Abstract
Protease-activated receptors (PARs) are a unique family of G protein-coupled receptors (GPCRs) that are irreversibly activated following proteolytic cleavage of their extracellular N-terminus. PARs play critical functions in hemostasis, thrombosis, inflammation, embryonic development and cancer progression. Due to the irreversible proteolytic nature of PAR activation, signaling by the receptors is tightly regulated. Three distinct processes including desensitization, internalization and lysosomal degradation, regulate the temporal and spatial aspects of activated PAR signaling. Posttranslational modifications play a critical role in regulating each of these processes and here we review the nature of PAR posttranslational modifications and their importance in signal regulation. The PARs are activated by numerous proteases, and some can elicit distinct cellular responses, how this biased agonism is determined is unknown. Further study of the function of posttranslational modifications of the PARs will lead to a greater understanding of the physiological regulation of baised agonism and how PAR signaling is precisely controlled in different cellular contexts.
Introduction
The protease-activated receptors (PARs) are a family of heptahelical G- protein-coupled receptors (GPCRs) that mediate responses important for hemostasis, thrombosis, inflammation, embryonic development and the pathogenesis of certain malignant cancers. There are four members of the PAR family: PAR1, PAR2, PAR3 and PAR4. PAR1, also known as the thrombin receptor, was discovered in 1991 and is the best characterized and the founding member of the PAR family (1). A distinct gene encodes each PAR and the receptor's display different species and cell type expression patterns. PAR1 is coexpressed with PAR4 in human platelets and mouse endothelial cells, whereas PAR1 is coexpressed with PAR3 in murine platelets and human macrovascular endothelial cells. PARs are expressed in the vasculature and surrounding tissues and in various cell types including platelets, fibroblasts, smooth muscle cells, endothelial cells, leukocytes and some epithelial cells and nerve cells.
PARs are unique among the GPCRs, in that their activation occurs by irreversible proteolytic cleavage of the N-terminus. PAR cleavage is mediated by trypsin-like serine proteases, which reveals a cryptic ligand sequence that binds to the surface of the second extracellular loop. This induces a conformational change in the receptor triggering a wide and diverse array of signaling responses. The specific signaling pathways activated by each PAR depends on many factors including, which protease activates the receptor and the cell types in which the receptors are activated (1–3). There is a highly selective group of serine proteases that can cleave and activate PAR1 including thrombin, plasmin, kallikreins, factor Xa and activated protein C (APC) (3), as well as the matrix-metalloprotease-1 (MMP1) (4, 5). PAR4, and to a lesser extent PAR3, has also been shown to be cleaved and activated by most, but not all of the same proteases. PAR2 differs from the other members in that it is activated by a separate group of serine proteases including trypsin, tryptase and the coagulation factors VIIa and Xa (3), as well as the membrane anchored serine protease matriptase (6). Thus, the diverse range of activating proteases enable PARs to function in distinct physiological processes, including and not limited to; activation of platelets, upregulation of multiple endothelial and smooth muscle cell activities, regulation of neuronal functions, fine-tuning of inflammatory responses and the promotion of angiogenesis, tumor growth and metastasis in an increasing list of cancers.
Despite the irreversible proteolytic activation of PARs, signaling is rapidly shut off, at least to G protein signaling, suggesting that each activated receptor transmits a defined amount of secondary messenger and is then deactivated. This places a marked degree of importance on understanding the mechanisms that contribute to the regulation of agonist binding to receptor, agonist/receptor-mediated signal transduction, termination of signaling, and the resensitization of cellular responses following receptor activation. Despite the multitude of studies on PARs, the mechanisms detailing how the receptors are modified during or after translation, how these modifications effect receptor interactions with secondary effectors and how modifications modulate signaling responses are relatively unknown. Posttranslational modification of a nascent protein involves the covalent attachment of modifying chemical moieties or another protein to specific amino acid residues and is a highly dynamic and often reversible process. Posttranslational modifications are integral to the regulation of all GPCRs and the PARs are no exception, but the role of these modifications are far from the usual; this review will focus on the various roles of phosphorylation, ubiquitination and glycosylation in the regulation of PAR signaling and highlight those areas that deserve more study.
PAR signaling
As with all GPCRs the PARs are either preassembled with, or are stimulated to assemble with members of the heterotrimeric G protein family, which are comprised of α, β and γ subunits. There are twenty-three Gα subunits, seven Gβ and twelve Gγ subunits identified in the mammalian genome and the subunits form distinct heterotrimeric complexes that are divided into four families. PARs have been linked to the activation of three distinct G protein subtypes including Gαi/o, Gα12/13, and Gαq (3, 7). Once activated conformational changes in the intracellular cytosolic domains of the PARs enable the receptors to act as GTP exchange factors for the Gα subunits. This energy transfer elicits the association /dissociation of the G protein subunits and/or the recruitment of G proteins to activated GPCRs (8).
G protein activation enables the PARs to elicit a diverse array of cellular responses through the stimulation of a variety of secondary signaling effectors. Gαi is able to inhibit adenylyl cyclase diminishing cAMP accumulation, whereas the βγ subunits stimulate phospholipase C (PLC)-catalyzed hydrolysis of phosphoinositides (PIs), which in turn mediates Ca2+ mobilization and the activation of several kinases including receptor tyrosine kinases (RTKs), extracellular signal-regulated protein kinases (ERK1, 2) and protein kinase C (PKC) (9–11). PAR1 stimulation of Gα12/13 activates Rho GTP exchange factors (GEFs) and PLC activation (12–14), additionally Gα13 but not Gα12, has been linked to dishevelled mediated stabilization of β-catenin in several cancer cell lines (15, 16). Activated PAR2 also triggers signaling cascades typically mediated by Gαi/o, Gα12/13, and Gαq signaling, although there was no direct evidence showing that PAR2 coupled directly to heterotrimeric G proteins. However, McCoy et al. recently demonstrated that PAR1 and PAR2 form stable complexes with overlapping but distinct sets of heterotrimeric G protein subtypes in the same cells (17–19). PAR3 has also recently been linked to G protein signaling. Using a lung epithelial cell line lacking PAR1 and PAR4, but endogenously expressing PAR3, thrombin stimulation resulted in Rho and Ca2+ dependent release of ATP (20). However, this is the only example of PAR3 dependent G protein signaling. PAR4 is also able to couple to and signal via Gαq and Gα12/13 proteins (21).
