SUMMARY
Several recent studies have demonstrated that the activation of protease-activated receptor 1 (PAR-1) by thrombin and activated protein C (APC) on cultured vascular endothelial cells elicits paradoxical proinflammatory and antiinflammatory responses, respectively. Noting that the protective intracellular signaling activity of APC requires the interaction of the protease with its receptor, endothelial protein C receptor (EPCR), we recently hypothesized that the occupancy of EPCR by protein C may also change the PAR-1-dependent signaling specificity of thrombin. In support of this hypothesis, we demonstrated that EPCR is associated with caveolin-1 in lipid rafts of endothelial cells and that the occupancy of EPCR by the Gla-domain of protein C/APC leads to its dissociation from caveolin-1 and recruitment of PAR-1 to a protective signaling pathway through the coupling of PAR-1 to the pertussis toxin sensitive Gi-protein. Thus, when EPCR is bound by protein C, a PAR-1-dependent protective signaling response in cultured endothelial cells can be mediated by either thrombin or APC. This article will briefly review the mechanism by which the occupancy of EPCR by its natural ligand modulates the PAR-1-dependent signaling specificity of coagulation proteases.
Keywords: EPCR, PAR-1, thrombin, protein C, signaling, specificity
INTRODUCTION
Protein C is a multi-domain vitamin K-dependent plasma serine protease zymogen that upon activation by thrombin in complex with the endothelial cell surface receptor, thrombomodulin (TM), down-regulates the clotting cascade by the proteolytic degradation of procoagulant cofactors Va and VIIIa by limited proteolysis (1). In addition to its anticoagulant function, activated protein C (APC) also exhibits potent cytoprotective and antiinflammatory properties (2–4). The protective anticoagulant and antiinflammatory activities of APC have led to FDA approval of recombinant APC as a therapeutic drug for treating severe sepsis (5). Unlike the relatively well-known mechanism of function of APC in the anticoagulant pathway (1), the exact mechanism by which APC functions in the antiinflammatory pathway is not known. However, in vitro and in vivo data in various cellular and animal models of inflammation have indicated that the interaction of APC with the endothelial protein C receptor (EPCR) is required for the cytoprotective activity of the protease (4, 6–8). It has been demonstrated that the interaction of APC with EPCR renders the protease capable of activating the G-protein coupled receptor, protease-activated receptor 1 (PAR-1), thereby initiating protective intracellular signaling responses in cells expressing both receptors (3, 4). Noting that thrombin is the only known physiological activator of protein C, and it cleaves PAR-1 with 3–4 orders of magnitude higher catalytic efficiency to elicit proinflammatory responses (9), there is controversy as to whether or not APC can exert protective activity through the cleavage of PAR-1 when thrombin is also present in the same environment (9–11). In this review, we will present some of our recent findings which suggest that the occupancy of EPCR by the Gla-domain of either the zymogen protein C or APC on vascular endothelial cells can switch the PAR-1-dependent signaling specificity of thrombin. Thus, the activation of PAR-1 by either thrombin or APC elicits protective signaling responses on cells expressing both receptors.
ACTIVATION OF PAR-1 BY COAGULATION PROTEASES
In addition to their role in the clotting cascade, coagulation proteases regulate a diverse array of cellular activities including inflammation, development, tumor growth, metastasis, apoptosis, and tissue remodeling (12–15). It has become clear in recent years that the direct cellular effects of coagulation proteases are mediated through their activation of the PAR sub-family of G-protein coupled receptors expressed on the surface of various cell types (12, 14). So far, four members of the PAR family (PAR-1, PAR-2, PAR-3 and PAR-4) have been identified (12). The protease cleavage of PARs on cell surfaces exposes a new N-terminus on the NH2-terminal domain that binds to the second membrane-spanning extracellular loop of the receptor, thereby activating them by a tethered ligand mechanism and initiating intracellular signaling responses under various pathophysiological conditions (12). PAR-1, which was first identified as a thrombin receptor on the surface of human platelets, can be cleaved by sub-nanomolar concentrations of thrombin, thereby leading to rapid platelet aggregation in response to vascular injury (12). However, subsequent studies indicated that thrombin also modulates inflammatory pathways through the activation of PAR-1 (12, 14). Thus, it has been demonstrated that thrombin up-regulates the expression of various cytokines (i.e., IL-1, IL-6 and TNF-α) and cell adhesion molecules; E-selectin, P-selectin, intracellular adhesion molecule-1 and vascular cell adhesion molecule-1 on endothelial cells (15, 16). Thrombin also induces apoptosis through the activation of caspases and enhances the barrier permeability of endothelial cells through the PAR-1-dependent activation of the Rho family of GTPases (17, 18). Thrombin mediates its proinflammatory properties through activation of the nuclear factor (NF)-κB pathway in endothelial cells (16, 18). Paradoxically, when APC forms a complex with EPCR it evokes antiinflammatory responses in endothelial cells, apparently through the activation of PAR-1 (3, 4). Thus, it has been demonstrated that the APC-EPCR complex inhibits the cytokine-mediated activation of NF-κB and down-regulates the expression of proinflammatory cytokines by activation of PAR-1 in endothelial cells (3, 4, 16, 19). It is thought that these properties of APC are responsible, at least partially, for the beneficial protective effects of the protease in reducing the mortality rate of severe sepsis (4, 5, 7, 14). Noting that thrombin is the only known physiological activator of protein C, and that it cleaves PAR-1 with 3–4 orders of magnitude higher catalytic efficiency than APC to elicit proinflammatory responses (9), the hypothesis that APC can exert protective activity through the cleavage of the same receptor has remained controversial (9–11). Since most of the PAR-1-dependent proinflammatory activities of thrombin are based on in vitro data, as described below, a partial answer for this question my be provided by our recent discovery that the occupancy of EPCR plays a critical role in determining the PAR-1-dependent signaling specificity of coagulation proteases in vascular endothelial cells independent of the protease (thrombin or APC) activating the receptor (19). Thus, the physiological relevance of the in vitro data in cellular models proposing PAR-1-dependent proinflammatory activities for thrombin requires further investigation.
