Abstract
Antisense oligonucleotides are commonly employed to study the roles of genes in development. Although morpholino phosphorodiamidate oligonucleotides (morpholinos) are widely used to block translation or splicing of target gene products, the usefulness of other modifications in mediating RNase-H independent inhibition of gene activity in embryos has not been investigated. In this study, we investigated the extent that fully modified 2′-O-methyl oligonucleotides (2′-OMe oligos) can function as translation inhibiting reagents in vivo, using Xenopus and zebrafish embryos. We find that oligos against Xenopus β-catenin, wnt11 and bmp4, and against zebrafish chordin (chd) can efficiently and specifically generate embryonic loss-of-function phenotypes comparable to morpholino injection and other methods. These results show that fully modified 2′-OMe oligos can function as RNase-H independent antisense reagents in vertebrate embryos and can thus serve as an alternative modification to morpholinos in some cases.
Keywords: antisense oligonucleotides, embryo, 2′-O-methyl RNA, morpholino
Antisense deoxyoligonucleotides (oligos) are used routinely to disrupt gene function in developing embryos. This strategy is particularly important in organisms where gene targeting by homologous recombination is limited or impossible, including Xenopus, zebrafish and many non-traditional model organisms. Antisense oligos inhibit gene function through one of two main mechanisms. Oligos composed of DNA bases with phosphodiester or phosphorothioate linkages can bind to and degrade the target mRNA by eliciting RNase H activity (Cazenave et al., 1987; Dash et al., 1987; Shuttleworth and Colman, 1988). Alternatively, oligos composed of various nuclease resistant modified nucleotides and linkages can bind with high affinity to the target mRNA and inhibit translation (Boiziau et al., 1991) or splicing. Chimeric oligos, which incorporate nuclease resistant termini flanking an RNase H competent core, have also been designed to improve oligo longevity and specificity (reviewed in Hulstrand et al., 2010).
Chimeric oligos have been particularly useful in targeting maternal mRNAs in Xenopus oocytes, since RNase H-dependent antisense mechanisms are quite effective in these cells. In fertilized embryos however, simple phosphorothioate-modified, RNase H-dependent oligos are highly unstable and exhibit greater toxicity (Dash et al., 1987). Although the use of second and third-generation oligo modifications has somewhat mitigated this limitation (Hukriede et al., 2003; Lennox et al., 2006), RNase H-independent morpholino phosphorodiamidate oligos (morpholinos) have become the predominant reagent used for antisense experiments in Xenopus and zebrafish embryos (reviewed in Heasman, 2002; Eisen and Smith, 2008). Although morpholinos were initially adopted to fill the clear need for a stable and specific antisense reagent in genetically intractable organisms, other types of RNase H-independent oligos have been successfully used for clinical applications (Sazani and Kole, 2003) and may thus be suitable for antisense experiments in embryos.
In this study, we investigated whether fully modified 2′-O-methyl (2′-OMe) RNA antisense oligos could function as effective translational inhibitory reagents in Xenopus and zebrafish embryos. 2′-OMe modified oligos have several features that make them attractive antisense reagents, including, rapid hybridization kinetics, thermal stability, and resistance to RNase H and other nucleases. Importantly, 2′-OMe-modified oligos inhibit in vitro translation with a similar efficacy and specificity to morpholinos (Stein et al., 1997) and can inhibit translation and splicing in vivo (reviewed in Manoharan, 1999). In contrast to morpholinos, 2′-OMe modified oligos are readily available from many commercial sources and can be produced in small scales and purified as desired.
To test the activity of 2′-OMe oligos in vertebrate embryos, we obtained a fully modified 2′-OMe RNA antisense 25mer oligo targeted against the start codon of Xenopus β-catenin, using a previously published sequence (Heasman et al., 2000). This oligo (βcat-2ome) was injected at 3, 6 and 12 ng into one-to-two cell embryos. Injection of 12 ng was lethal, producing a cell-dissociation phenotype in all cases, which was not rescued by RNA injection and is therefore a non-specific effect (data not shown). Embryos injected with the 3 ng (not shown) and 6 ng doses survived and exhibited a high incidence of mild and severe ventralization, respectively (Fig. 1A, B). These embryos displayed all the morphological hallmarks of dorsal axis deficiency, including delayed and symmetrical gastrulation, appearance of animal pole “cysts,” failure or delay of neural plate formation and reduced head and dorsal axial structures. Our data are consistent with the results obtained using the isosequential morpholino oligo (Heasman et al., 2000).