Once activated GPCRs are rapidly phosphorylated mainly by G protein-receptor kinases (GRKs) on serine (Ser) and threonine (Thr) residues localized on the third intracellular loop and cytoplasmic tail (C-tail) (22). PAR phosphorylation triggers the recruitment of arrestins, which promotes the dissociation and hence, inactivation of G proteins, a process referred to as desensitization (22). Arrestins are multifunctional adaptor proteins, of which there are four members that can be separated into two groups. The visual arrestins (arrestin 1 and 4) are primarily expressed in photoreceptor cells and the ubiquitously expressed non-visual arrestins, arrestin-2 and -3 (also known as β-arrestin-1 and -2, respectively). A new group of arrestin-related molecules known as the α-arrestins have been recently identified and contain PY-motifs, which bind WW domains of E3 ubiquitin ligases and may function in regulation of mammalian GPCRs (23).
The non-visual β-arrestins play a central role in the regulation of GPCR signaling by facilitating receptor desensitization, promoting receptor internalization and acting as scaffolding proteins for additional G protein independent signaling from the plasma membrane and endosomes (3, 22). The binding of arrestins to activated and phosphorylated GPCRs promotes desensitization through steric hindrance of G protein coupling, although phosphorylation is not absolutely essential for agonist dependent desensitization of at least two GPCRs (3, 24, 25). Arrestin-2 and -3 are also able to interact directly with clathrin and adaptor protein complex-2 (AP-2) to promote receptor internalization through clathrin-coated pits. Arrestins exist predominantly in the cytoplasm, where arrestin-2 is basally phosphorylated by ERK1/2 in HEK 293 cells, which reduces its interaction with clathrin (22). Upon agonist stimulation of GPCRs, arrestin-2 is recruited to phosphorylated receptors at the plasma membrane. This is thought to enable arrestin dephosphorylation and results in a conformational change to reveal the AP-2 and clathrin binding sites and facilitates internalization (22). The non-visual arrestin-2 and -3 regulate activated PAR1 and PAR2 desensitization, as discussed below. However, PAR3 and PAR4 are the exception to the rule with no evidence to date linking arrestins or indeed any other adaptor protein to the receptors after activation.
In addition to arrestins, there are five other proteins shown to bind to PARs and include Jab for PAR2 and zyxin, HSP90, creatine kinase and bicaudal for PAR1 (3). These interacting proteins are thought to regulate various aspects of PAR signaling such as gene transcription, trafficking, Rho activation and changes in the actin cytoskeleton. However, the role of posttranslational modifications in regulating these interactions with PARs has not been investigated and thus, will not be discussed. As with many other GPCRs, PAR1 is rapidly desensitized by phosphorylation, followed by its internalization to early endosomes. However, because of the irreversible proteolytic nature of receptor activation, PAR1 is then shuttled directly from endosomes to the lysosome for degradation rather than dissociating from agonist and recycling back to the cell surface in a resensitized state typical of most classic GPCRs (26). PAR1 desensitization and trafficking are highly regulated and are the basis for PAR1 signal termination, and raise the question of how PAR posttranslational modifications regulate each step of these processes and whether all PARs utilize similar mechanisms.
PAR phosphorylation
Phosphorylation of GPCRs is principally although not exclusively, mediated by GRKs. In mammalian systems there are currently seven known GRKs (GRK1–7). Ishii et al. provided the first direct evidence for agonist-dependent phosphorylation of PAR1 in 1994. Moreover, overexpression of GRK3, previously known as BARK2, was able to markedly reduce PAR1 stimulated Gαq-dependent mobilization of intracellular Ca2+ in Xenopus oocytes (Fig. 1) (27). In a subsequent study using human endothelial cells expressing endogenous PAR1 (these cells endogenously express only GRK 2, 5 and 6), overexpression of GRK5, and not GRK3 or GRK6 resulted in increased PAR1 phosphorylation and partially blocked thrombin-stimulated increased endothelial barrier permeability (28). Although, GRK5 overexpression reduced thrombin stimulated increases in intracellular Ca2+, the response was not completely inhibited and it remains to be determined whether GRK5 can modulate all types of PAR1 dependent signaling (G-protein linked or otherwise). Indeed, whether GRK5 and/or GRK3 are responsible for PAR1 phosphorylation in all tissues or in response to all activating proteases remains to be determined. Moreover, there is no clear understanding of how the GRKs are activated or recruited to PAR1, and no kinases have been identified for the phosphorylation of the other PARs. Therefore, further analysis of how phosphorylation of the PARs is regulated by different activating proteases in distinct cell types is important.
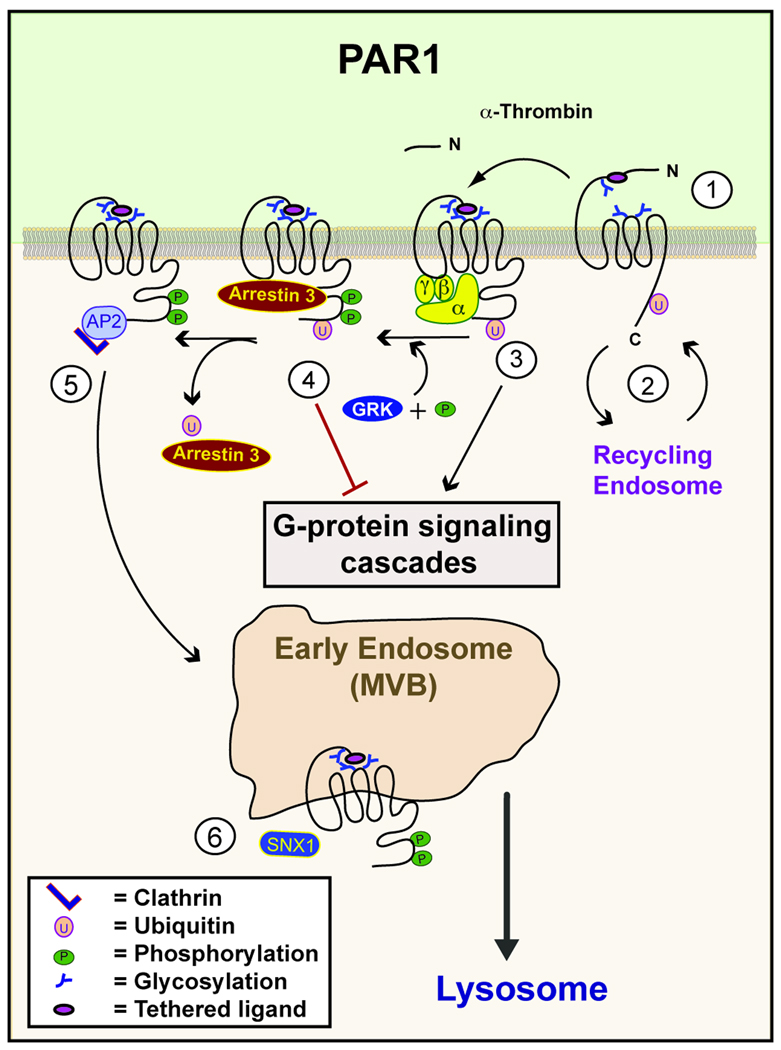
Fig. 1. Regulation of PAR1 signaling by posttranslational modifications.