EPCR OCCUPANCY ALTERS THE SIGNALING SPECIFICITY OF PAR-1
In a series of recent studies, we employed molecular biology and biochemical approaches to investigate the mechanism by which the activation of PAR-1 by the two proteases thrombin and APC may initiate two opposite responses in endothelial cells. Our aim was to investigate two questions. 1) Is the level of receptor activation by thrombin and APC a contributing factor in determining the specificity of signaling responses in endothelial cells? 2) Does the occupancy of EPCR by the Gla-domain of APC alter the signaling specificity of PAR-1 in endothelial cells? To provide answers to these questions, we engineered a meizothrombin derivative in which the Gla-domain of the thrombin intermediate was changed with the corresponding domain of APC as described in Fig. 1 (19, 20). Relative to APC, the protein C-meizothrombin chimera cleaved PAR-1 with a 3-4-fold higher catalytic efficiency similar to that observed with thrombin (20). The mutant construct also interacted with EPCR with an affinity that was essentially identical to that observed with protein C/APC (20). Interestingly, however, this meizothrombin derivative elicited a PAR-1-dependent barrier protective response in human umbilical vein endothelial cells (HUVECs) (Fig. 2A) with an efficiency that was at least 10-fold higher than the protective effect of APC (20). These results suggested that the binding of Gla-domain of APC to EPCR determines the type of PAR-1 response rather than the protease type that cleaves the receptor (20). To provide further support for this hypothesis, the effect of PAR-1 cleavage by thrombin was monitored in endothelial cells which had been pretreated with the catalytically inactive Ser-195 to Ala (PC-S195A) substitution mutant of protein C (19, 20). The results revealed that when endothelial EPCR is occupied by its ligand, the cleavage of PAR-1 by thrombin elicits only protective signaling responses in endothelial cells (19, 20) (Fig. 2A). To provide firm support for this hypothesis, HUVECs were pre-incubated with a sub-physiological concentration of the zymogen protein C (50-nM of either wild-type protein C or PC-S195A) and used the thrombin receptor agonist peptides (TRAP) SFLLRN or TFLLRN to activate PAR-1 (19–22). In agreement with the hypothesis, both PAR-1 agonist peptides elicited a barrier disruptive response in HUVECs that could be effectively reversed to a protective response if cells were pretreated with the protein C zymogen prior to stimulation by TRAPs (Fig. 2B). Further studies demonstrated that when EPCR is occupied by protein C, thrombin activation of PAR-1 inhibited the activation of RhoA GTPase and enhanced the activation of Rac1 GTPase in the TNF-α-stimulated endothelial cells, which is similar to the response observed with APC (19, 21). Furthermore, thrombin inhibited the NF-κB pathway by a PAR-1-dependent mechanism if cells were pretreated with the protein C zymogen (19, 21). These results suggested that the activation of PAR-1 by thrombin on intact vascular endothelial cells expressing EPCR would initiate potent protective intracellular signaling responses in the presence of physiological concentrations of the zymogen protein C.
