (ICCs) from the factor analysis. Cronbach’s α was also calculated to for the scale and subscales. We expected α values and ICCs of ≥ .70, which is acceptable for research or group comparisons [18]; the DCS user manual reports an α = .86 for the DCS-LL using 63 women considering breast cancer treatment options [1].
Construct validity was assessed using an approach used by O’Connor and others [4, 15] and hypothesizes that the three subscales that contribute to uncertainty (Informed, Values Clarity, and Supported) are strongly and positively associated with the Uncertainty subscale [Pearson correlation coefficients (r) ≥ .40, p < .01].
To determine whether the DCS-LL could differentiate between men based on their intention to undergo PCS (discriminant validity), mean scores at T0 were compared using two-way analysis of variance with an a priori contrast. The contrast was created using men’s responses to, “Given what you know about prostate cancer and PSA testing, do you plan to have a PSA test?” We hypothesized that men who had made a decision (responded “yes” or “no”) would have higher decisional conflict scores than men who were not sure about screening (responded “not sure”).
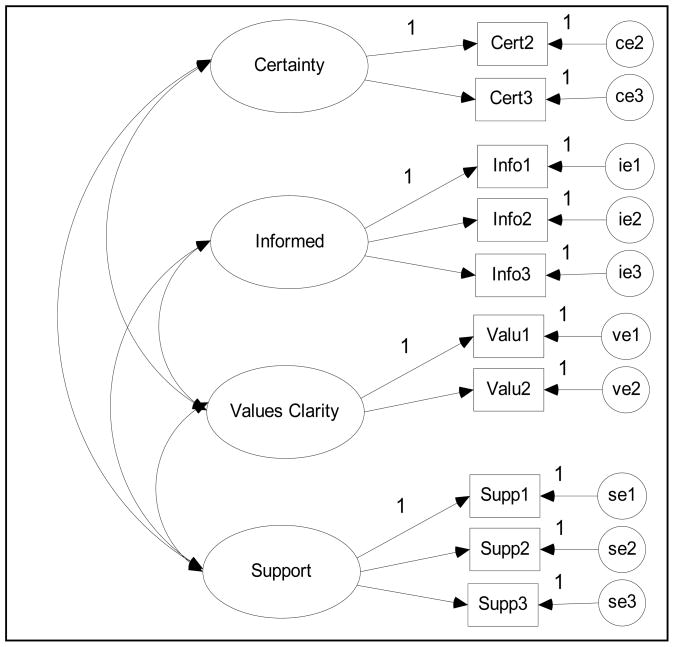
To test factor validity, confirmatory factor analyses (CFAs) were conducted to assess model fit (Figure 1). We used a mean- and variance-adjusted weighted least-squares estimator (WLSMV) in Mplus because the data were ordered categorically and the scale items were non-normally distributed [19]. Model fit was assessed by examining the χ2 test of model fit, the root mean square error of approximation (RMSEA), the comparative fit index (CFI), the Tucker–Lewis Index (TLI), and the weighted root mean square residual (WRMR). A combination of a statistically non-significant χ2 value (p > .05), RMSEA < (.05 – .08), CFI > .95, TLI > .95, and WRMR < .90 indicates adequate model fit [20, 21]. Additional exploratory factor analyses were conducted using WLSMV with an oblique rotation (GEOMIN) [19].
Figure 1.
Four–Factor Model of the Low Literacy Decisional Conflict Scale (DCS-LL)
3. Results
Cronbach’s α for to the total scale and subscales indicated good internal consistency reliability (α’s ≥ .80), except for the Supported subscale (Table 2). This pattern was similar for the intraclass correlation coefficients.
Table 2.
Baseline (T0) and two–week follow–up (T2) internal consistency reliability for the low literacy Decisional Conflict Scale
| Subscale |
Cronbach’s α |
Intraclass Correlation Coefficients (ICCs) |
||
|---|---|---|---|---|
| Informed | Values Clarity | Supported | ||
| Baseline (T0) | ||||
| DCS total | .834 | --- | --- | --- |
| Uncertainty | .817 | .748 | .637 | .655 |
| Informed | .838 | --- | .868 | .429 |
| Values Clarity | .820 | --- | --- | .246 |
| Supported | .468 | --- | --- | --- |
| Two-week Follow-up (T2)a | ||||
| DCS total | .859 | --- | --- | --- |
| Uncertainty | .835 | --- | --- | --- |
| Informed | .818 | --- | --- | --- |
| Values Clarity | .917 | --- | --- | --- |
| Supported | .596 | --- | --- | --- |
The ICCs from the confirmatory factor analysis are presented for at T0 only because the T2 model was not identified.