(1) N-linked glycosylation of PAR1 regulates surface expression and signaling. (2) Basal ubiquitination of PAR1 promotes retention at the cell surface and negatively regulates constitutive recycling. (3) Thrombin binds to and cleaves the N-terminus of PAR1, exposing a new N-terminus that functions as a tethered ligand, which induces conformational changes within the receptor that results in activation of heterotrimeric G proteins. (4) Activation of PAR1 results in rapid phosphorylation mediated by GRKs and the recruitment of arrestins, which promote the dissociation of receptor from G proteins. (5) After activation, PAR1 is internalized through clathrin-coated pits via an AP-2 dependent pathway that occurs independent of arrestins. (6) Once internalized, activated PAR1 is sorted from early endosomes/multivesicular bodies (MVBs) to lysosomes through a poorly understood process mediated by SNX1.
PAR1 phosphorylation occurs at multiple Ser/Thr sites in the C-tail region and mutation of all of Ser/Thr sites to Ala (alanine) within the C-tail domain resulted in loss of phosphorylation and defects in signaling and receptor internalization (29). Mutation of various clusters of Ser/Thr sites within the C-tail allowed Hammes et al. to define specific PAR1 phosphorylation sites important for regulating signaling that do not appear to function in receptor internalization in Rat1 fibroblasts. This suggests that PAR1 phosphorylation differentially regulates association with clathrin adaptors that facilitate receptor internalization versus uncoupling from G protein signaling (29, 30). The actual sites of PAR1 that are phosphorylated after activation have not been mapped and whether the receptor is differentially phosphorylated following activation with different proteases and in distinct cell types has not been determined.
PAR2 is the only other member of the PAR family to have been conclusively demonstrated to be phosphorylated (31). Although it is predicted that PAR3 and PAR4 may also be phosphorylated in an agonist dependent manner this remains to be determined. Similar to other GPCRs, agonist stimulation of PAR2 results in rapid and robust phosphorylation, followed by binding of arrestin-2 and arrestin-3 leading to clathrin- and arrestin-dependent endocytosis (Fig. 2) (3). There are multiple Ser/Thr sites in the C-tail of PAR2 and mutation of all Ser/Thr to Ala virtually abolished agonist driven PAR2 phosphorylation, and prevented agonist dependent desensitization. Interestingly, mutation of specific clusters of Ser/Thr sites in the PAR2 C-tail did not prevent phosphorylation or desensitization, which suggests redundancy in the function of C-tail phosphorylation (31). Interestingly, agonist promoted endocytosis of the PAR2 phosphorylation-deficient mutant occurred through a dynamin-dependent but clathrin- and arrestin-independent mechanism. This provides novel insight into how phosphorylation can differentially regulate PAR signaling and its role in specifying a distinct receptor trafficking pathway (31). The implications of this work have yet to be fully understood, and it is possible that the selective use of the dynamin, clathrin and arrestin dependent endocytic pathway may yet play another role in regulating PAR2 function.
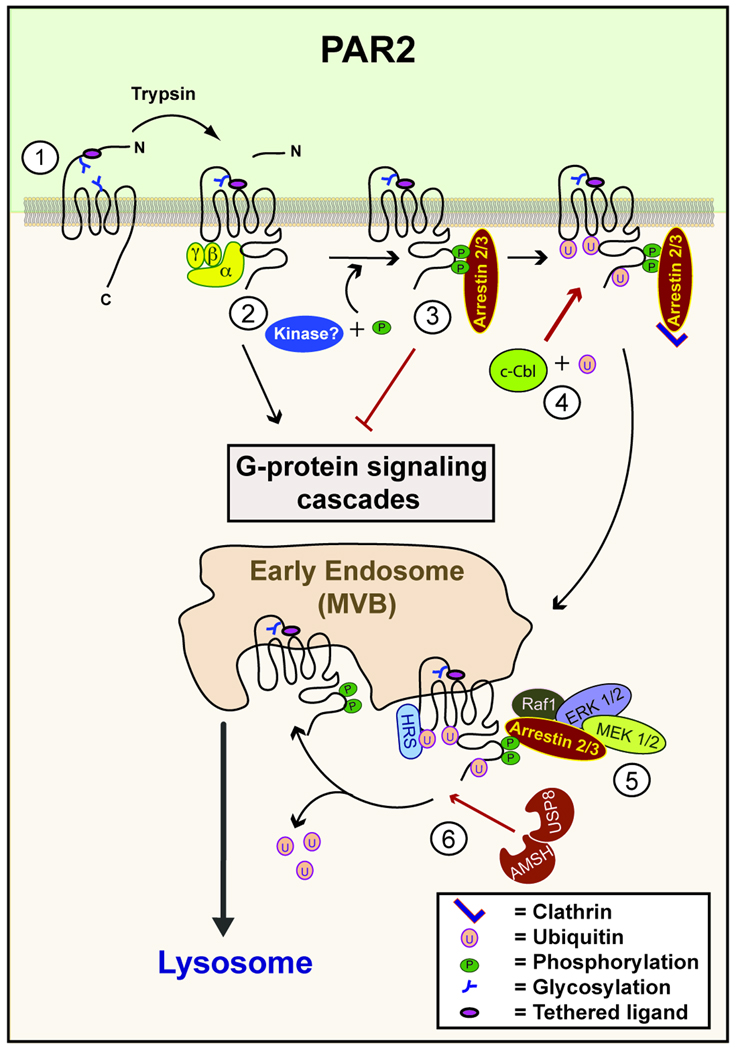
Fig. 2. Regulation of PAR2 signaling by posttranslational modifications.