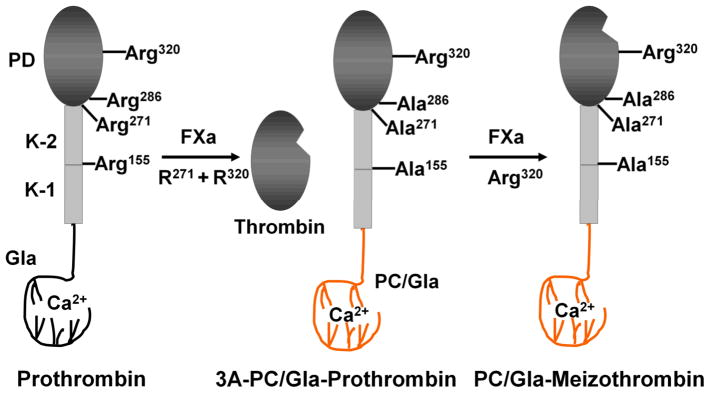
Fig. 1.
Cartoons of construction and factor Xa activation of wild-type and the three Arg to Ala substitution mutants of prothrombin. (Left Panel) The proteolytic cleavage of wild-type prothrombin by factor Xa after Arg-320 generates an intermediate active product called meizothrombin. A second cleavage at Arg-271 by factor Xa separates the catalytic domain of prothrombin from the non-catalytic Gla, Kringle-1 and Kringle-2 domains to yield thrombin. Further cleavages at Arg-155 and Arg-286 can occur by a feed-back cleavage mechanism by both thrombin and meizothrombin. (Central Panel) A prothrombin derivative (3A-PC/Gla-prothrombin) was prepared in which the Gla-domain of the molecule was replaced with the corresponding domain of protein C and its Arg-155, Arg-271 and Arg-286 residues were substituted with 3 Ala residues. (Right Panel) The prothrombin chimeric mutant can be activated by factor Xa through cleavage after Arg-320 to yield the active protease PC/Gla-meizothrombin. K-1 and K-2 represent Kringle-1 and 2 domains of prothrombin, respectively. PD, protease domain. The figure is adopted from Ref. 19 with modifications.
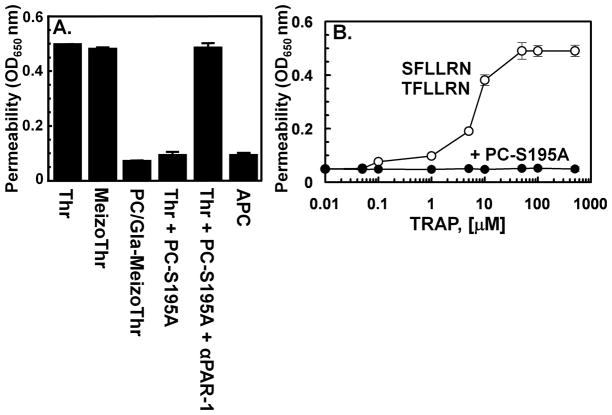
Fig. 2.
The occupancy of EPCR by the Gla-domain of protein C switches the PAR-1-dependent signaling specificity of thrombin from a barrier-enhancing effect to a barrier protective response in HUVECs. The barrier protective effect of proteases (1 nM for thrombin and meizothrombin derivatives and 10 nM for APC) in HUVECs in response to proinflammatory stimuli (10 nM thrombin or 10 ng/mL LPS) with or without pretreatment of cells with PC-S195A (50 nM) and a function-blocking antibody to PAR-1 was monitored as described in Ref. 22. (B) The concentration dependence of the barrier permeability effect of the PAR-1 agonist peptides (either SFLLRN or TFLLRN) was monitored in the absence (○) or presence of 50 nM PC-S195A (●) as described in Ref. 20.
It is worth noting that a recent study demonstrated that the PAR-1 cleavage dependent signaling by thrombin leads to rapid internalization of the receptor, whereas the APC-activated PAR-1 remains for a longer time on the surface of cultured endothelial cells (23). The relevance of this observation to paradoxical PAR-1 signaling by the two proteases requires further investigation, nevertheless, these results raise the possibility that differences in the duration of PAR-1 signaling in endothelial cells may also alter the signaling specificity of coagulation proteases.