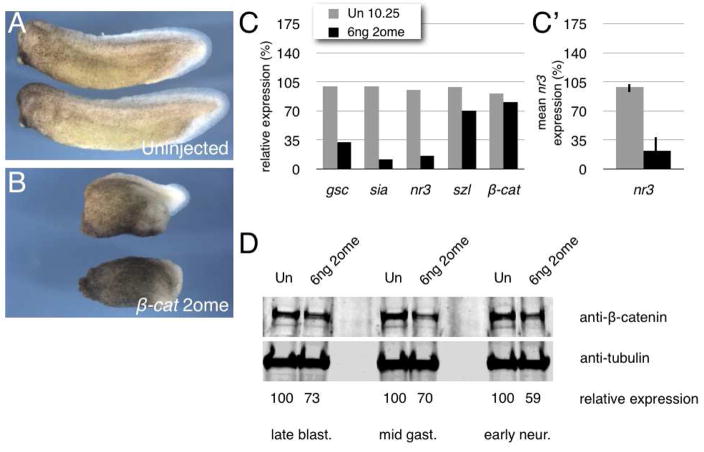
Fig. 1. 2′-O-methyl oligos against Xenopus β-catenin inhibit dorsal axis formation and β-catenin signaling.
(A) Phenotypes of uninjected embryos (stage 31/32); 30/30, 100% normal phenotype (two experiments). Anterior is to the left in all cases.
(B) Phenotypes of embryos injected with 6ng β-cat-2ome oligo; 24/27, 89% axis deficiency (two experiments).
(C) Quantitative real-time PCR of dorsal (gsc, sia and nr3) markers, ventral markers (szl) and β-catenin (β-cat) in control (Un) and 6ng β-cat-2ome oligo-injected (6ng 2ome) embryos at stage 10.25.
(C′) Average (mean) relative expression levels of nr3 in controls and in 6ng β-cat-2ome oligo-injected embryos from six separate experiments (including those in Fig. 1C and 3D). Error bars indicate standard deviation; p-value by t-test was ~0.0001.
(D) Immunoblotting analysis of β-catenin protein levels (top panel) and α-tubulin (lower level) in uninjected control (Un) and in 6ng β-cat-2ome oligo-injected embryos at late blastula, early gastrula and early neurula stages. The relative abundance of β-catenin compared with tubulin is shown below each lane.
To determine the extent that Wnt/β-catenin target genes were affected by injection of βcat-2ome, we analyzed gastrula stage embryos injected with 6 ng oligo by RT-PCR (Fig. 1C). Injected embryos expressed lower levels of nodal-related 3 (nr3) and siamois (sia), which are regulated directly by Wnt/β-catenin signaling. Levels of the Organizer marker goosecoid (gsc) were also reduced, whereas a ventrally expressed gene, sizzled (szl), was unaffected at this stage. β-catenin RNA levels were not affected by βcat-2ome oligo injection, suggesting that the oligo was not acting by causing RNA degradation. By contrast, analysis of β-catenin protein abundance in late blastula stage embryos showed that βcat-2ome oligo was sufficient to reduce total β-catenin abundance by ~33% (Fig. 1D). These reductions in protein levels persisted at least in to the neurula stages, which has also been observed for the corresponding morpholino (Heasman et al., 2000), and is consistent with levels of depletion sufficient to elicit ventralization in Xenpous (Heasman et al., 1994; Heasman et al., 2000). Ventralized embryos could also be obtained by injecting 6ng βcat-2ome oligo into oocytes and fertilizing them by host-transfer into egg-laying females (data not shown), indicating that, like MOs, 2′-OMe oligos can be used both before and after fertilization in Xenopus. Taken together, these data show that injection of an antisense 2′-OMe-modified oligo against β-catenin can reduce β-catenin protein levels without degrading β-catenin RNA, as well as inhibit β-catenin activity and disrupt dorsal axis formation.