(1) N-linked glycosylation of PAR2 is important for tryptase but not for trypsin binding and cleavage of the receptor. (2) Protease activation of PAR2 stimulates conformational changes and the activation of heterotrimeric G proteins. (3) Activated PAR2 is phosphorylated by an unknown kinase, which promotes the recruitment and binding of arrestin 2/3 and the uncoupling from G protein signaling. (4) Activation of PAR2 results in c-Cbl recruitment and ubiquitination of PAR2, which presumably occurs at the plasma membrane. (5) The stable association of arrestin 2/3 with activated PAR2 results in co-internalization and recruitment of a signaling complex including Raf1, ERK1/2 and MEK 1/2 on endosomes. (6) HRS mediates lysosomal sorting of ubiquitinated PAR2 and the deubiquitinating enzymes AMSH and USP8 regulate PAR2 deubiquitination and trafficking of the receptor to lysosomes.
Arrestin binding to activated PAR2 is stable and persists during endocytosis, where arrestin acts as a scaffold for G protein independent signaling from the endosome (3). PAR2 and arrestin form a signaling complex that includes Raf-1 and ERK1/2 (Fig. 2). This complex is thought to promote PAR2 dependent chemotaxis of some breast cancer cells and permeability of intestinal epithelial cells induced by stress and inflammation (22). Interestingly, when the C-tail of PAR2 was removed, arrestin was still able to promote desensitization and receptor internalization. However, the PAR2 mutant lost its ability to stably interact with arrestins even though activated mutant receptor still trafficked to the endosome (31). Also, stimulation of wild- type PAR2 leads to rapid and prolonged arrestin dependent activation of ERK1/2. The C-tail deletion of PAR2 or loss of arrestins resulted in only rapid and transient increase in ERK1/2 signaling (31, 32). What has not been directly tested is whether phosphorylation of the C-tail at specific sites stabilizes arrestin binding to PAR2 to facilitate prolonged ERK1/2 signaling independent of receptor internalization.
Arrestins normally display functional redundancy for most GPCRs. However, PAR1 is distinctly regulated by the two non-visual arrestin isoforms. Activated PAR1 is phosphorylated and rapidly desensitized primarily by arrestin-2 and not by arrestin-1 (Fig. 1) (33). Using mouse embryonic fibroblasts (MEFs) from arrestin-2 and -3 knockout mice, Paing et al. demonstrated that loss of arrestin-2 caused a marked increased in activated PAR1 signaling, compared to wild-type MEFs, but that clathrin-mediated internalization and the lysosomal degradation of PAR1 remained intact (33). As discussed above, phosphorylation of activated GPCRs is critical for the recruitment of arrestins; it was therefore predicted that activated and phosphorylated PAR1 would function similarly. This was not the case. A phosphorylation-deficient PAR1 mutant, in which all C-tail Ser/Thr were mutated, retained the capacity to recruit arrestin-2, demonstrating that not only do the arrestin isoforms bind to PAR1 differentially, but also that phosphorylation is dispensable for this process (34). This was supported by the use of an arrestin-2 R169E mutant that binds to activated GPCRs with high affinity, independent of receptor phosphorylation, both arrestin-2 wild-type and R169E mutant were equally effective at promoting wild-type and phosphorylation deficient PAR1 desensitization (34). This data leaves at least one important question unanswered; since arrestins do not regulate PAR1 internalization and phosphorylation is not required for arrestin binding to PAR1, what is the role of phosphorylation? One function of PAR1 phosphorylation that remains unknown is whether it influences the recruitment and binding of another clathrin adaptor protein to aid receptor internalization or subsequent sorting to lysosomes? Finally, phosphorylation is often reversible but whether or not the PARs are dephosphorylated after endocytosis prior to degradation has not been demonstrated, therefore the effects of phosphatases on PAR signaling remains to be investigated.
PAR ubiquitination
Initial investigations of PAR1 signal regulation led to the discovery that PAR1 is not recycled from the endosome after agonist stimulation, as-per the canonical GPCR trafficking model. After endocytosis PAR1 is trafficked directly from the endosome to the lysosome where it is degraded, a process is also known as downregulation. Replacement of the C-tail of the substance P receptor (also known as the neurokinin-1 receptor) with the C-tail of PAR1 switched its trafficking route from recycling to lysosomal degradation (35). Thus, the C-tail of each GPCR dictated its trafficking fate. This raises the question as to what are the determinants that direct sorting of PAR1 to the lysosome rather than recycling, and whether posttranslational modifications influence this process? Another posttranslational modification that affects adaptor protein binding and controls the trafficking fate of GPCRs is ubiquitination.
There is a substantial literature demonstrating that ubiquitination plays a critical role in the lysosomal degradation of multiple GPCRs and has at least an indirect role in regulating the endocytosis of several other GPCRs. Ubiquitin is a 76-amino acid protein that can be attached via an isopeptide bond to lysine residues of target proteins. Ubiquitin itself contains seven lysine residues that in turn can also be ubiquitinated. Target proteins can be either mono-ubiquitinated by a single ubiquitin molecule or have multiple single ubiquitin molecules attached to separate lysine residues on the target protein. Additionally, ubiquitin can be sequentially added to an individual ubiquitin molecule to form poly-ubiquitin chains, which can vary in length and composition depending on which of the seven lysines are ubiquitinated. Whether the target protein is mono, multi- or poly-ubiquitinated can influence the function of the ubiquitination and the distinct roles of each are still a matter of extensive investigation.
The covalent ubiquitination of target proteins is a reversible dynamic multi-step process, in which ubiquitin is “passed” sequentially from an E1 ubiquitin activating ligase, then to an E2 ubiquitin conjugating enzyme which in turn transfers ubiquitin to an E3 ubiquitin ligase. In the vast majority of cases, the E3-ubiquitin ligase provides specificity by recognition and binding of the target protein. The ubiquitin moiety is then transferred to the target protein by either the E2 conjugating enzyme (in the case of RING-finger E3 ligases) or directly by the E3 ligase (HECT-domain containing E3 ligases) (26). Similar to phosphorylation, ubiquitination is often reversed and is regulated by a family of ubiquitin carboxyl-terminal hydrolases, otherwise known as deubiquitinating enzymes (DUBs). DUBs remove ubiquitin from target proteins to prevent degradation of proteins and also just prior to protein degradation. Thus, deubiquitination effectively recycles the ubiquitin and maintains the levels of free ubiquitin important for cellular hemeostasis.
