Notch1 has been shown to be a tumor suppressor in neuroendocrine tumors. Previous in vitro studies in neuroendocrine tumor cell lines have also suggested that valproic acid, a histone deacetylase inhibitor, can induce Notch1 and that Notch1 activation correlates with a decrease in tumor markers for neuroendocrine tumors. This study showed that valproic acid activates Notch1 signaling in vivo and may have a role in treating low-grade neuroendocrine tumors.
Keywords: Neuroendocrine tumors, Valproic acid, Histone deacetylase inhibitor, Pancreatic carcinoid, Notch signaling
Abstract
Introduction.
Notch1 has been shown to be a tumor suppressor in neuroendocrine tumors (NETs). Previous in vitro studies in NET cell lines have also suggested that valproic acid (VPA), a histone deacetylase inhibitor, can induce Notch1 and that Notch1 activation correlates with a decrease in tumor markers for NETs. Thus, this study aimed to evaluate the role of VPA in treating NETs and to determine whether VPA induced the Notch signaling pathway signaling in vivo.
Patients and Methods.
Eight patients with low-grade NETs (carcinoid and pancreatic) were treated with 500 mg of oral VPA twice a day with dosing adjusted to maintain a goal VPA level between 50 and 100 μg/mL. All patients were followed for 12 months or until disease progression.
Results.
Notch1 signaling was absent in all tumors prior to treatment and was upregulated with VPA. One patient had an unconfirmed partial response and was noted to have a 40-fold increase in Notch1 mRNA levels. Four patients had stable disease as best response. Tumor markers improved in 5 out of 7 patients. Overall, treatment with VPA was well tolerated.
Conclusion.
VPA activates Notch1 signaling in vivo and may have a role in treating low-grade NETs.
Introduction
Neuroendocrine tumors (NETs) include a range of rare and diverse neoplasms arising from the neuroendocrine system. NETs include low-grade, indolent tumors like carcinoid tumors, gastroenteropancreatic endocrine tumors, medullary carcinomas of the thyroid, and high-grade, aggressive tumors like catecholamine secreting tumors, Merkel cell carcinoma, and other rare tumors [1, 2]. These tumors are characterized histologically by the presence of neurosecretory granules. The tumors react positively to silver stains and to markers of neuroendocrine tissue including neuron-specific enolase, synaptophysin, and chromogranin that reflect tumor activity in vivo [3–5]. NETs represent 0.5% of all malignancies; however, their incidence has risen from 1.09 out of 100,000 in 1973 to 5.25 out of 100,000 in 2004 [6, 7]. The gastroenteropancreatic NETs include pancreatic islet cell tumors and carcinoid tumors and are the most common of the NETs [1, 8]. Despite being the most common NET, gastroenteropancreatic NETs represent only 2% of all gastrointestinal malignant neoplasms [9, 10].
For low-grade NETs, the definitive management for localized disease is surgical resection [11]; however, the diagnosis of these tumors is often delayed and many patients are, therefore, diagnosed with metastatic or inoperable tumors. For these patients, observation is usually sufficient management until the patient either develops symptoms secondary to hormone production or tumor bulk or shows evidence of rapidly progressive disease regardless of symptoms.
Patients who become symptomatic from hormonal hypersecretion can be treated effectively with somatostatin analogs, most commonly, octreotide [12]. Recent data from the PROMID study also suggest that treatment with octreotide LAR may improve progression-free survival (PFS) [13]. α-Interferon has also been shown to improve symptoms of hormonal hypersecretion in patients with both carcinoid and pancreatic NETs, both alone and in combination with somatostatin analogs [12]. Although both somatostatin analogs and α-interferon can result in tumor stabilization, neither agent is considered effective in controlling tumor growth [12, 14]. Tumor response has been reported in ∼10%–15% of patients treated with interferon, but tumor regression has been reported to occur only in <5% of patients treated with somatostatin analogs [12, 14, 15].