We next directly compared the activity of βcat-2ome with β-catenin-MO in embryos from the same female. Embryos were injected with 8 ng, 6ng or 4ng of 2′OMe oligo or MO and frozen at the gastrula stage for RT-PCR and immunoblot analysis. The 8 ng and 6ng (not shown) doses of MO and 2′-OMe caused similar degrees of ventralization (Fig. 2A), resulting in predominantly anterior truncations. However, at the 4 ng doses, the β-catenin-MO was unable to ventralize embryos, whereas 4 ng of the βcat-2ome oligo yielded >50% ventralized embryos. In a separate experiment, we found that 3ng of 2ome oligo was sufficient to cause partial axis reduction in about 50% of the embryos as well as cause a reduction in Wnt target gene expression (data not shown). By RT-PCR, we found that the 8 ng and 6 ng doses of both oligo types caused similar reductions in nr3 expression levels (Fig. 2B) as well as in sia and gsc (not shown). We also compared beta-catenin protein levels in sibling embryos from the same experiment, frozen at the late blastula stage (Fig. 2C). These results fit well with our RT-PCR data, showing that the higher doses cause similar levels of inhibition, whereas the low MO dose causes very little depletion.
Fig. 2. Comparison of 2′-O-methyl oligo and morpholino oligo activity.
(A) Phenotypes of uninjected, β-cat morpholino-injected and β-cat-2ome oligo-injected embryos, 8 ng and 4 ng doses (stage 28). Un, 18/18, 100% normal; 8 ng MO, 8/16, 50% axis deficiency; 4 ng MO, 0/10, 0% axis deficiency; 8 ng 2ome, 14/14, 100% axis deficiency, 4 ng 2ome, 9/17, 53% axis deficiency.
(B) Quantitative real-time PCR of nr3 in control (Un) and oligo-injected embryos at stage 10.25. Oligo doses are 8 ng, 6 ng, 3 ng.
(C) Immunoblotting analysis of β-catenin protein levels (top panel) and α-tubulin (lower level) in uninjected control (Un) and in β-cat-MO and 2ome oligo-injected embryos at the late blastula stages (8 ng, 6 ng, 3 ng). The relative abundance of β-catenin compared with tubulin is shown below each lane.
To confirm that βcat-2ome oligo was acting specifically by inhibiting β-catenin, we performed rescue experiments by injecting mouse β-catenin mRNA. This transcript does not contain a binding site for the oligo and will therefore not compete for binding to the endogenous transcript. We injected embryos with βcat-2ome (6 ng) at the two-cell stage, followed by injection of β-catenin RNA (50 pg) into one dorsal blastomere at the 4-cell stage. Embryos were then frozen at the gastrula and neurula stages for gene expression analysis or left for phenotype assessment. β-catenin was able to rescue normal axis formation (Fig. 3A–C) and RT-PCR analysis showed that dorsal and ventral markers were restored to near-normal levels in 2′-OMe-injected embryos coinjected with β-catenin RNA. These data thus demonstrate that the ventralization generated by oligo injection is indeed due to β-catenin depletion and not due to non-specific oligo effects.
Fig. 3. Specificity of 2′-O-methyl oligos against β-catenin.
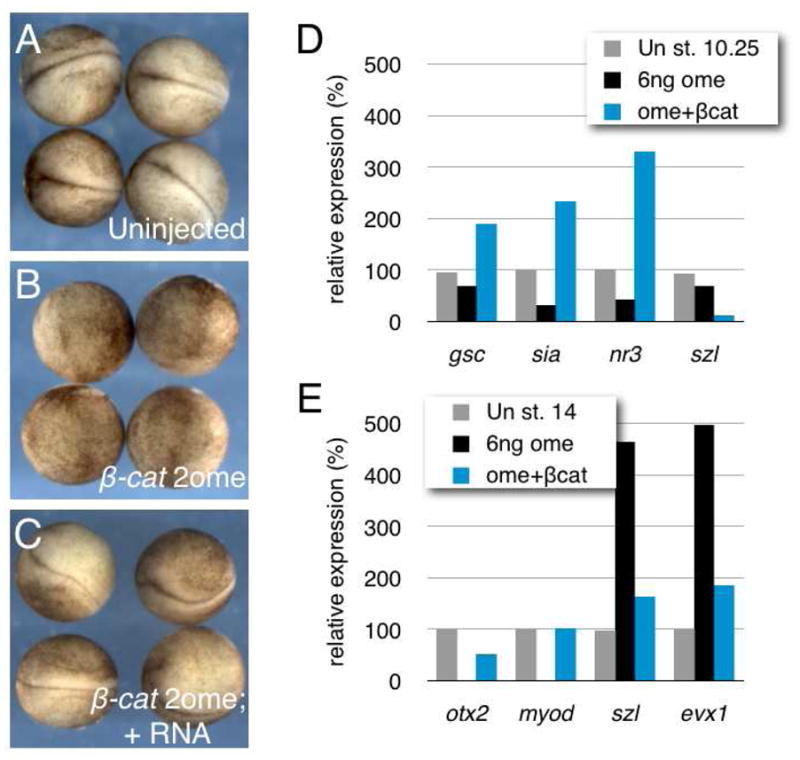
(A) Phenotypes of uninjected embryos (stage 17); 20/20, 100% normal phenotype.