Ubiquitin was first demonstrated to be both necessary and sufficient to drive both constitutive and agonist-induced internalization of the yeast Ste2 GPCR (26). Both mono-ubiquitination and the linking of short lysine-63 poly-ubiquitin chains promoted Ste2 receptor endocytosis. Yeast lack β-arrestins and evidence links the yeast homologues of mammalian epsin (ent1 and 2), which contain ubiquitin-interacting motifs that bind ubiquitin, and enable them to act as endocytic clathrin adaptor proteins to facilitate receptor internalization (26). There is no evidence to date that conclusively links the direct ubiquitination of mammalian GPCRs to the regulation of agonist promoted receptor internalization. However, ubiquitin can modulate the function of various adaptor proteins, which in turn can regulate receptor internalization. One example of this type of regulation occurs with internalization of Notch, a single transmembrane receptor for delta. Agonist stimulation of Notch leads to the recruitment of USP9X, a deubiquitinating enzyme, which deubiquitinates epsin and enables it to promote Notch internalization (26). In contrast, ubiquitination of β-arrestins appears to be important for stable interaction with some GPCRs and facilitates their internalization (36).
PAR1 is unusual in the field of GPCRs in that basal ubiquitination of the receptor codes for receptor retention at the cell surface. Ubiquitination of lysine residues within the C-tail tyrosine AP-2 binding motif is thought to prevent AP-2 binding and consequently impedes constitutive internalization (Fig. 1). Indeed, mutation of lysines within the tyrosine-based motif, resulted in enhanced PAR1 constitutive internalization and this phenotype was reversed by fusion of an in-frame single ubiquitin moiety to the PAR1 C-tail (37, 38). This mode of PAR1 retention is so far unique among the GPCRs and is predicted to play an important role in receptor resensitization, as discussed below. Although PAR1 is basally ubiquitinated, neither the PAR1 ubiquitin-deficient mutant nor the ubiquitin fusion mutant displayed any defect in agonist stimulated internalization or degradation. However, activated PAR1 internalization was only partially inhibited in AP-2 deficient cells, whereas internalization of ubiquitin-deficient PAR1 mutant is effectively blocked by depletion of AP-2 (38). Thus, although ubiquitination is not critical for the regulation of PAR1 internalization it may modulate which clathrin adaptor proteins are used to mediate agonist-stimulated PAR1 internalization. How this might affect PAR1 is unclear, but it is entirely plausible that ubiquitin may act as a scaffold for adaptor proteins and signaling effectors or retard their binding through steric hindrance. More work is needed to firmly establish not only which E3 ligases and DUBs are responsible for regulating PAR1 ubiquitination, but also when and where PAR1 is ubiquitinated and how this affects PAR1 signaling globally.
Consistent with the model of ubiquitin-independent lysosomal degradation of PAR1, agonist-induced PAR1 degradation is independent of hepatocyte growth factor-regulated tyrosine kinase substrate (HRS) and tumor suppressor gene 101 (Tsg101), ubiquitin-binding components of the endosomal-sorting complex required for transport (ESCRT) complex. Rather, PAR1 lysosomal sorting depends on a poorly understood process that requires the function of sorting nexin-1 (SNX-1) (39), and whether ESCRT is ultimately important for lysosomal degradation of PAR1 remains to be determined. The ESCRT complex binds and sorts ubiquitinated proteins to intralumenal vesicles of multivesicular bodies (MVBs), prior to degradation in the lysosome.
After activation GPCRs are internalized to the endosome, where the receptor is dephosphorylated before being recycled back to the plasma membrane, this process is termed resensitization. Resensitization is an important physiological process that allows cells to recover appropriate responses in a timely manner after repetitive agonist stimulation. Because of the irreversible proteolytic activation of the PARs, rapid resensitization requires a different strategy. PAR1 relies on an endosomal pool of constitutively recycled receptor that is continually internalized and returned to the plasma membrane. As described above, the constitutive recycling of PAR1 appears to be at least in part regulated by ubiquitination (3). The mechanisms and role of posttranslational modifications, in regulating each step of PAR1 recycling remains unclear. PAR2 resensitization requires mobilization of a pool of naïve receptor from the Golgi complex as well as de novo receptor biosynthesis (3). The role of posttranslational modifications in this process has not been thoroughly examined. The mechanisms responsible for PAR3 and PAR4 internalization and endocytic trafficking have not determined and require investigation.
Ubiquitin plays a more classic role in the regulation of PAR2 by mediating its lysosomal degradation. Upon activation of PAR2, c-Cbl, a soluble RING domain E3 ligase, is phosphorylated and then translocated to the plasma membrane or early endosome, where it interacts with and mono-ubiquitinates PAR2 (Fig. 2). A PAR2 lysine-less mutant was not ubiquitinated, but receptor internalization remained intact. However, the ubiquitin-deficient PAR2 mutant was retained at the early endosome and failed to traffic to the lysosome and was not degraded (40, 41). In contrast to PAR1, PAR2 lysosomal sorting requires HRS, an ubiquitin-binding subunit of the ESCRT complex. Although ubiquitin is essential for cargo sorting by ESCRT, ubiquitin moieties are removed by DUBs before entry into MVBs (42). Using siRNAs knockdown strategies or overexpression of mutants to perturb DUB function, Hasdemir et al. identified two endosomal DUBs, associated molecule with the Src homology 3 domain of STAM (signal-transducing adapter molecule) (AMSH) and ubiquitin-specific protease 8 (USP8), that appear to mediate lysosomal trafficking and degradation of PAR2 in HEK 293 cells (40). Disruption of AMSH or USP8 function resulted in PAR2 retention in enlarged endosomes and defective degradation that corresponded with prolonged PAR2 ubiquitination, suggesting that deubiquitination of PAR2 is important for lysosomal sorting. Intriguingly, PAR2 coexpression with arrestin-3 and either mutant USP8 or AMSH resulted in co-localization of all three proteins in enlarged endosomes. However, arrestin-3 dissociation from the endosomal complex and consequent ERK1/2 signaling was similar to that observed under normal conditions. Thus, binding of arrestin to activated PAR2 and the consequent ERK1/2 signaling occurs independent of receptor ubiquitination. Indeed, PAR2 stimulated ERK1/2 signaling was not increased or prolonged in cells expressing mutant DUBs (40), but whether ERK1/2 signaling is altered in cells expressing ubiquitin-deficient PAR2 mutant or cells lacking c-Cbl remains unclear. Interestingly, the mechanisms responsible for dissociation of arrestin-3 and the signaling complex from PAR2 under these conditions is not known, and it may be possible that dephosphorylation of PAR2 and/or other adaptor proteins regulates dissociation of the signaling complex. Thus, further work is needed to determine how posttranslational modifications regulate PAR signaling from endosomes.