There was some evidence for good construct validity for two of three subscales that contribute to uncertainty (Table 3). The Informed subscale was strongly correlated with the Uncertainty subscale at both time points and the Values Clarity subscale was strongly correlated (T0) and weakly correlated (T2) with Uncertainty. Poor construct validity was evident for the Supported subscale (T0 and T2).
Table 3.
Means, standard deviations (SD), and Pearson correlations coefficients for the low-literacy Decisional Conflict Scale and subscales
| Means (SD) |
Correlation Coefficients |
|||
|---|---|---|---|---|
| Informed | Values Clarity | Supported | ||
| Baseline (T0) | ||||
| Total DCS | 33.66 (25.94) | |||
| Uncertainty | 24.65 (37.04) | .492* | .441* | .354* |
| Informed | 53.60 (40.71) | --- | .629* | .241* |
| Values Clarity | 39.80 (41.35) | --- | --- | .122 |
| Supported | 13.51 (21.42) | --- | --- | --- |
| Follow-up (T2) | ||||
| Total DCS | 17.41 (22.48) | |||
| Uncertainty | 12.36 (26.92) | .419* | .339* | .098 |
| Informed | 24.14 (34.27) | --- | .878* | .290* |
| Values Clarity | 25.57 (37.93) | --- | --- | .312* |
| Supported | 10.35 (19.56) | --- | --- | --- |
Pearson correlation coefficient was significant at the 0.01 level (1–tailed).
The two-way analysis of variance results suggested that the DCS-LL could discriminate between groups of men based on whether they had made a decision to undergo PCS [F(1, 423) = 12.52, p < .001]. Men who were undecided about screening had significantly higher total DCS scores than those who had made a decision.
Evidence for good factor validity was indicated by adequate model fit for the DCS-LL at T0 according to all indices except χ2 (p = .022) (Table 4). However, the 4-factor model at T2 could not be identified. We then conducted exploratory analyses with a WLSMV with oblique rotation (GEOMIN) estimator to explore the differences in the underlying structure of the DCS-LL at the two time points (Table 5). We determined the number of factors using scree plots and eigenvalues close to 1. A 3-factor model was indicated as the best solution at both time points. Additionally, factor 1 loaded all Uncertainty items, and factor 2 loaded all Informed and Values Clarity items. While all Supported items loaded on factor 3 at T2, one item (enough support) did not load on any factor and one time (feeling pressure) had a high cross-loading with factor 1.
Table 4.
Fit indicesa from the confirmatory factor analysis for the low-literacy Decisional Conflict Scale
| Model | χ2 (df)b | P–value | CFI | TLI | RMSEA | WRMR |
|---|---|---|---|---|---|---|
| Baseline (T0) 4–Factor Model | 25.216(13) | .022 | .990 | .989 | .079 | .659 |
| Follow-up (T2) 4–Factor Modelc | --- | --- | --- | --- | --- | --- |
CFI = comparative fit index; TLI = Tucker–Lewis index; RMSEA = root mean square error of approximation; WRMR = weighted root mean square residual
χ2(df) = Chi-square(degrees of freedom)
An item in the uncertainty subscale had a negative residual variance; fixing the variance to 0 made the model un–identifiable.
Table 5.