EPCR AND PAR-1 ARE ASSOCIATED WITH CAVEOLIN-1 IN LIPID-RAFTS
Recent results have demonstrated that both EPCR and PAR-1 are localized and/or associated with caveolin-1 within the cholesterol rich lipid-rafts in HUVECs (24). However, in a recent study, by co-immunoprecipitation and immunoblotting approaches using specific antibodies for EPCR, PAR-1 and caveolin-1, our results indicated that EPCR no longer remains associated with caveolin-1 if endothelial cells were pretreated with PC-S195A (19, 20). Thus, the ligand occupancy of EPCR led to dissociation and/or emigration of the receptor from the caveolar compartment, a process that appears to change the PAR-1-dependent signaling specificity of thrombin and APC from a permeability-enhancing to a barrier-protective response (19, 20). Similar results have been obtained in human pulmonary arterial endothelial cells (22). Thus, the thrombin cleavage of PAR-1 in endothelial cells of both venular and arterial beds expressing EPCR initiates protective signaling responses as long as there is sufficient ligand (protein C) to occupy the receptor (19–22). Consistent with our results, a recent study showed that the PAR-1-dependent activation of Rac1 by APC requires caveolin-1, whereas the PAR-1-dependent activation of RhoA by thrombin was independent of caveolin-1 (25), suggesting a key role for the caveolin-1 modulation of the signaling specificity of coagulation proteases. To provide further support for the hypothesis that the interaction of the Gla-domain of APC with EPCR alters the PAR-1-dependent signaling specificity of coagulation proteases, in a recent study we showed that the APC deletion mutant lacking the Gla-domain (activated GD-PC), similar to thrombin, elicits a PAR-1-dependent disruptive proinflammatory response in endothelial cells (26). However, preincubation of cells with PC-S195A switched the signaling specificity of activated GD-PC to a protective response similar to that observed with APC (26), confirming our hypothesis that if EPCR is occupied by protein C the activation of PAR-1 is protective, independent of the protease cleaving the receptor.
How the occupancy of EPCR changes the PAR-1-dependent signaling specificity of coagulation proteases is not known. PAR-1 is known to signal through coupling with different members of the G proteins including Gi/o, Gq and G12/13 (17, 19, 25, 27). It has been hypothesized that thrombin disrupts the endothelial barrier function through activation of PAR-1 coupled to Gq and/or G12/13, but APC signals through the cleavage of PAR-1 coupled to Gi/o (17, 19, 27). Among these G proteins, only the signaling function of Gi/o is sensitive to the pertussis toxin (PTX) (28). Interestingly, we demonstrated that while the PAR-1-dependent permeability-enhancing effect of thrombin is insensitive to PTX, the protective response of thrombin is sensitive to PTX in PC-S195A pretreated endothelial cells, suggesting that the ligand occupancy of EPCR recruits PAR-1 to a protective pathway, possibly by coupling the receptor to Gi/o-protein in endothelial cells (19, 20). Similar to the protective activity of APC (18, 29), the EPCR-dependent barrier protective activity of thrombin was also demonstrated to require signaling via another Gi-protein coupled receptor, sphingosine 1-phosphate receptor 1 (S1P1) (19, 22). Thus, it is not known whether the PTX sensitivity of the barrier protective activity of thrombin is due to EPCR facilitating a direct coupling of PAR-1 to Gi or if it is due to EPCR mediating it indirectly via the PI3-kinase dependent phosphorylation and activation of S1P1 independent of its ligand S1P (18, 29, 30). A hypothetical model showing how the occupancy of EPCR changes the PAR-1-dependent signaling specificity of thrombin in endothelial cells is presented in Fig. 3.
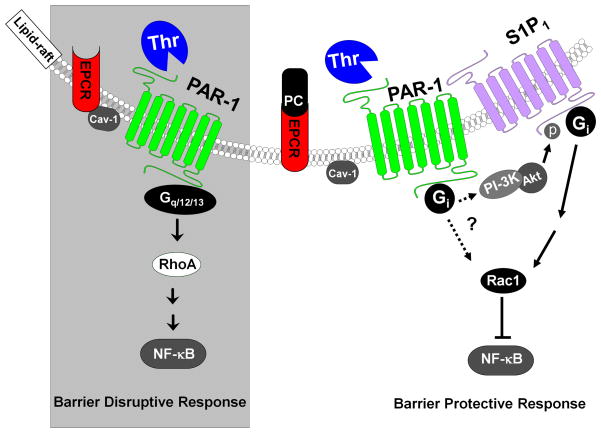
Fig. 3.
Cartoons of PAR-1-dependent signaling by APC and thrombin in endothelial cells when EPCR is either free or occupied by protein C. (Left Panel) EPCR interacts with caveolin-1 (Cav-1) within lipid-rafts of endothelial cells when it is not occupied by the Gla-domain of protein C. Thrombin cleavage of PAR-1 activates RhoA, up-regulates the NF-κB pathway and elicits disruptive signaling responses by signaling through G12/13 and/or Gq proteins. (Right Panel) The occupancy of EPCR by protein C leads to dissociation of EPCR from caveolin-1 and a switch in the specificity of PAR-1, presumably by signaling via the Gi-protein. Under these conditions, the thrombin cleavage of PAR-1 activates Rac1, inhibits the activation of the NF-κB pathway and initiates protective responses in endothelial cells. See the text for more detail. The figure is adopted from Ref. 39 with modifications.