PAR glycosylation
N-linked glycosylation is a complex process in which an oligosaccharide is attached to the asparagine (Asn) residue of a translating protein from the carrier molecule dolichol and is then further modified by glycosyltransferases and glycosidases ending in a vast array of possible glycan structures. Approximately 90% of all GPCRs examined are glycosylated on their extracellular domains at conserved consensus sites Asn-X-Ser/Thr (where X = any amino acid except proline). N-linked glycosylation sites occur most commonly at the N-terminus and less frequently on other domains of GPCRs (43, 44). In contrast to other posttranslational modifications, glycosylation is stable and important for maturation and proper folding of newly synthesized proteins, including GPCRs (45), and recent work suggest an emerging role in regulating GPCR activation (44).
All PAR family members contain at least one putative N-linked glycosylation site within the N-terminus proximal to the protease recognition/cleavage site. However, PAR1, PAR2 and PAR3 harbor additional consensus sites on their extracellular domains. With such a unique mechanism of activation it is possible that a charged oligosaccharide adjacent to the cleavage site could play a role in PAR activation, protease specificity, or docking of the newly generated tethered ligand. Out of the four receptors, only PAR1 and PAR2 N-linked glycosylation have been studied.
PAR1 contains the most N-linked glycosylation consensus sites out of the four PARs, with three sites residing in the N-terminus and two localized on the second extracellular loop. Previous studies showed that PAR1 is variably glycosylated resulting in the receptor migrating as multiple species between 34–100 kDa (46). The differential expression of N-linked glycosylation modifying enzymes in distinct cells types, as well as variances in experimental conditions and/or antibodies used to detect PAR1 could be responsible for the apparent heterogeneity of PAR1 glycosylation observed. The function of PAR1 N-linked glycosylation has been examined in a few studies. One study showed using T-lymphoblastoid cells and the drug tunicamycin, which globally inhibits N-linked glycosylation, that glycosylation is necessary for PAR1 expression at the cell surface (47). More recently, Soto et al. demonstrated that mutation of both asparagines of consensus sites localized on the second extracellular loop (NA ECL2) (48), markedly attenuated PAR1 glycosylation without affecting trafficking to the cell surface, suggesting that glycosylation of the N-terminus is important for cell surface expression. Moreover, the activated PAR1 NA ECL2 mutant displayed markedly enhanced signaling, compared to the fully glycosylated wild-type PAR1 (48). The observed changes in signaling may be due to the lack of the bulky sugars allowing for more efficient interaction of the newly generated tethered ligand with the second extracellular loop. Alternatively, the lack of PAR1 glycosylation may allow for more flexibility of the ECL2 domain and stabilization of the active receptor conformation promoting more efficient G protein coupling. More studies are needed to determine how glycosylation specifically regulates PAR1 signaling and activation of PAR1 with different proteases.
PAR2 contains an N-linked glycosylation consensus site on the N-terminus and in the second extracellular loop. In contrast to PAR1, glycosylation of PAR2 is not essential for cell surface expression, since mutation of both N-linked consensus sites reduced but did not abolish PAR2 surface expression (46). N-linked glycosylation of PAR2 was demonstrated by use of receptor mutants, tunicamycin and sialidase, which cleaves the terminal sialic acid residues from glycosylated proteins (46). Interestingly, N-linked glycosylation of PAR2 appears to inhibit the ability of tryptase to activate the receptor but not trypsin. It was further demonstrated that sialic acid modification of PAR2 regulates tryptase cleavage and receptor activation (46). Thus, sialylation of PAR2 in various tissues or under certain pathological conditions such as inflammation could be a possible mechanism for regulating PAR2 signaling by specific activating proteases. No studies on PAR3 or PAR4 N-linked glycosylation have been published to our knowledge. Whether glycosylation of the other PARs regulates the ability of distinct activating proteases to bind to and cleave the receptors has not been investigated.
Conclusions and future directions
The regulation of PAR signaling is critical for the fidelity of protease signaling and appropriate physiological responses in the vasculature and other organ systems. A vast literature has been generated describing the complex signaling responses of PAR stimulation in various organ and cell systems. However, there is surprisingly little information detailing the regulation of PARs by posttranslational modifications. Phosphorylation, ubiquitination and glycosylation are clearly important for the precise regulation of PAR1 and PAR2 signaling and are likely to play critical roles in the regulation of PAR3 and PAR4. In addition, other posttranslational modifications including S-nitrosylation, acetylation, palmitoylation and sumoylation as well as others could occur on PARs and have important functions. Thus, further studies are needed to define the full repertoire of PAR posttranslational modifications, the interplay of posttranslational modifications and the consequences of these modifications on receptor function in various systems.
There are many aspects of PAR function that are potentially affected by posttranslational modifications. Posttranslational modifications of PARs could function to localize receptors to distinct plasma membrane microdomains such as caveolae or other lipid rafts. PARs are also likely to self-associate to form homodimers and can interact with each other to form heterodimers, as is likely the case for PAR3 facilitating thrombin activation of PAR4 in mouse platelets. Whether posttranslational modifications function in PAR-PAR interactions is not known. In addition, PARs could also form complexes with other types of receptors. One possible candidate for interaction with PAR1 is the epidermal growth factor receptor (EGFR), which colocalizes with PAR1 and undergoes transactivation following thrombin stimulation in invasive breast carcinoma (40). It remains to be determined as to whether posttranslational modifications of PARs alter their function by modulating their ability to bind to other receptors or cofactors. More research in this area could provide a better understanding of PAR function in normal cells and in pathological conditions such invasive breast cancer.