Cytotoxic chemotherapy is also a therapeutic consideration in patients who are symptomatic secondary to tumor bulk or who have rapidly progressive disease. In general, the response to chemotherapy has had limited success in patients with gastroenteropancreatic NETs. Multiple chemotherapeutic agents have been assessed alone or in combination for patients with advanced carcinoid and pancreatic islet cell NETs. The response rate to chemotherapy in metastatic carcinoid tumors has been reported to be no higher than ∼20%–30% [16]. In endocrine pancreatic tumors, streptozocin combined with doxorubicin has been reported to generate responses in 69% of patients [17]; however, the determination of response in this trial contained methods unacceptable to today's standards. Researchers at the Memorial Sloan-Kettering Cancer Center (MSKCC) reported a patient series treated with this regimen with a response rate of only 6% as determined by standard common toxicity criteria (CTC) [18]. Further studies evaluating first-line chemotherapy for islet cell tumors have confirmed a low response rate, including studies evaluating topotecan, bortezomib, and gemcitabine, all of which reported a 0% response rate [19–21]. Recent data from a phase III trial involving the use of sunitinib in patients with advanced pancreatic NETs, however, suggest improved treatment outcomes (PFS and possibly overall survival [OS]) in this patient population [22]. In addition to the overall low response rates, however, the chemotherapeutic regimens recommended in NETs are also associated with significant toxicities [23]. Clearly, in patients with a potentially indolent disease, reducing the toxicities associated with treatment is of utmost importance. Less toxic, effective therapies for this population of patients are urgently needed.
Recent studies have shown that Notch1 signaling has a role in tumor suppression [24]. It has also been found that Notch1 signaling is very minimal or nonexistent in NETs [25] and that the activation of Notch1 signaling in NET cell lines results in a significant decrease in protein secretion of both chromogranin A and synaptophysin levels, serotonin secretion, and tumor growth in BON (human gastrointestinal [GI] carcinoid) and H727 (human pulmonary carcinoid) cell lines [26–29]. The search for compounds that activate the Notch1 signaling pathway revealed valproic acid (VPA) as a potential agent [30]. VPA, a fatty acid, is used primarily in the treatment of seizure disorders and bipolar disorder [31]. A study conducted with the BON and H727 cell lines confirmed VPA's ability to increase Notch1 levels and consequently decrease tumor biomarkers and hormone secretion. The study also confirmed VPA-associated reduction in tumor growth in nude mice that were transfected with both (BON and H727) human tumor xenografts [30]. The precise mechanism through which VPA increases Notch1 signaling remains unclear but may be related to its property as a histone deacetylase inhibitor [32, 33].
Thus, on the basis of the importance of Notch1 signaling in NET hormone production and tumor proliferation, as well as the effects of VPA on Notch1 in vivo and in vitro, we conducted a pilot study to evaluate the effects of VPA on tumor marker production, tumor response, survival, and Notch signaling.
Materials and Methods
Patients
To be eligible for the trial, patients had to have histologically confirmed metastatic low-grade NETs. All tumor specimens were submitted to a central NET pathologist for confirmation of the diagnosis and tumor grade. Patients with small-cell lung cancers, paragangliomas, and pheochromocytomas were excluded. Patients were required to have measurable disease per the Response Evaluation Criteria in Solid Tumors (RECIST) v1.0 [34]. All eligible patients were ≥4 weeks from the completion of major surgery, chemotherapy, or other systemic therapy or local liver therapy and ≥3 weeks from the completion of radiation therapy. Eligible patients had an absolute neutrophil count ≥1,000/mm3, platelets ≥75,000/mm3, total bilirubin <2.0× the upper limit of normal (ULN) or aspartate aminotransferase (AST) <3× ULN, and a creatinine <2× ULN. Patients needed to be ≥18 years old, needed to have an Eastern Cooperative Oncology Group (ECOG) performance status of ≤2, and were required to be able to provide informed consent regarding the treatment itself as well as pre- and post-treatment biopsies. All patients were enrolled at the University of Wisconsin. All aspects of the study (design, protocol, and ethics) were approved by the University of Wisconsin scientific review committee and the Institutional Review Board. This protocol was completed in March 2010.