EPCR OCCUPANCY ALTERS THE SIGNALING SPECIFICITY OF PAR-2
In addition to thrombin and APC, other coagulation proteases activate PAR-1 and PAR-2 to initiate both proinflammatory and antiinflammatory responses (14, 31). For instance, factor VIIa (FVIIa), by itself or in complex with tissue factor (TF), has been demonstrated to modulate cellular responses through activation of PAR-2 on endothelial and other cell types (14, 31, 32). Furthermore, it has been demonstrated that the TF-FVIIa complex can indirectly signal through both PAR-1 and PAR-2 by activating FX to FXa on endothelial cells (33). In a recent report, it was demonstrated that FXa, through activation of both PAR-1 and PAR-2 can initiate potent barrier protective effects in HUVECs in response to proinflammatory stimuli (34). Other studies have reported a proinflammatory role for FXa in similar cellular model systems (35). The discrepancy in results between different studies is not known. Interestingly, in a recent study we demonstrated that, in addition to changing the signaling specificity of PAR-1, the occupancy of EPCR by protein C also switches the signaling specificity of PAR-2 from a barrier disruptive to a barrier protective effect in endothelial cells (36). This was evidenced by the observation that, similar to PAR-1 agonist peptides (36), the PAR-2 agonist peptide, SLIGKV, also elicited a barrier disruptive response in HUVECs that could be effectively reversed to a protective response if cells were pretreated with the protein C zymogen prior to stimulation by the PAR-2 agonist peptide (36). These results suggested that the occupancy of EPCR by protein C recruits both PAR-1 and PAR-2 to protective pathways. Thus, it appears that all coagulation proteases capable of cleaving these receptors would elicit only protective signaling responses if cells express EPCR and the receptor is exposed to physiological concentrations of protein C. These results challenge the physiological relevance of in vitro studies in cellular models, which have all monitored the signaling effects of PAR-1 and PAR-2 in the absence of EPCR occupancy and thus reported disruptive proinflammatory roles for activation of both receptors in cultured endothelial cells.
CROSSTALKS BETWEEN PAR-1 AND OTHER RECEPTORS
In addition to EPCR, recent results have indicated that the PAR-1-dependent protective signaling activity of both APC and thrombin requires receptor crosstalks with a number of other G-protein and non-G-protein coupled receptors. Thus, it has been demonstrated that siRNA for S1P1 eliminates the protective effect of APC in response to proinflammatory stimuli in endothelial cells (18, 19, 22, 29). Two other recent studies demonstrated that APC-mediated signaling via both apolipoprotein E receptor 2 (ApoER2) and angiopoietin/Tie2 pathways also contributes to cytoprotective and antiinflammatory properties of APC (37, 38). Thus, the knockdown or blockade of either one of these receptors (EPCR, PAR-1, S1P1, ApoER2 or Tie2) eliminated the protective effect of APC in cellular models. We have demonstrated a similar requirement for crosstalks between PAR-1 with S1P1 and Tie2 for the protective effect of thrombin in endothelial cells pretreated with PC-S195A (19, 39), suggesting that coordinated communications among these receptors are involved in determining the PAR-1-dependent signaling specificity of coagulation proteases. EPCR and PAR-1-dependent cytoprotective and antiinflammatory activity for APC has been established in animal models of inflammation (6, 7, 40, 41). In murine models of endotoxemia, it has been demonstrated that APC does not exert a protective effect in genetically altered mice either lacking PAR-1 or expressing sub-optimal levels of EPCR (7, 40, 41). Results from a recent study using a similar model of endotoxemia indicated that, the interaction of APC with the macrophage integrin CD11b mediates the PAR-1-dependent protective activity of APC by an EPCR-independent mechanism (42), suggesting that EPCR co-receptor signaling may not contribute to protective PAR-1 signaling in immune cells. However, another study using a similar model, demonstrated that the expression of EPCR on CD8+ dendritic cells is required for the protective activity of APC (40). Another recent study demonstrated that unlike the crosstalk between PAR-1 and S1P1 which elicits a protective response in endothelial cells (29), crosstalk between PAR-1 and S1P3 on both vascular and dendritic cells evokes proinflammatory responses (43), providing a clue for the mechanism by which PAR-1 activation can elicit paradoxical signaling responses through interaction with different co-receptors. In addition to PAR-1, in vitro and in vivo studies have indicated that the optimal EPCR-dependent cytoprotective activity of APC also requires PAR-3 in neurons (44). These results underscore the complex nature of crosstalks that could be controlling the PAR-1 signaling network under different pathophysiological conditions. A better understanding of the mechanism of receptor crosstalks among the players of the PAR-1 axis is imperative to developing new strategies for treating severe inflammatory disorders.