Factor loadingsa from the exploratory factor analysis of the low-literacy Decisional Conflict Scale
| Original Subscale | Items | Baseline (T0) | Two week follow–up (T2) | ||||
|---|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 1 | Factor 2 | Factor 3 | ||
| Uncertainty | D1: sure what to do | .693 | 1.080 | ||||
| D2: clear what choice is best | .839 | 0.761 | |||||
| Informed | D3: aware of options | .644 | 0.706 | ||||
| D4: know advantages | .937 | 0.877 | |||||
| D5: know disadvantages | .974 | 0.922 | |||||
| Values | D6: advantages importance | .861 | 0.923 | ||||
| Clarity | D7: disadvantages importance | .945 | 0.911 | ||||
| D8: without pressure | .601 | 0.686 | |||||
| Supported | D9: enough support | 0.693 | |||||
| D10: enough advice | .537 | .402 | 0.926 | ||||
Only factor loadings ≥ .400 are presented
Discussion and conclusion
4.1. Discussion
We found strong evidence for internal consistency reliability, construct and discriminant validity, and fair evidence factor validity and invariance before and after the use of a PCS decision for the DCS-LL overall and for most subscales. The poor performance of the Support subscale in most tests suggests that feelings of being supported may not be a contributing factor to the uncertainty whether or not to undergo PCS. This may be unique to screening decisions that may lead to other decisions and consequences if abnormal results are found. These future decisions may be where support is more relevant.
Although there was evidence for a 4-factor model at T0, exploratory analyses suggested a 3-factor model. Perceptions of feeling informed about options and the values a patient associates with those options appear to be measuring the same underlying construct. The low degree of decisional conflict reported by our sample may have made it difficult to discriminate between latent DCS factors [15]. A French study of cancer patients deciding whether to undergo BRCA genetic testing found it difficult to distinguish DCS latent factors, and the authors suggested that the amount of conflict generated by BRCA genetic testing is overestimated [22]. Other authors have suggested that patients experience decisional conflict only when they perceive that they have choice [15]. For PCS, some men may not view refusing screening as a real option because they may believe that “prevention is always beneficial” [23].
The results of the Informed and Values Clarity loading as one factor may have broader implications for evaluating decision aids. The International Patient Decision Aids Standards Collaboration has identified that two of the six decision process criteria for evaluating decision aids can be measured using these two subscales.[24, 25] Our results suggest that it may not be appropriate to use the two subscales as separate criteria.
4.2. Conclusion
Our study provides support for use of the DCS-LL in PCS decision aid studies, but suggests caution about using the individual subscales as evaluative criteria. In particular, the support subscale does not perform well. This study’s conclusions are limited to the small sample size at follow-up, which limited confirmatory factor analysis at T2. Additionally, the small sample size did not allow for us to test discriminant validity of the DCS-LL or explore decision aid groups at T2. Another limitation was that individual health literacy was not assessed, and it is unknown if the low literacy version is influenced by health literacy. Future research may want to explore the influence of health literacy on the factor structure of the DCS-LL.
4.3. Practice implications
Factors regarding uncertainty about a course of action may be unique to the type of healthcare decision. Future research should explore what factors men may find conflictual for PCS and other screening decisions. Future studies using the DCS should test psychometric properties, including factor comparisons to the original subscales.
Acknowledgments
This work was part of Suzanne Kneuper Linder’s dissertation and was funded through a Pre-doctoral Fellowship, University of Texas School of Public Health Cancer Education and Career Development Program, National Cancer Institute/NIH Grant R25-CA-57712. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. SKL is a current Post-doctoral Fellow in the Department of General Internal Medicine, The University of Texas MD Anderson Cancer Center in Houston, Texas. This study was also supported by a grant from the Agency for Healthcare Research and Quality (grant No. 5 R01 HS10612).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Suzanne K. Linder, Department of General Internal Medicine, The University of Texas MD Anderson Cancer Center, Houston, USA.
Paul R Swank, The School of Medicine, The University of Texas Health Science Center, Houston, USA.
Sally W Vernon, The School of Public Health, University of Texas Health Science Center, Houston, USA.
Patricia D Mullen, The School of Public Health, The University of Texas Health Science Center, Houston, USA.
Robert O Morgan, The School of Public Health, The University of Texas Health Science Center, Houston, USA.
Robert J Volk, Department of General Internal Medicine and Houston Center for Education and Research on Therapeutics, The University of Texas MD Anderson Cancer Center, Houston, USA.
References
- 1.O’Connor AM. User Manual - Decisonal Conflict Scale. 2005 Available from: http://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf.