Patients were excluded from the trial if they were on concurrent chemotherapy or radiotherapy. Patients with gastrointestinal tract disease resulting in an inability to take oral medication (i.e., peptic ulcer disease, uncontrolled nausea, vomiting, diarrhea, bowel obstruction, or inability to swallow tablets), a history of hypothyroid disease, or significant, active cardiac disease were also excluded. Lastly, pregnant or lactating women were excluded from participation in the trial due to concern for side effects in the developing embryo and infant.
Treatment
VPA was given at a starting dose of 500 mg orally, two times a day with a goal target serum level between 50 and 100 μg/mL. VPA was administered daily without breaks in treatment, and each cycle was defined as 28 days in length. A serum VPA level was obtained after 4–5 days of treatment by drawing a blood sample within 2 hours of taking a dose of VPA. If the VPA level was <50 μg/mL, the VPA dose was increased by 250 mg/d until the target VPA level was achieved. The converse was done if VPA levels were >100 μg/mL, with a reduction in total daily dose by 250 mg. Once target levels of VPA were achieved, VPA levels were monitored weekly for 4 weeks and then monthly.
Toxicity and Dose Modifications
All patients who received at least one dose of VPA treatment were evaluated for toxicity and tolerability. Toxicities observed were summarized in terms of types and severities by the NCI CTC v3.0. A change in treatment protocol occurred (as described above) if patients experienced a CTC grade 3 event of hypertension, a CTC grade 4 event of ataxia or dizziness, a CTC grade 4 event of neutropenia, low platelet count, an aspartate aminotransferase (AST) or alanine aminotransferase (ALT) >3× ULN, a CTC grade 2 event of bilirubin elevation, a CTC grade 3 event of ataxia or dizziness lasting >10 days with continued dosing of VPA, or any CTC grade 3 toxicity that was thought to be related to VPA.
If patients experienced grade 3–4 toxicities, subsequent doses of VPA were held. Further treatment with VPA began once the toxicities had resolved to CTC grade 1 toxicity or less (or baseline value for AST/ALT). Patients were to be followed every week until resolution of toxicity. Treatment would be restarted with a dose reduction of 500 mg/d (from the last dose). If a second episode of this or any other toxicity occurred at this dose, VPA was further reduced by 500 mg/d. If an unacceptable toxicity occurred after the second dose reduction, VPA was permanently discontinued. Treatment was also discontinued if a patient required a dose delay of >28 days for toxicity. Doses were also held for all other toxicities; however, patients were continued at the same dose at which they were noted to have a toxicity. If a patient was held from treatment for purposes other than study drug toxicity, they were allowed to be held for 42 days. The patient had to have repeat imaging to assess disease if >28 days had elapsed since study drug treatment.
Quantitative Real-Time Polymerase Chain Reaction for Notch1 Signaling
Frozen tissue biopsy specimens were pulverized at cryogenic temperature in the CryoPrep System (Covaris, Woburn, MA). Total RNA from homogenized samples was harvested using an RNeasy Plus mini kit (Qiagen, Valencia, CA), per the manufacturer's directions. Integrity was assured and concentration was determined using a Nanodrop spectrophotometer (Nanodrop, Wilmington, DE). Exactly 0.5 μg of total RNA was reverse transcribed with the iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer's instructions.
The quantitative real-time polymerase chain reaction reactions were performed on the CFX96 real-time PCR system (Bio-Rad) using SoFast EvaGreen Supermix (Bio-Rad) at the following conditions: 1 cycle of 30 seconds at 95°C; 40 cycles of 5 seconds at 95°C; 10 seconds at 60°C; followed by 10 seconds of 65°–95°C per step up. Primers were obtained from Integrated DNA Technologies (Coralville, IA) and sequences used were as follows: NOTCH1 (forward: 5′ GTC AAC GCC GTA GAT GAC CT 3′; reverse: 5′ TTG TTA GCC CCG TTC TTC AG 3′). The housekeeping gene, ribosomal protein s27 (forward: 5′ TCT TTA GCC ATG CAC AAA CG 3′; reverse: 5′ TTT CAG TGC TGC TTC CTC CT 3′), was used to normalize the gene-specific signals for each sample according to the formula 2(Ct(s27)−Ct(Notch)). Then Notch1 expression folds were obtained by dividing normalized expressions of unknown samples by normalized expression of negative control comprising GI carcinoid cell line (BON). Fold expression was then plotted as average ± SEM.