Acknowledgements
This work is supported by the National Institutes of Health Grants HL073328 and GM090689 and a UC Tobacco-Related Disease Research Program Exploratory Award.
References
- 1.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Ishii M, Wang L, Ishii K, Coughlin SR. Thrombin receptor activation. Confirmation of the intramolecular tethered liganding hypothesis and discovery of an alternative intermolecular liganding mode. J Biol Chem. 1994;269(23):16041–16045. [PubMed] [Google Scholar]
- 3.Soh UJ, Dores MR, Chen B, Trejo J. Signal transduction by protease-activated receptors. Br J Pharmacol. 2010;160(2):191–203. doi: 10.1111/j.1476-5381.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, et al. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120(3):303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Trivedi V, Boire A, Tchernychev B, Kaneider NC, Leger AJ, et al. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell. 2009;137(2):332–343. doi: 10.1016/j.cell.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seitz I, Hess S, Schulz H, Eckl R, Busch G, et al. Membrane-type serine protease-1/matriptase induces interleukin-6 and -8 in endothelial cells by activation of protease-activated receptor-2: potential implications in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(4):769–775. doi: 10.1161/01.ATV.0000258862.61067.14. [DOI] [PubMed] [Google Scholar]
- 7.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3(8):1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 8.Ayoub MA, Trinquet E, Pfleger KD, Pin JP. Differential association modes of the thrombin receptor PAR1 with Galphai1, Galpha12, and beta-arrestin 1. FASEB J. 2010;24(9):3522–3535. doi: 10.1096/fj.10-154997. [DOI] [PubMed] [Google Scholar]
- 9.Baffy G, Yang L, Raj S, Manning DR, Williamson JR. G protein coupling to the thrombin receptor in Chinese hamster lung fibroblasts. J Biol Chem. 1994;269(11):8483–8487. [PubMed] [Google Scholar]
- 10.Offermanns S, Toombs CF, Hu YH, Simon MI. Defective platelet activation in G alpha(q)-deficient mice. Nature. 1997;389(6647):183–186. doi: 10.1038/38284. [DOI] [PubMed] [Google Scholar]
- 11.Trejo J, Connolly AJ, Coughlin SR. The cloned thrombin receptor is necessary and sufficient for activation of mitogen-activated protein kinase and mitogenesis in mouse lung fibroblasts. Loss of responses in fibroblasts from receptor knockout mice. J Biol Chem. 1996;271(35):21536–21541. doi: 10.1074/jbc.271.35.21536. [DOI] [PubMed] [Google Scholar]
- 12.Gohla A, Offermanns S, Wilkie TM, Schultz G. Differential involvement of Galpha12 and Galpha13 in receptor-mediated stress fiber formation. J Biol Chem. 1999;274(25):17901–17907. doi: 10.1074/jbc.274.25.17901. [DOI] [PubMed] [Google Scholar]
- 13.Lopez I, Mak EC, Ding J, Hamm HE, Lomasney JW. A novel bifunctional phospholipase c that is regulated by Galpha 12 and stimulates the Ras/mitogen-activated protein kinase pathway. J Biol Chem. 2001;276(4):2758–2765. doi: 10.1074/jbc.M008119200. [DOI] [PubMed] [Google Scholar]
- 14.Offermanns S, Laugwitz KL, Spicher K, Schultz G. G proteins of the G12 family are activated via thromboxane A2 and thrombin receptors in human platelets. Proc Natl Acad Sci U S A. 1994;91(2):504–508. doi: 10.1073/pnas.91.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turm H, Maoz M, Katz V, Yin YJ, Offermanns S, et al. Protease-activated receptor-1 (PAR1) acts via a novel Galpha13-dishevelled axis to stabilize beta-catenin levels. J Biol Chem. 2010;285(20):15137–15148. doi: 10.1074/jbc.M109.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yin YJ, Katz V, Salah Z, Maoz M, Cohen I, et al. Mammary gland tissue targeted overexpression of human protease-activated receptor 1 reveals a novel link to beta-catenin stabilization. Cancer Res. 2006;66(10):5224–5233. doi: 10.1158/0008-5472.CAN-05-4234. [DOI] [PubMed] [Google Scholar]
- 17.Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53(2):245–282. [PubMed] [Google Scholar]
- 18.Traynelis SF, Trejo J. Protease-activated receptor signaling: new roles and regulatory mechanisms. Curr Opin Hematol. 2007;14(3):230–235. doi: 10.1097/MOH.0b013e3280dce568. [DOI] [PubMed] [Google Scholar]
- 19.McCoy KL, Traynelis SF, Hepler JR. PAR1 and PAR2 couple to overlapping and distinct sets of G proteins and linked signaling pathways to differentially regulate cell physiology. Mol Pharmacol. 2010;77(6):1005–1015. doi: 10.1124/mol.109.062018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seminario-Vidal L, Kreda S, Jones L, O'Neal W, Trejo J, et al. Thrombin promotes release of ATP from lung epithelial cells through coordinated activation of rho- and Ca2+-dependent signaling pathways. J Biol Chem. 2009;284(31):20638–20648. doi: 10.1074/jbc.M109.004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakanishi-Matsui M, Zheng YW, Sulciner DJ, Weiss EJ, Ludeman MJ, et al. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404(6778):609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- 22.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 23.Nabhan JF, Pan H, Lu Q. Arrestin domain-containing protein 3 recruits the NEDD4 E3 ligase to mediate ubiquitination of the beta2-adrenergic receptor. EMBO Rep. 11(8):605–611. doi: 10.1038/embor.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurevich EV, Gurevich VV. Arrestins: ubiquitous regulators of cellular signaling pathways. Genome Biol. 2006;7(9):236. doi: 10.1186/gb-2006-7-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirsch JA, Schubert C, Gurevich VV, Sigler PB. The 2.8 A crystal structure of visual arrestin: a model for arrestin's regulation. Cell. 1999;97(2):257–269. doi: 10.1016/s0092-8674(00)80735-7. [DOI] [PubMed] [Google Scholar]