(B) Phenotypes of embryos injected with 6ng β-cat-2ome oligo; 15/15, 100% axis deficiency.
(C) Phenotypes of embryos co-injected with 6ng β-cat-2ome oligo and 50pg β-catenin mRNA (dorsal injection); 3/15, 20% axis deficiency.
(D) Quantitative real-time PCR of dorsal and ventral markers in control (Un) and oligo-injected embryos at stage 10.25.
(E) Quantitative real-time PCR of anterodorsal (otx2, myod) and ventroposterior (szl, evx1) markers in control (Un) and oligo-injected embryos at stage 14.
We used β-catenin as the initial target in these proof-of-principle experiments because the effects of its depletion on dorsoventral patterning are well characterized (Heasman et al., 1994; Heasman et al., 2000). To explore the generality of using 2′-OMe-modified oligos, we next tested a 2′-OMe oligo against Xenopus wnt11 (wnt11b) and compared its activity to the isosequential morpholino (Pandur et al., 2002) in affecting pronephros development (Tételin and Jones, 2009). Injection of wnt11-MO into a ventral vegetal blastomere is sufficient to inhibit pronephros development on the injected side. We injected 20ng of wnt11-MO or 6ng of wnt11-2ome oligo into ventral vegetal blastomeres of 8 cell embryos obtained from the same female. These embryos were raised to the tailbud stage and analyzed by in situ hybridization for pax8, a marker of the pronephric anlagen and otic vesicle at this stage. Both reagents caused morphogenetic abnormalities and reduced the pronephric expression of pax8 to a similar degree (Fig. 4A, B), thus providing a second example of 2′-OMe functionality in embryos.
Fig. 4. 2′-O-methyl oligos against Xenopus wnt11 and bmp4 cause pronephric and tail fin/proctodeal defects.
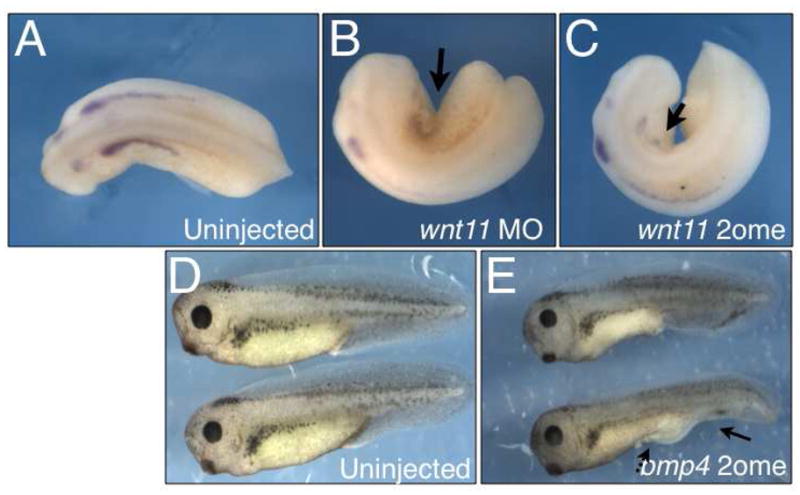
(A) pax8 expression in an uninjected control embryo (stage 24/25); 20/20, 100% normal expression (one experiments).
(B) pax8 expression in embryos injected unilaterally with 20ng of wnt11-MO; 5/9, 56% reduced pronephros (one experiment).
(C) pax8 expression in embryos injected unilaterally with 6ng of wnt11-2ome; 6/8, 75% reduced pronephros (one experiment).
(D) Phenotypes of uninjected embryos (stage 36/7); 40/40, 100% normal phenotype (two experiments).
(E) Phenotypes of embryos injected with 4ng bmp4-2ome oligo. Arrows indicate ventral tail fin (solid line) and proctodeal (dashed line) abnormalities; 16/28, 57% tail defects (two experiments).
We also tested a third example in Xenopus, targeting bone morphogenetic protein 4 (bmp4) using a sequence isosequential to the published MO sequence (Reversade et al., 2005). Injection of bmp4-2ome (3–4 ng/embryo) caused ventral tail and proctodeal defects in over 60% of embryos (Fig. 4D). Molecular analysis of gastrula stage embryos showed reduction in ventroposterior marker expression (szl, evx1; data not shown), confirming defects in ventral cell fate specification and recapitulating known effects of bmp4 inhibition.