Outcome Measures
Because one of the endpoints of this study was to determine the effects of treatment on Notch signaling in tumor specimens, patients were considered evaluable only if they received a pretreatment and post-treatment biopsy. Core biopsies of a single lesion were obtained for all pre- and post-treatment biopsies. Patients were also required to have achieved the target blood level of VPA. Baseline measurements were obtained as close as possible to initiation of treatment and not beyond four weeks. Labs and tumor markers were obtained prior to the study and obtained every 4 weeks throughout the course of the study. Lesions were identified and measured by either computed tomography or magnetic resonance imaging, and the same imaging modality was used throughout the course of the study. Images were obtained every 4 weeks. The clinical response was based on the RECIST v1.0 [34]. Stable disease (SD) was defined as patients who met SD criteria at least once after study entry at a minimum interval of 12 weeks. All patients, including those who discontinued the protocol therapy early, were followed for response until progression and for survival for 12 months from the date of registration. All patients were followed through completion of all protocol therapy. All patients who received at least one dose of VPA treatment were evaluated for toxicity and tolerability. Time from registration to first response, complete response, disease recurrence, or progression and death was also recorded. Tumor markers, that is, chromogranin A, 5-HIAA, gastrin, and so forth, were determined at baseline, assessed every 4 weeks during treatment, and assessed at the end of the protocol treatment.
Statistical Analysis
Demographic characteristics and clinical responses were summarized using frequency tables. Percentage changes in tumor marker levels were calculated and displayed graphically using a waterfall plot. NOTCH1 expression folds were obtained by dividing normalized expressions of unknown samples (pre or on VPA) by normalized expression of negative control comprising GI carcinoid cell line (BON). Bar charts were used to plot mean fold expressions ± standard errors. A paired t-test was used to evaluate whether there was a significant change in Notch1 expression levels from baseline to the post-VPA assessment. For a given patient, OS for a patient was defined as the number of days from the day of first VPA administration to the patient's death. Patients who had not died at the time of analysis were censored at the time that they were last known to be alive. PFS was defined as the number of days from the day of first VPA administration to the day the patient experienced an event of disease progression or death, whichever came first. If a patient had not experienced an event of disease progression or death at the time of analysis, then the patient's data were censored at the date of the last available evaluation. Kaplan-Meier curves were constructed to estimate the median survival time for both PFS and OS. The data analysis was performed using SAS version 9.2 software (SAS Corp., Cary, NC).
Results
Patient Characteristics
A total of eight patients were enrolled in the trial between April 2008 and March 2009. The median age of the patients enrolled was 62.5 years with a range of 47–65 years. The majority of patients enrolled in this trial were female (75%) with an ECOG performance status of 0 or 1 (87.5%). Half of the patients had no previous treatment, and the other half had been treated with at least one agent, the most common being octreotide. Most patients had a midgut tumor. The patients and their characteristics are noted in Table 1.
Table 1.
Demographics of enrolled patients (N = 8)
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status; pNET, pancreatic neuroendocrine tumor.
Clinical Response to Treatment with VPA
Five (62.5%) of the 6 patients assessable for radiographic response were noted to have stable disease by RECIST criteria over the course of their treatment with VPA (Table 2). One patient (12.5%) with a midgut carcinoid tumor was noted to have an unconfirmed partial response (PR). One patient (12.5%) manifested progressive disease while on trial. Two patients were not assessable for radiologic response as they discontinued the study prior to repeat imaging. One patient stopped participation for personal reasons, and the other was taken off because he or she had not taken the study drug for >42 days because of concurrent illness.
Table 2.
Clinical response
Tumor Marker Response after Treatment with VPA
Five of seven patients (71%) who were assessable for tumor marker response were noted to have improvements in their chromogranin A levels after initiation of treatment with VPA. Three of five patients (60%) experienced an ∼90% best response in their chromogranin A levels from baseline. Two patients (25%) experienced significant increases in their tumor levels, as shown in Figure 1. The data from one patient were not available as no tumor levels were checked prior to enrollment and while the patient was enrolled.