- 26.Marchese A, Paing MM, Temple BR, Trejo J. G protein-coupled receptor sorting to endosomes and lysosomes. Annu Rev Pharmacol Toxicol. 2008;48:601–629. doi: 10.1146/annurev.pharmtox.48.113006.094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishii K, Chen J, Ishii M, Koch WJ, Freedman NJ, et al. Inhibition of thrombin receptor signaling by a G-protein coupled receptor kinase. Functional specificity among G-protein coupled receptor kinases. J Biol Chem. 1994;269(2):1125–1130. [PubMed] [Google Scholar]
- 28.Tiruppathi C, Yan W, Sandoval R, Naqvi T, Pronin AN, et al. G protein-coupled receptor kinase-5 regulates thrombin-activated signaling in endothelial cells. Proc Natl Acad Sci U S A. 2000;97(13):7440–7445. doi: 10.1073/pnas.97.13.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammes SR, Shapiro MJ, Coughlin SR. Shutoff and agonist-triggered internalization of protease-activated receptor 1 can be separated by mutation of putative phosphorylation sites in the cytoplasmic tail. Biochemistry. 1999;38(29):9308–9316. doi: 10.1021/bi9902236. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe BL, Trejo J. Clathrin-dependent mechanisms of G protein-coupled receptor endocytosis. Traffic. 2007;8(5):462–470. doi: 10.1111/j.1600-0854.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 31.Ricks TK, Trejo J. Phosphorylation of protease-activated receptor-2 differentially regulates desensitization and internalization. J Biol Chem. 2009;284(49):34444–34457. doi: 10.1074/jbc.M109.048942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stalheim L, Ding Y, Gullapalli A, Paing MM, Wolfe BL, et al. Multiple independent functions of arrestins in the regulation of protease-activated receptor-2 signaling and trafficking. Mol Pharmacol. 2005;67(1):78–87. doi: 10.1124/mol.104.006072. [DOI] [PubMed] [Google Scholar]
- 33.Paing MM, Stutts AB, Kohout TA, Lefkowitz RJ, Trejo J. beta -Arrestins regulate protease-activated receptor-1 desensitization but not internalization or Down-regulation. J Biol Chem. 2002;277(2):1292–1300. doi: 10.1074/jbc.M109160200. [DOI] [PubMed] [Google Scholar]
- 34.Chen CH, Paing MM, Trejo J. Termination of protease-activated receptor-1 signaling by beta-arrestins is independent of receptor phosphorylation. J Biol Chem. 2004;279(11):10020–10031. doi: 10.1074/jbc.M310590200. [DOI] [PubMed] [Google Scholar]
- 35.Trejo J, Coughlin SR. The cytoplasmic tails of protease-activated receptor-1 and substance P receptor specify sorting to lysosomes versus recycling. J Biol Chem. 1999;274(4):2216–2224. doi: 10.1074/jbc.274.4.2216. [DOI] [PubMed] [Google Scholar]
- 36.Shenoy SK, Barak LS, Xiao K, Ahn S, Berthouze M, et al. Ubiquitination of beta-arrestin links seven-transmembrane receptor endocytosis and ERK activation. J Biol Chem. 2007;282(40):29549–29562. doi: 10.1074/jbc.M700852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paing MM, Temple BR, Trejo J. A tyrosine-based sorting signal regulates intracellular trafficking of protease-activated receptor-1: multiple regulatory mechanisms for agonist-induced G protein-coupled receptor internalization. J Biol Chem. 2004;279(21):21938–21947. doi: 10.1074/jbc.M401672200. [DOI] [PubMed] [Google Scholar]
- 38.Wolfe BL, Marchese A, Trejo J. Ubiquitination differentially regulates clathrin-dependent internalization of protease-activated receptor-1. J Cell Biol. 2007;177(5):905–916. doi: 10.1083/jcb.200610154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gullapalli A, Wolfe BL, Griffin CT, Magnuson T, Trejo J. An essential role for SNX1 in lysosomal sorting of protease-activated receptor-1: evidence for retromer-, Hrs-, and Tsg101-independent functions of sorting nexins. Mol Biol Cell. 2006;17(3):1228–1238. doi: 10.1091/mbc.E05-09-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasdemir B, Murphy JE, Cottrell GS, Bunnett NW. Endosomal deubiquitinating enzymes control ubiquitination and down-regulation of protease-activated receptor 2. J Biol Chem. 2009;284(41):28453–28466. doi: 10.1074/jbc.M109.025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacob C, Cottrell GS, Gehringer D, Schmidlin F, Grady EF, et al. c-Cbl mediates ubiquitination, degradation, and down-regulation of human protease-activated receptor 2. J Biol Chem. 2005;280(16):16076–16087. doi: 10.1074/jbc.M500109200. [DOI] [PubMed] [Google Scholar]
- 42.Williams RL, Urbe S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol. 2007;8(5):355–368. doi: 10.1038/nrm2162. [DOI] [PubMed] [Google Scholar]
- 43.Landolt-Marticorena C, Reithmeier RA. Asparagine-linked oligosaccharides are localized to single extracytosolic segments in multi-span membrane glycoproteins. Biochem J. 1994;302((Pt 1)):253–260. doi: 10.1042/bj3020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amith SR, Jayanth P, Franchuk S, Finlay T, Seyrantepe V, et al. Neu1 desialylation of sialyl alpha-2,3-linked beta-galactosyl residues of TOLL-like receptor 4 is essential for receptor activation and cellular signaling. Cell Signal. 2010;22(2):314–324. doi: 10.1016/j.cellsig.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 45.Wheatley M, Hawtin SR. Glycosylation of G-protein-coupled receptors for hormones central to normal reproductive functioning: its occurrence and role. Hum Reprod Update. 1999;5(4):356–364. doi: 10.1093/humupd/5.4.356. [DOI] [PubMed] [Google Scholar]
- 46.Compton SJ. Glycosylation and Proteinase-Activated Receptor Function. Drug Development Research. 2003;59:350–354. [Google Scholar]
- 47.Tordai A, Brass LF, Gelfand EW. Tunicamycin inhibits the expression of functional thrombin receptors on human T-lymphoblastoid cells. Biochem Biophys Res Commun. 1995;206(3):857–862. doi: 10.1006/bbrc.1995.1122. [DOI] [PubMed] [Google Scholar]
- 48.Soto AG, Trejo J. N-linked glycosylation of protease-activated receptor-1 second extracellular loop: a critical determinant for ligand-induced receptor activation and internalization. J Biol Chem. 2010;285(24):18781–18793. doi: 10.1074/jbc.M110.111088. [DOI] [PMC free article] [PubMed] [Google Scholar]