CONCLUDING REMARKS
Our in vitro results in cellular models suggest that the activation of both PAR-1 and PAR-2 by coagulation proteases would elicit only protective signaling responses in endothelial cells if they express EPCR and that the receptor is occupied by the Gla-domain of its ligand protein C/APC. The relevance of these findings to the physiology of PAR-1 and PAR-2 under in vivo conditions is not known and requires further investigation. We hypothesize that the protective EPCR co-receptor signaling on endothelial cells may constitute a self-guarding mechanism that ensures PAR-1 signaling by thrombin and other coagulation proteases does not disrupt the integrity of healthy vasculature. Thus, only during injury, trauma and inflammation, which can lead to denudation of the endothelium and/or down-regulation of the cell surface EPCR, the activation of these receptors by coagulation proteases initiates proinflammatory responses. We recently demonstrated that TM is localized with EPCR and PAR-1 within lipid-rafts of endothelial cells (24). Based on our in vitro data, it appears that, similar to TM, EPCR is involved in modulating the physiological function of thrombin in circulation. TM, by directly interacting with thrombin, switches the specificity of thrombin in the clotting cascade (1), and EPCR, by interacting with protein C, indirectly switches the PAR-1-dependent signaling specificity of thrombin in the signaling pathway (19). It is worth noting that a similar regulatory role for TM in altering the PAR-1-dependent signaling function of thrombin has also been reported (45). In a recent study, we showed that thrombin in complex with TM is a less effective activator of PAR-1 than APC (26). Noting that TM is abundantly expressed on capillary endothelial cells (46) and that it has a much higher affinity than PAR-1 for thrombin, the possibility that APC is the primary PAR-1 activator in microcirculation needs to be considered.
Acknowledgments
I thank Audrey Rezaie for proofreading the manuscript. The research discussed herein was supported by grants awarded by the National Heart, Lung, and Blood Institute of the National Institutes of Health (Grants No. HL 101917 and HL 68571).
References
- 1.Esmon CT. Molecular events that control the protein C anticoagulant pathway. Thromb Haemost. 1993;70:29–35. [PubMed] [Google Scholar]
- 2.Taylor FB, Jr, Chang A, Esmon CT, D’Angelo A, Vigano-D’Angelo S, Blick KE. Protein C prevents the coagulopathic and lethal effects of E coli infusion in the baboon. J Clin Invest. 1987;79:918–925. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296:1880–1882. doi: 10.1126/science.1071699. [DOI] [PubMed] [Google Scholar]
- 4.Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood. 2007;109:3161–3172. doi: 10.1182/blood-2006-09-003004. [DOI] [PubMed] [Google Scholar]
- 5.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJJ. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Eng J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 6.Taylor FB, Stearns-Kurosawa DJ, Kurosawa S, Ferrell G, Chang AC, Laszik Z, Kosanke S, Peer G, Esmon CT. The endothelial cell protein C receptor aids in host defense against Escherichia coli sepsis. Blood. 2000;95:1680–1686. [PubMed] [Google Scholar]
- 7.Kerschen EJ, Fernandez JA, Cooley BC, Yang XV, Sood R, Mosnier LO, Castellino FJ, Mackman N, Griffin JH, Weiler H. Endotoxemia and sepsis mortality reduction by non-anticoagulant-activated protein C. J Exp Med. 2007;204:2439–2448. doi: 10.1084/jem.20070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng T, Liu D, Griffin JH, Fernández JA, Castellino F, Rosen ED, Fukudome K, Zlokovic BV. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat Med. 2003;9:338–342. doi: 10.1038/nm826. [DOI] [PubMed] [Google Scholar]
- 9.Ludeman MJ, Kataoka H, Srinivasan Y, Esmon NL, Esmon CT, Coughlin SR. PAR1 cleavage and signaling in response to activated protein C and thrombin. J Biol Chem. 2005;280:13122–13128. doi: 10.1074/jbc.M410381200. [DOI] [PubMed] [Google Scholar]
- 10.Esmon CT. Is APC activation of endothelial cell PAR1 important in severe sepsis? No. J Thromb Haemost. 2005;3:1910–1911. doi: 10.1111/j.1538-7836.2005.01573.x. [DOI] [PubMed] [Google Scholar]
- 11.Ruf W. Is APC activation of endothelial cell PAR1 important in severe sepsis? Yes. J Thromb Haemost. 2005;3:1912–1914. doi: 10.1111/j.1538-7836.2005.01576.x. [DOI] [PubMed] [Google Scholar]