Figure 1.
Waterfall plot of tumor marker change from baseline. The majority of patients experienced an improvement in their tumor markers with 3 out of 7 approaching a near 100% improvement from baseline.
Survival Data
All patients were followed for a period of 12 months after completing the study drug unless they died. Five (62.5%) patients remained alive at a maximum follow-up time of 21 months. The median OS time has not yet been reached for this cohort (Figure 2).
Figure 2.
Overall survival plotted by the Kaplan-Meier method. The median overall survival has not been reached after a maximum follow-up time of 21 months.
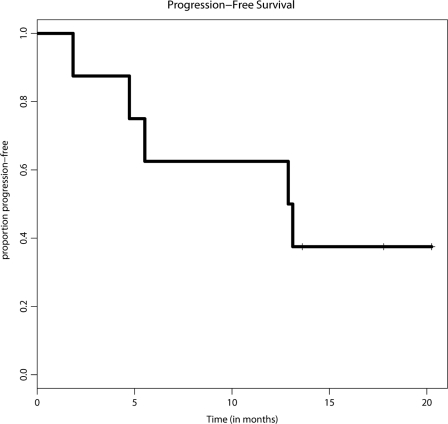
Three (37.5%) patients remained free of disease progression at the end of the trial with a maximum follow-up time of 20 months. However, one patient out of this group died from nontreatment-related causes. The median time to progression was 13 months (range 2–20+), as shown in Figure 3.
Figure 3.
Progression-free survival (PFS) plotted by the Kaplan-Meier method. The median PFS time was 13 months.
Toxicities
Overall, there were few cases of >grade 1 toxicities associated with VPA administration. The >grade 1 toxicities included one patient who was noted to have to grade 3 fatigue and grade 3 hyponatremia that required coming off the study drug. One patient came off the protocol as a result of grade 2 diarrhea, which was attributed to her NET. See Table 3.
Table 3.
Toxicities possibly, probably, or definitely related to valproic acid treatment
No grade 4 or 5 toxicities were experienced. Toxicities were graded using NCI CTC AE v. 3.0.
Abbreviations: AST, aspartate aminotransferase; GI, gastrointestinal.
Notch1 Analysis
A total of 5 out of 8 patients had pretreatment biopsies, and 4 out of 8 patients had post-treatment biopsies (Table 1). None of the patients experienced any complications as a result of the biopsies. Tumor biopsy samples were analyzed for Notch1 mRNA by real-time PCR. As shown in Figure 4, minimal to no Notch1 mRNA was present in the tumor samples at baseline, similar to BON GI carcinoid cells, which served as the negative control. This is consistent with previously reported data that Notch1 is generally not present in neuroendocrine cancers such as medullary thyroid cancer and pheochromocytoma [24, 27, 29]. After receiving VPA, a tumor biopsy was obtained and analyzed for Notch1 mRNA again. On average, there was robust 10-fold induction of Notch1 mRNA compared to pretreatment levels. In the one patient with an unconfirmed PR, the Notch1 mRNA induction with VPA treatment was >40-fold.
Figure 4.
Gene expression analysis for NOTCH1 in pretreatment and post-treatment biopsies. Each of pre-VPA and on VPA bars represents the mean of biopsies from four different patients. NOTCH1–3 expression folds were obtained by dividing normalized expressions of unknown samples (pre- or on VPA) by normalized expression of negative control comprising gastrointestinal carcinoid cell line (BON). Fold expression was then plotted as average ± SEM. The differences in NOTCH1 expressions between pre-VPA and on VPA treatment groups were statistically significant (p < .05). Abbreviation: VPA, valproic acid.