We further tested the effectiveness of 2′-OMe oligos in zebrafish by targeting chordin (chd), using an oligo isosequential to the published MO sequence (Nasevicius and Ekker, 2000). We injected chd-2ome (3–5 ng) into one-to-two cell zebrafish embryos and raised these to the tailbud stage (Fig. 5). Injection of chd-2ome caused reduction of head and axial structures, indicative of ventralization, in over 50% of embryos, mimicking the effects of the morpholino oligo as well as the phenotype of the chordino mutant (Nasevicius and Ekker, 2000; Hammerschmidt et al., 1996).
Fig. 5. 2′-O-methyl oligos against zebrafish chd cause dorsoventral patterning defects.
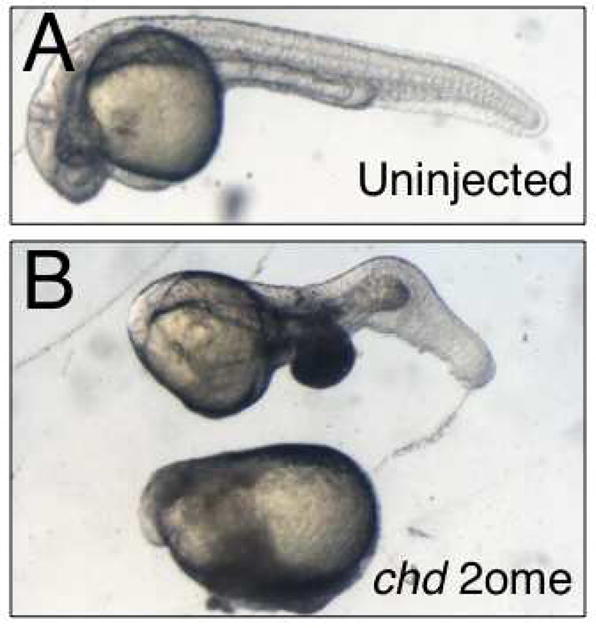
(A) Phenotypes of uninjected embryos (~ 30 hours post fertilization); 10/10, 100% normal phenotype.
(B) Phenotypes of embryos injected with 4ng chd-2ome oligo; 8/15, 53% axis deficiency.
In summary, these data demonstrate that 2′-OMe-modified oligos can be used comparably to MOs for gene knockdown experiments in vertebrate embryos. Although we tested oligos targeted against the start codon to inhibit translation, 2′-OMe-modified oligos would most likely be effective in blocking splicing as well, since they are used in this context in other experimental settings, including human clinical trials. Antisense 2′-OMe oligos have also been used successfully to inhibit miRNA/siRNA function in vivo (Hutvágner et al., 2004). Also, Lennox et al. (2006) have found that chimeric oligos consisting of 2′-OMe modifications on the 5′ and 3′ termini with a central phosphodiester region can be effective RNase H-dependent antisense reagents in early embryos. The 2′-OMe modification can be readily used in chimeric oligo synthesis, allowing for some greater flexibility in choice of antisense mechanisms. The fully modified 2′-OMe-modified oligos we tested functioned similarly to the morpholino oligos, although we found non-specific effects beginning at doses of 12 ng for all oligos tested (data not shown), whereas morpholinos are often injected at much higher doses (Heasman, 2002). Ribose methylation occurs naturally during rRNA and tRNA biogenesis (Maden, 1990) and during piRNA/siRNA biogenesis in animals (Horwich et al., 2007) and miRNA biogenesis in plants (Yang et al., 2006). It is therefore possible that oligo breakdown may alter endogenous nucleotide pools or the activity of proteins involved in RNA metabolism. Also, the high affinity of 2′-OMe modified bases for their RNA targets may allow a certain degree of mismatched binding to occur with high doses, resulting in spurious inhibition. In our hands, morpholinos have a wider range of functional doses, whereas 2′-OMe-modified oligos could be used at lower effective doses. In conclusion, fully modified 2′-OMe oligos can provide a useful alternative antisense reagent for investigators performing gene knockdown studies in embryos.