- 2.O’Connor AM, et al. Do patient decision aids meet effectiveness criteria of the international patient decision aid standards collaboration? A systematic review and meta-analysis. Med Decis Making. 2007;27:554–74. doi: 10.1177/0272989X07307319. [DOI] [PubMed] [Google Scholar]
- 3.O’Connor AM, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews. 2009;(1) doi: 10.1002/14651858.CD001431.pub2. Art. No.: CD001431 2009. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor AM, Jacobsen MJ, Stacey D. An evidence-based approach to managing women’s decisional conflict. J Obstet Gynecol Neonatal Nurs. 2002;31:570–81. doi: 10.1111/j.1552-6909.2002.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 6.Schroder FH, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 7.Weber BA, et al. Psychosocial consequences of prostate cancer: 30 years of research. Geriatr Nurs. 2005;26:166–75. doi: 10.1016/j.gerinurse.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Andriole GL, et al. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360:1310–9. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunkel EJ, et al. Biopsychosocial aspects of prostate cancer. Psychosomatics. 2000;41:85–94. doi: 10.1176/appi.psy.41.2.85. [DOI] [PubMed] [Google Scholar]
- 10.Agency for Healthcare Research and Quality. Quality-of-life issues complicate decisions about prostate cancer screening. 189. AHRQ Research Activities; November/December, 1995. Research Activities newsletter. Available from: http://www.ahrq.gov/research/nov95/dept1.htm. [Google Scholar]
- 11.Volk RJ, et al. Trials of decision aids for prostate cancer screening: a systematic review. Am J Prev Med. 2007;33:428–434. doi: 10.1016/j.amepre.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy AD. On what basis should the effectiveness of decision aids be judged? Health Expect. 2003;6:255–68. doi: 10.1046/j.1369-6513.2003.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor AM, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2003:CD001431. doi: 10.1002/14651858.CD001431. [DOI] [PubMed] [Google Scholar]
- 14.Kryworuchko J, et al. Appraisal of primary outcome measures used in trials of patient decision support. Patient Educ Couns. 2008;73:497–503. doi: 10.1016/j.pec.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Koedoot N, et al. The decisional conflict scale: further validation in two samples of Dutch oncology patients. Patient Educ Couns. 2001;45:187–93. doi: 10.1016/s0738-3991(01)00120-3. [DOI] [PubMed] [Google Scholar]
- 16.Siminoff LA, Fetting JH. Factors affecting treatment decisions for a life-threatening illness: the case of medical treatment of breast cancer. Soc Sci Med. 1991;32:813–8. doi: 10.1016/0277-9536(91)90307-x. [DOI] [PubMed] [Google Scholar]
- 17.Volk RJ, et al. Entertainment education for prostate cancer screening: a randomized trial among primary care patients with low health literacy. Patient Educ Couns. 2008;73:482–9. doi: 10.1016/j.pec.2008.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bland JM, Altman DG. Cronbach’s alpha. Bmj. 1997;314:572. doi: 10.1136/bmj.314.7080.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muthen LK, Muthen BO. Mplus User’s Guide. 5. Vol. 5. Los Angeles: Muthen & Muthen; 2007. [Google Scholar]
- 20.Yu CY. Doctoral dissertation. Los Angeles: University of California; 2002. Evaluating cutoff criteria of model fit for latent variable models with binary and continuous outcomes. [Google Scholar]
- 21.Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- 22.Mancini J, et al. Cross-cultural validation of the Decisional Conflict Scale in a sample of French patients. Qual Life Res. 2006;15:1063–8. doi: 10.1007/s11136-005-6003-9. [DOI] [PubMed] [Google Scholar]
- 23.Volk RJ, et al. Trials of decision aids for prostate cancer screening: a systematic review. Am J Prev Med. 2007;33:428–434. doi: 10.1016/j.amepre.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 24.Elwyn G, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. Bmj. 2006;333:417. doi: 10.1136/bmj.38926.629329.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elwyn G, et al. Assessing the quality of decision support technologies using the International Patient Decision Aid Standards instrument (IPDASi) PLoS ONE. 2009;4:e4705. doi: 10.1371/journal.pone.0004705. [DOI] [PMC free article] [PubMed] [Google Scholar]