Discussion
To our knowledge, this is the first study that sought to evaluate both the role of VPA in activating the Notch1 pathway in NETs and the association with clinical outcome. Our results confirm previous in vitro findings of low Notch1 activity in NETs as seen in the pretreatment Notch1 activity [24, 27, 29]. We were also able to confirm the role of VPA in activating the Notch1 pathway as noted by the 10-fold induction in Notch expression. For two patients with evidence of some clinical benefit (one patient with an unconfirmed PR), both had a significant elevation in Notch expression on post-treatment tumor biopsy. Although the numbers are small, our data suggest that VPA may have a role in activating the Notch1 pathway in vivo. In patients who did not respond, the corresponding Notch1 expression levels were increased to a lower extent than the subjects with clinical benefit. This might suggest that the tumor lacks the signaling necessary for upregulation of Notch expression, or that higher VPA levels might be required.
In the patient noted to have progressive disease, Notch1 mRNA levels were not increased while on VPA. This supports our hypothesis that, although Notch1 might be the appropriate pathway to be targeted, VPA was not the right substrate in these cases to adequately activate Notch signaling. There are a variety of reasons this could be true, one of which is that the tumor has a different molecular milieu. We have preliminary data to suggest that VPA induces Notch1 by upregulating Notch1 transcription. We speculate that patients who did not respond to VPA may have mutations in the Notch1 promoter, preventing VPA-associated Notch1 transcriptional activation.
The majority of our patients treated with VPA experienced an improvement in their tumor markers as seen in Figure 1. Changes in tumor markers such as chromogranin A have been linked to clinical benefit in some cases [35, 36], suggesting benefit for these subjects during VPA administration. Our data also support that VPA did not adversely affect survival, and it was accompanied by infrequent grade 2 and higher toxicities in this population. The majority of patients had stabilization of their progressive disease.
Our study has its limitations. This was an underpowered phase II study that was designed to evaluate the feasibility of pre- and post-treatment biopsies and the evaluation of Notch signaling in NETs. Thus, the true effect of VPA in this patient population is difficult to discern. We were also unable to obtain adequate tissue with tumor on all pre- and postbiopsies to analyze other molecular markers of Notch1 activation, an inherent limitation to the trial design, which further limited our ability to draw definitive conclusions about the role of VPA in activating the Notch1 pathway. The role of VPA as a histone deacetylase inhibitor also confounds our results. As noted earlier, Notch1 levels might increase with VPA administration due to inhibition of histone deacetylase; however, it is also possible that VPA-induced inhibition of histone deacetylase might trigger other signaling pathways, which could have yielded our results. Given our findings, however, a larger phase II trial might be warranted to further clarify the effect of VPA in treating NETs, as this study has closed and is not recruiting any further patients.
In conclusion, our data support the hypothesis that Notch1 signaling is minimal to absent in patients with NETs and that activation of the pathway results in some clinical responses (improvement in tumor markers); however, no tumor regression was observed in this trial. Although the mechanism remains unclear, VPA activates Notch1 signaling in vitro [26–28], and it may have a role in the treatment of NETs through activating Notch1 in vivo. Our small numbers, however, make it difficult to confirm this. Further studies are needed to evaluate the role of VPA in activating this pathway and any potential clinical benefit it could offer patients with NETs.
Acknowledgments
The authors acknowledge funding from National Institute of Health RO1-CA109053 (H.C.), National Institute of Health RO1-CA121115 (H.C.), and UWCCC Investigator-Initiated Grant (K.D.H.).