Methods
Xenopus and zebrafish embryos
Eggs were collected from hCG-stimulated females (1000U) and fertilized using sperm macerated in 0.3× MMR (1× MMR: 100mM NaCl, 2mM KCl, 2mM CaCl2, 1mM MgCl2, 15 mM HEPES, pH 7.6). Embryos were dejellied in 2% cysteine/0.1x MMR, pH 7.8, extensively washed in 0.1x MMR and placed into Ficoll solution for microinjection (2% Ficoll 400 [GE Healthcare] in 0.5x MMR). Following injection, embryos were maintained in Ficoll overnight at 16–18°C and transferred to 0.1x MMR until the desired stage. For molecular analysis, embryos were placed in microcentrifuge tubes with minimal buffer and frozen on dry ice.
Zebrafish embryos were obtained from natural spawning of adults. Embryos were rinsed with embryo medium (Westerfield, 1995) and staged according to Kimmel et al. (1995). Injections were done into the yolk cell at the one-to-two cell stage.
Antisense oligonucleotides
The antisense oligos used were fully modified 25mers with 2′-O-methyl ribonucleotides and HPLC purified (Integrated DNA Technologies, IDT; Coralville, IA). Oligos were dissolved to 1 mM in deionized water and stored at −80°C. Working solutions of 0.25 mM (~1.0–1.5 μg/μl) were made for injections. The following sequences were synthesized, mN= 2′Ome residue, regions matching the start codon are underlined where applicable:
β-catenin-2ome: 5′ mUmUmU mCmAmA mCmCmG mUmUmU mCmCmA mAmAmG mAmAmC mCmAmG mG 3′ (n.t. 260–236, 5′UTR; Heasman et al., 2000).
wnt11-2ome: 5′ mCmCmA mGmUmG mAmCmG mGmGmU mCmGmG mAmGmC mCmAmU mUmGmG mU (wnt11b; n.t. 285–309, Pandur et al., 2002)
bmp4-2ome: 5′ mCmAmG mCmAmU mUmCmG mGmUmU mAmCmC mAmGmG mAmAmU mCmAmU mG 3′ (n.t. 119–95; Reversade et al., 2005).
(zebrafish) chordin-2ome: 5′ mAmUmC mCmAmC mAmGmC mAmGmC mCmCmC mUmCmC mAmUmC mAmUmC mC (n.t. 174–150; Nasevicius and Ekker, 2000).
Realtime RT-PCR
Total RNA was isolated by homogenizing in an RNA lysis buffer containing proteinase K (50 mM Tris, pH 7.5, 50 mM NaCl, 5 mM EDTA, 0.5% SDS, 250 μg/ml PK), followed by phenol:chloroform extraction and ethanol precipitation (Kerr et al., 2009). RNAs were treated with DNase I (Turbo DNA-free kit, Ambion) and reverse transcribed using MMLV reverse transcriptase (Invitrogen) primed with 0.25 μg random hexamers. cDNA was diluted 1:10 and 5 μl was used for PCR. Reactions were run on a LightCycler 480 according to the manufacturer’s instructions and reagents (Roche Applied Science). Amplified products were detected by SYBR Green or by exonuclease probes (Universal Probe Library probes; Roche Applied Science). The cycling conditions were: 94°C (10 minutes, one cycle), 94°C, 60°C, 72°C, (10 seconds each, for 40 cycles). Quantitation was performed by comparing samples to a standard curve of serially diluted control cDNA, followed by normalization against ornithine decarboxylase (odc). Individual representative reactions are shown, each of which was repeated at least twice with similar results. The primers and detection probes used are shown in Table 1.
Table 1.