- 12.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 13.Yin YJ, Salah Z, Maoz M, Ram SC, Ochayon S, Neufeld G, Katzav S, Bar-Shavit R. Oncogenic transformation induces tumor angiogenesis: a role for PAR1 activation. FASEB J. 2003;17:163–174. doi: 10.1096/fj.02-0316com. [DOI] [PubMed] [Google Scholar]
- 14.Ruf W, Dorfleutner A, Riewald M. Specificity of coagulation factor signaling. J Thromb Haemost. 2003;1:1495–1503. doi: 10.1046/j.1538-7836.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 15.Kaplanski G, Marin V, Fabrigoule M, Boulay V, Benoliel AM, Bongrand P, Kaplanski S, Farnarier C. Thrombin-activated human endothelial cells support monocyte adhesion in vitro following expression of intracellular adhesion molecule-1 (ICAM-1; CD54) and vascular cell adhesion molecule-1 (VCAM-1; CD106) Blood. 1998;92:1259–1267. [PubMed] [Google Scholar]
- 16.Joyce DE, Gelbert L, Ciaccia A, DeFoff B, Grinnell BW. Gene expression profile of antithrombotic protein C defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001;276:11199–11203. doi: 10.1074/jbc.C100017200. [DOI] [PubMed] [Google Scholar]
- 17.Camerer E, Coughlin SR. APC signaling: tickling PAR1 for barrier protection? Blood. 2005;105:3004–3005. [Google Scholar]
- 18.Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JGN. Activated protein C mediates novel lung endothelial barrier enhancement: Role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- 19.Rezaie AR. Regulation of the protein C anticoagulant and antiinflammatory pathways. Curr Med Chem. 2010;17:2059–2069. doi: 10.2174/092986710791233706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bae JS, Yang L, Manithody C, Rezaie AR. The ligand occupancy of endothelial protein C receptor switches the PAR-1-dependent signaling specificity of thrombin from a permeability-enhancing to a barrier-protective response in endothelial cells. Blood. 2007;110:3909–3916. doi: 10.1182/blood-2007-06-096651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bae JS, Rezaie AR. Thrombin inhibits nuclear factor kappaB and RhoA pathways in cytokine-stimulated vascular endothelial cells when EPCR is occupied by protein C. Thromb Haemost. 2009;101:513–520. [PMC free article] [PubMed] [Google Scholar]
- 22.Bae JS, Rezaie AR. Protease activated receptor 1 (PAR-1) activation by thrombin is protective in human pulmonary artery endothelial cells if endothelial protein C receptor is occupied by its natural ligand. Thromb Haemost. 2008;100:101–109. doi: 10.1160/TH08-02-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schuepbach RA, Feistritzer C, Brass LF, Riewald M. Activated protein C-cleaved protease activated receptor-1 is retained on the endothelial cell surface even in the presence of thrombin. Blood. 2008;111:2667–2673. doi: 10.1182/blood-2007-09-113076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bae JS, Yang L, Rezaie AR. Receptors of the protein C activation and activated protein C signaling pathways are colocalized in lipid rafts of endothelial cells. Proc Natl Acad Sci (USA) 2007;104:2867–2872. doi: 10.1073/pnas.0611493104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russo A, Soh UJ, Paing MM, Arora P, Trejo J. Caveolae are required for protease-selective signaling by protease-activated receptor-1. Proc Natl Acad Sci (USA) 2009;106:6393–6397. doi: 10.1073/pnas.0810687106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae JS, Yang L, Rezaie AR. Lipid raft localization regulates the cleavage specificity of protease activated receptor 1 in endothelial cells. J Thromb Haemost. 2008;6:954–961. doi: 10.1111/j.1538-7836.2008.02924.x. [DOI] [PubMed] [Google Scholar]
- 27.McLaughlin JN, Shen L, Holinstat M, Brooks JD, DiBenedetto E, Hamm HE. Functional selectivity of G protein signaling by agonist peptides and thrombin for the protease-activated receptor-1. J Biol Chem. 2005;280:25048–25059. doi: 10.1074/jbc.M414090200. [DOI] [PubMed] [Google Scholar]
- 28.Hepler JR, Gilman AG. G proteins. Trends Biochem Sci. 1992;17:383–387. doi: 10.1016/0968-0004(92)90005-t. [DOI] [PubMed] [Google Scholar]
- 29.Feistritzer C, Riewald M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood. 2005;105:3178–3184. doi: 10.1182/blood-2004-10-3985. [DOI] [PubMed] [Google Scholar]