Author Contributions
Conception/design: Kyle D. Holen, Daniel L. Mulkerin, Sam J. Lubner, William R. Schelman, Jens Eickhoff, Herbert Chen, Noelle K. LoConte
Provision of study material or patients: Kyle D. Holen, Daniel L. Mulkerin, William R. Schelman, Herbert Chen, Noelle K. LoConte
Collection and/or assembly of data: Tabraiz A. Mohammed, Kyle D. Holen, Renata Jaskula-Sztul, Daniel L. Mulkerin, Sam J. Lubner, William R. Schelman, Herbert Chen, Noelle K. LoConte
Data analysis and interpretation: Tabraiz A. Mohammed, Kyle D. Holen, Renata Jaskula-Sztul, Daniel L. Mulkerin, Sam J. Lubner, Jens Eickhoff, Herbert Chen, Noelle K. LoConte
Manuscript writing: Tabraiz A. Mohammed, Renata Jaskula-Sztul, Noelle K. LoConte
Final approval of manuscript: Tabraiz A. Mohammed, Kyle D. Holen, Daniel L. Mulkerin, Sam J. Lubner, William R. Schelman, Herbert Chen, Noelle K. LoConte
References
- 1.Ansari A, Meeran K, Bloom SR. Classification of Neuroendocrine Tumors. In: Hay I, Wass J, editors. Clinical Endocrine Oncology. Second Edition. Blackwell Publishing, Ltd.; 2008. pp. 437–432. [Google Scholar]
- 2.Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. The Oncologist. 2008;13:539–547. doi: 10.1634/theoncologist.2007-0239. [DOI] [PubMed] [Google Scholar]
- 3.Delcore R, Friesen SR. Gastrointestinal neuroendocrine tumors. J Am Coll Surg. 1994;178:187–211. [PubMed] [Google Scholar]
- 4.Pisegna J, Sawicki M. Neuroendocrine Pancreas. In: Haskell CM, Berek JS, editors. Cancer Treatment. Philadelphia: W.B. Saunders; 2001. pp. 1065–1081. [Google Scholar]
- 5.Rindi G, Villanacci V, Ubiali A. Biological and molecular aspects of gastroenteropancreatic neuroendocrine tumors. Digestion. 2000;62(Suppl 1):19–26. doi: 10.1159/000051851. [DOI] [PubMed] [Google Scholar]
- 6.Taal BG, Visser O. Epidemiology of neuroendocrine tumours. Neuroendocrinology. 2004;80(Suppl 1):3–7. doi: 10.1159/000080731. [DOI] [PubMed] [Google Scholar]
- 7.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 8.Pinchot SN, Holen K, Sippel RS, et al. Carcinoid tumors. The Oncologist. 2008;13:1255–1269. doi: 10.1634/theoncologist.2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 10.Kang H, O'Connell JB, Leonardi MJ, et al. Rare tumors of the colon and rectum: a national review. Int J Colorectal Dis. 2007;22:183–189. doi: 10.1007/s00384-006-0145-2. [DOI] [PubMed] [Google Scholar]
- 11.Lee JE, Evans DB. Advances in the diagnosis and treatment of gastrointestinal neuroendocrine tumors. Cancer Treat Res. 1997;90:227–238. doi: 10.1007/978-1-4615-6165-1_12. [DOI] [PubMed] [Google Scholar]
- 12.Oberg K. Chemotherapy and biotherapy in the treatment of neuroendocrine tumours. Ann Oncol. 2001;12(Suppl 2):S111–S114. doi: 10.1093/annonc/12.suppl_2.s111. [DOI] [PubMed] [Google Scholar]
- 13.Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study of the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 14.Appetecchia M, Baldelli R. Somatostatin analogues in the treatment of gastroenteropancreatic neuroendocrine tumours, current aspects and new perspectives. J Exp Clin Cancer Res. 2010;29:19. doi: 10.1186/1756-9966-29-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modlin IM, Kidd M, Latich I, et al. Current status of gastrointestinal carcinoids. Gastroenterology. 2005;128:1717–1751. doi: 10.1053/j.gastro.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 16.Brentjens R, Saltz L. Islet cell tumors of the pancreas: the medical oncologist's perspective. Surg Clin North Am. 2001;81:527–542. doi: 10.1016/s0039-6109(05)70141-9. [DOI] [PubMed] [Google Scholar]
- 17.Moertel CG, Lefkopoulo M, Lipsitz S, et al. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519–523. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- 18.Cheng PN, Saltz LB. Failure to confirm major objective antitumor activity for streptozocin and doxorubicin in the treatment of patients with advanced islet cell carcinoma. Cancer. 1999;86:944–948. [PubMed] [Google Scholar]