Realtime PCR primers and exonuclease detection probes
| Target | Detection | Upstream Primer (5′-3′) | Downstream Primer (5′-3′) | Reference |
|---|---|---|---|---|
| odc | UPL #69 | CAGCTTCAGCAATGACGACT | AGATCAGCAACATAAAAGGCATC | New |
| gsc | UPL #126 | CCAAGAAACCAAGTACCCAGA | GCTCTTCGATTTTTGAACCAA | New |
| nr3 | UPL #43 | TTTGTGGACTTTCAGAAGATCG | GGGAGCAAACTCTTAATGTAGGAA | New |
| szl | UPL #12 | GAAGGCCCAGTTGAGTTCAT | GCAATAACATACACTGTGGGTCTG | New |
| -catenin | SYBR | GGACAAACCCCAGGACTACA | ATTGCATCCTGACCATAGCC | New |
| otx2 | SYBR | CGGGATGGATTTGTTGCA | TTGAACCAGACCTGGACT | Heasman et al., 2000 |
| myod | SYBR | AGCTCCAACTGCTCCGACGGCATGAA | AGGAGAGAATCCAGTTGATGGAAACA | Rupp and Weintraub, 1991 |
| evx1 | SYBR | ATATGATGAGCCACGCAGCAG | CAGATGCTGCAGCTCTTTGGC | Gawantka et al., 1995 |
Immunoblotting
Control and oligo-injected embryos were frozen at the late blastula stage and homogenized in 10μl per embryo cell lysis buffer (20 mM Tris, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, plus protease and phosphatase inhibitor cocktail [Thermo/Pierce]). Lysates were boiled in SDS-PAGE sample buffer and one embryo equivalent was loaded onto 10% pre-cast gels (Thermo/Pierce) and run in Tris-HEPES-SDS buffer according to the manufacturer’s instructions. Proteins were transferred to nitrocellulose membranes. For immunoblotting, membranes were blocked in Sea Block (Thermo/Pierce), incubated with primary antibodies (rabbit anti-β-catenin, Sigma, 1:4000; mouse anti-α-tubulin mAb DM1A, Sigma, 1:10,000) diluted in SeaBlock containing 0.1% Tween-20. Washes were done using PBS+0.1% Tween 20, followed by incubation with secondary antibodies (goat anti-mouse DyLight 800, Thermo/Pierce, 1:3000; goat anti-rabbit DyLight 700, Thermo/Pierce, 1:3000) diluted in PBS+ 0.1% Tween-20 + 0.02% SDS. Blots were washed and scanned on an Odyessy near-infrared imager (Licor), according to the manufacturer’s instructions. Band intensities were determined using the Odyssey software and β-catenin levels were normalized against α-tubulin.
In situ hybridization
Whole-mount in situ hybridization was performed essentially as described (Sive et al., 2000; Cuykendall et al., 2009). Antisense RNA probes labeled with digoxigenin-11-UTP (Roche Applied Science) were synthesized using polymerases and reaction buffers from Promega. cDNA for Xenopus tropicalis pax8 (in pCS107; BC125805) was obtained commercially (imaGenes) and linearized by digestion with EcoRI, followed by transcription with T7 RNA polymerase.
Acknowledgments
Grant support: NIH 5R01 GM083999 to DWH
The authors would like to thank Diane Slusarski for contributing zebrafish embryos and for critical reading of the manuscript, and Dan Weeks for critical reading of the manuscript. This work was supported by The University of Iowa and by NIH 5R01 GM083999-02 to D.W.H.
References
- Boiziau C, Kurfurst R, Cazenave C, Roig V, Thuong NT, Toulmé JJ. Inhibition of translation initiation by antisense oligonucleotides via an RNase-H independent mechanism. Nucleic Acids Res. 1991;19:1113–1119. doi: 10.1093/nar/19.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave C, Loreau N, Thuong NT, Toulmé JJ, Hélène C. Enzymatic amplification of translation inhibition of rabbit beta-globin mRNA mediated by anti-messenger oligodeoxynucleotides covalently linked to intercalating agents. Nucleic Acids Res. 1987;15:4717–4736. doi: 10.1093/nar/15.12.4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuykendall TN, Houston DW. Vegetally localized Xenopus trim36 regulates cortical rotation and dorsal axis formation. Development. 2009;136:3057–3065. doi: 10.1242/dev.036855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash P, Lotan I, Knapp M, Kandel ER, Goelet P. Selective elimination of mRNAs in vivo: complementary oligodeoxynucleotides promote RNA degradation by an RNase H-like activity. Proc Natl Acad Sci U S A. 1987;84:7896–7900. doi: 10.1073/pnas.84.22.7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen JS, Smith JC. Controlling morpholino experiments: don’t stop making antisense. Development. 2008;135:1735–1743. doi: 10.1242/dev.001115. [DOI] [PubMed] [Google Scholar]
- Gawantka V, Delius H, Hirschfeld K, Blumenstock C, Niehrs C. Antagonizing the Spemann organizer: role of the homeobox gene Xvent-1. EMBO J. 1995;14:6268–79. doi: 10.1002/j.1460-2075.1995.tb00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, van Eeden FJ, Granato M, Brand M, Furutani-Seiki M, Haffter P, Heisenberg CP, Jiang YJ, Kelsh RN, Odenthal J, Warga RM, Nüsslein-Volhard C. dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development. 1996;123:95–102. doi: 10.1242/dev.123.1.95. [DOI] [PubMed] [Google Scholar]