- 30.Bae JS, Kim YU, Park MK, Rezaie AR. Concentration dependent dual effect of thrombin in endothelial cells via Par-1 and Pi3 Kinase. J Cell Physiol. 2009;219:744–751. doi: 10.1002/jcp.21718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awasthi V, Mandal SK, Papanna V, Mohan Rao LV, Pendurthi UR. Modulation of tissue factor-factor VIIa signaling by lipid rafts and caveolae. Arterioscler Thromb Vasc Biol. 2007;27:1447–1455. doi: 10.1161/ATVBAHA.107.143438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris DR, Ding Y, Ricks TK, Gullapalli A, Wolfe BL, Trejo J. Protease-activated receptor-2 is essential for factor VIIa and Xa-induced signaling, migration, and invasion of breast cancer cells. Cancer Res. 2006;66:307–314. doi: 10.1158/0008-5472.CAN-05-1735. [DOI] [PubMed] [Google Scholar]
- 33.Riewald M, Ruf W. Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proc Natl Acad Sci (USA) 2001;98:7742–7747. doi: 10.1073/pnas.141126698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feistritzer C, Lenta R, Riewald M. Protease-activated receptor-1 and -2 can mediate endothelial barrier protection: role in factor Xa signaling. J Thromb Haemost. 2005;3:2798–2805. doi: 10.1111/j.1538-7836.2005.01610.x. [DOI] [PubMed] [Google Scholar]
- 35.Senden NH, Jeunhomme TM, Heemskerk JW, Wagenvoord R, van’t Veer C, Hemker HC, Buurman WA. Factor Xa induces cytokine production and expression of adhesion molecules by human umbilical vein endothelial cells. J Immunol. 1998;161:4318–4324. [PubMed] [Google Scholar]
- 36.Bae JS, Yang L, Rezaie AR. Factor X/Xa elicits protective signaling responses in endothelial cells directly via PAR-2 and indirectly via endothelial protein C receptor-dependent recruitment of PAR-1. J Biol Chem. 2010;285:34803–34812. doi: 10.1074/jbc.M110.163642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang XV, Banerjee Y, Fernandez JA, Deguchi H, Xu X, Mosnier LO, Urbanus RT, de Groot PG, White-Adams TC, McCarty OJT, Griffin JH. Activated protein C ligation of ApoER2 (LRP8) causes Dab1-dependent signaling in U937 cells. Proc Natl Acad Sci (USA) 2009;106:274–279. doi: 10.1073/pnas.0807594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minhas N, Xue M, Fukudome K, Jackson CJ. Activated protein C utilizes the angiopoietin/Tie2 axis to promote endothelial barrier function. FASEB J. 2010;24:873–881. doi: 10.1096/fj.09-134445. [DOI] [PubMed] [Google Scholar]
- 39.Bae JS, Rezaie AR. Thrombin upregulates the angiopoietin-Tie2 Axis: endothelial protein C receptor occupancy prevents the thrombin mobilization of angiopoietin 2 and P-selectin from Weibel-Palade bodies. J Thromb Haemost. 2010;8:1107–1115. doi: 10.1111/j.1538-7836.2010.03812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerschen E, Hernandez I, Zogg M, Jia S, Hessner MJ, Fernandez JA, Griffin JH, Huettner CS, Castellino FJ, Weiler H. Activated protein C targets CD8+ dendritic cells to reduce the mortality of endotoxemia in mice. J Clin Invest. 2010;120:3167–3178. doi: 10.1172/JCI42629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iwaki T, Cruz DT, Martin JA, Castellino FJ. A cardioprotective role for the endothelial protein C receptor in lipopolysaccharide-induced endotoxemia in the mouse. Blood. 2005;105:2364–2371. doi: 10.1182/blood-2004-06-2456. [DOI] [PubMed] [Google Scholar]
- 42.Cao C, Gao Y, Li Y, Antalis TM, Castellino FJ, Zhang L. The efficacy of activated protein C in murine endotoxemia is dependent on integrin CD11b. J Clin Invest. 2010;120:1971–1980. doi: 10.1172/JCI40380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niessen F, Schaffner F, Furlan-Freguia C, Pawlinski R, Bhattacharjee G, Chun J, Derian CK, Andrade-Gordon P, Rosen H, Ruf W. Dendritic cell PAR1-S1P3 signaling couples coagulation and inflammation. Nature. 2008;452:654–658. doi: 10.1038/nature06663. [DOI] [PubMed] [Google Scholar]
- 44.Guo H, Liu D, Gelbard H, Cheng T, Insalaco R, Fernandez JA, Griffin JH, Zlokovic BV. Activated protein C prevents neuronal apoptosis via protease activated receptors 1 and 3. Neuron. 2004;41:563–572. doi: 10.1016/s0896-6273(04)00019-4. [DOI] [PubMed] [Google Scholar]
- 45.Olivot JM, Estebanell E, Lafay M, Brohard B, Aiach M, Rendu F. Thrombomodulin prolongs thrombin-induced extracellular signal-regulated kinase phosphorylation and nuclear retention in endothelial cells. Circ Res. 2001;88:681–687. doi: 10.1161/hh0701.088769. [DOI] [PubMed] [Google Scholar]
- 46.Busch C, Cancilla P, DeBault L, Goldsmith J, Owen W. Use of endothelium cultured on microcarriers as a model for the microcirculation. Lab Invest. 1982;47:498–504. [PubMed] [Google Scholar]