- 19.Shah MH, Young D, Kindler HL, et al. Phase II study of the proteasome inhibitor bortezomib (PS-341) in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2004;10:6111–6118. doi: 10.1158/1078-0432.CCR-04-0422. [DOI] [PubMed] [Google Scholar]
- 20.Ansell SM, Mahoney MR, Green EM, et al. Topotecan in patients with advanced neuroendocrine tumors: a phase II study with significant hematologic toxicity. Am J Clin Oncol. 2004;27:232–235. doi: 10.1097/01.coc.0000054535.19808.f4. [DOI] [PubMed] [Google Scholar]
- 21.Kulke MH, Kim H, Clark JW, et al. A Phase II trial of gemcitabine for metastatic neuroendocrine tumors. Cancer. 2004;101:934–939. doi: 10.1002/cncr.20466. [DOI] [PubMed] [Google Scholar]
- 22.Niccoli P, Raoul J, Bang Y, et al. Updated safety and efficacy results of the phase III trial of sunitinib (SU) versus placebo (PBO) for treatment of pancreatic neuroendocrine tumors (NET) ASCO Meeting Abstracts. 2010;28:4000. [Google Scholar]
- 23.Delaunoit T, Van den Eynde M, Borbath I, et al. Role of chemotherapy in gastro-entero-pancreatic neuroendocrine tumors: the end of a story? Acta Gastroenterol Belg. 2009;72:49–53. [PubMed] [Google Scholar]
- 24.Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756–767. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 25.Kunnimalaiyaan M, Traeger K, Chen H. Conservation of the Notch1 signaling pathway in gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G636–G642. doi: 10.1152/ajpgi.00146.2005. [DOI] [PubMed] [Google Scholar]
- 26.Kunnimalaiyaan M, Yan S, Wong F, et al. Hairy Enhancer of Split-1 (HES-1), a Notch1 effector, inhibits the growth of carcinoid tumor cells. Surgery. 2005;138:1137–1142. doi: 10.1016/j.surg.2005.05.027. discussion 1142. [DOI] [PubMed] [Google Scholar]
- 27.Nakakura EK, Sriuranpong VR, Kunnimalaiyaan M, et al. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J Clin Endocrinol Metab. 2005;90:4350–4356. doi: 10.1210/jc.2005-0540. [DOI] [PubMed] [Google Scholar]
- 28.Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, et al. Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neu-roendocrine phenotype of medullary thyroid cancer cells. J Biol Chem. 2006;281:39819–39830. doi: 10.1074/jbc.M603578200. [DOI] [PubMed] [Google Scholar]
- 29.Kunnimalaiyaan M, Chen H. Tumor Suppressor Role of Notch-1 Signaling in Neuroendocrine Tumors. Oncologist. 2007;12:535–542. doi: 10.1634/theoncologist.12-5-535. [DOI] [PubMed] [Google Scholar]
- 30.Greenblatt DY, Vaccaro AM, Jaskula-Sztul R, et al. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. The Oncologist. 2007;12:942–951. doi: 10.1634/theoncologist.12-8-942. [DOI] [PubMed] [Google Scholar]
- 31.Henry TR. The history of valproate in clinical neuroscience. Psychopharmacol Bull. 2003;37(Suppl 2):5–16. [PubMed] [Google Scholar]
- 32.Adler JT, Hottinger DG, Kunnimalaiyaan M, et al. Histone deacetylase inhibitors upregulate Notch-1 and inhibit growth in pheochromocytoma cells. Surgery. 2008;144:956–961. doi: 10.1016/j.surg.2008.08.027. discussion 961–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ning L, Greenblatt DY, Kunnimalaiyaan M, et al. Suberoyl bis-hydroxamic acid activates Notch-1 signaling and induces apoptosis in medullary thyroid carcinoma cells. The Oncologist. 2008;13:98–104. doi: 10.1634/theoncologist.2007-0190. [DOI] [PubMed] [Google Scholar]
- 34.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 35.Massironi S, Conte D, Sciola V, et al. Plasma chromogranin A response to octreotide test: prognostic value for clinical outcome in endocrine digestive tumors. Am J Gastroenterol. 2010;105:2072–2078. doi: 10.1038/ajg.2010.154. [DOI] [PubMed] [Google Scholar]
- 36.Nikou GC, Marinou K, Thomakos P, et al. Chromogranin a levels in diagnosis, treatment and follow-up of 42 patients with non-functioning pancreatic endocrine tumours. Pancreatology. 2008;8:510–519. doi: 10.1159/000152000. [DOI] [PubMed] [Google Scholar]