- Heasman J. Morpholino oligos: making sense of antisense? Dev Biol. 2002;243:209–214. doi: 10.1006/dbio.2001.0565. [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of beta-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Heasman J, Kofron M, Wylie C. Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev Biol. 2000;222:124–134. doi: 10.1006/dbio.2000.9720. [DOI] [PubMed] [Google Scholar]
- Horwich MD, Li C, Matranga C, Vagin V, Farley G, Wang P, Zamore PD. The Drosophila RNA methyltransferase, DmHen1, modifies germline piRNAs and single-stranded siRNAs in RISC. Curr Biol. 2007;17:1265–72. doi: 10.1016/j.cub.2007.06.030. [DOI] [PubMed] [Google Scholar]
- Hukriede NA, Tsang TE, Habas R, Khoo PL, Steiner K, Weeks DL, Tam PP, Dawid IB. Conserved requirement of Lim1 function for cell movements during gastrulation. Dev Cell. 2003;4:83–94. doi: 10.1016/s1534-5807(02)00398-2. [DOI] [PubMed] [Google Scholar]
- Hulstrand AM, Schneider PN, Houston DW. The use of antisense oligonucleotides in Xenopus oocytes. Methods. 2010;51:75–81. doi: 10.1016/j.ymeth.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvágner G, Simard MJ, Mello CC, Zamore PD. Sequence-specific inhibition of small RNA function. PLOS Biol. 2004;2:0465–75. doi: 10.1371/journal.pbio.0020098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr TC, Cuykendall TN, Luettjohann LC, Houston DW. Maternal Tgif1 regulates nodal gene expression in Xenopus. Dev Dyn. 2008;237:2862–73. doi: 10.1002/dvdy.21707. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Lennox KA, Sabel JL, Johnson MJ, Moreira BG, Fletcher CA, Rose SD, Behlke MA, Laikhter AL, Walder JA, Dagle JM. Characterization of modified antisense oligonucleotides in Xenopus laevis embryos. Oligonucleotides. 2006;16:26–42. doi: 10.1089/oli.2006.16.26. [DOI] [PubMed] [Google Scholar]
- Maden BE. The numerous modified nucleotides in eukaryotic ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1990;39:241–303. doi: 10.1016/s0079-6603(08)60629-7. [DOI] [PubMed] [Google Scholar]
- Manoharan M. 2′-carbohydrate modifications in antisense oligonucleotide therapy: importance of conformation, configuration and conjugation. Biochim Biophys Acta. 1999;1489:117–130. doi: 10.1016/s0167-4781(99)00138-4. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Pandur P, Läsche M, Eisenberg LM, Kühl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–41. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- Reversade B, Kuroda H, Lee H, Mays A, De Robertis EM. Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embryos. Development. 2005;132:3381–3392. doi: 10.1242/dev.01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp R, Weintraub H. Ubiquitous MyoD transcription at the midblastula transition precedes induction-dependent MyoD expression in presumptive mesoderm of X. laevis. Cell. 1991;65:927–37. doi: 10.1016/0092-8674(91)90545-a. [DOI] [PubMed] [Google Scholar]
- Sazani P, Kole R. Therapeutic potential of antisense oligonucleotides as modulators of alternative splicing. J Clin Invest. 2003;112:481–486. doi: 10.1172/JCI19547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth J, Colman A. Antisense oligonucleotide-directed cleavage of mRNA in Xenopus oocytes and eggs. Embo J. 1988;7:427–434. doi: 10.1002/j.1460-2075.1988.tb02830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive H, Grainger R, Harland RM. Early Development of Xenopus Laevis: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Stein D, Foster E, Huang SB, Weller D, Summerton J. A specificity comparison of four antisense types: morpholino, 2′-O-methyl RNA, DNA, and phosphorothioate DNA. Antisense Nucleic Acid Drug Dev. 1997;7:151–157. doi: 10.1089/oli.1.1997.7.151. [DOI] [PubMed] [Google Scholar]
- Tételin S, Jones EA. Xenopus Wnt11b is identified as a potential pronephric inducer. Dev Dyn. 2009;239:148–59. doi: 10.1002/dvdy.22012. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. University of Oregon Press; Eugene, OR: 1993. [Google Scholar]
- Yang Z, Ebright YW, Yu B, Chen X. HEN1 recognizes 21–24 nt small RNA duplexes and deposits a methyl group onto the 20 OH of the 30 terminal nucleotide. Nucleic Acids Res. 2006;34:667–675. doi: 10.1093/nar/gkj474. [DOI] [PMC free article] [PubMed] [Google Scholar]