Abstract
In recent years, bio-inorganic nanohybrids composed of biological macromolecules and functional inorganic nanomaterials have revealed many unique properties that show promise for the future. Transmission electron microscopy (TEM) is a popular and relatively simple tool that can offer a direct visualization of the nanomaterials with high resolutions. When TEM is applied to visualize bio-inorganic nanohybrids, a treatment of negative staining is necessary due to the presence of biological molecules in the nanohybrids except for those with densely packed inorganic materials. However, the conventional negative-staining procedure for regular biological samples cannot be directly applied to such bio-inorganic nanohybrids. To image a specific bio-inorganic nanohybrid, negative-staining factors such as negative stain type, working pH, staining time, and drying method, should be identified. Currently, no detailed studies have been done to investigate how to adjust negative-staining factors based on specific bio-inorganic nanohybrids. In this study, bacteriophage-gold nanoparticle hybrids were chosen as a model to systematically study the effects of each factor on the negative staining of the nanohybrids. The best staining conditions for gold nanoparticle-phage nanohybrids were obtained and the effects of each factor on the negative staining of general nanohybrids were discussed. This work indicates that with proper staining it is possible to use TEM to directly visualize both biological and inorganic components without introducing any artifact.
Keywords: transmission electron microscopy, bio-inorganic nanohybrids, negative staining, bacteriophage, gold nanoparticles
Introduction
Nano-scale inorganic materials exhibit unique properties and show great promise for the future (Daniel and Astruc, 2004; Lu and others, 2007; Sargent, 2005; Tasis and others, 2006). One particular challenge with nano-scale inorganic materials has been in assembling them into well-defined structures or integrating them into large-scale devices. One strategy incorporates the use of self-ordered biomolecules, which are directly obtained from Nature such as nucleic acids, proteins and viruses, to organize nanomaterials into pre-designed patterns. The combination of these biological molecules with functional inorganic nano-materials have led to many hierarchical nanostructure organizations with controlled shape, size, alignment and orientation (Belcher and others, 2004; Cao and Mao, 2007; Lin and others, 2009; Mao and others, 2003; Mao and others, 2004; Sharma and others, 2009). The characterization of the designed bio-inorganic nano-hybrids is a crucial step in the development of novel nanomaterials. A variety of imaging techniques have been applied in this field such as transmission electron microscopy (TEM), atomic force microscopy (AFM), scanning electron microscopy (SEM) and fluorescence microscopy. Among them, TEM is a relatively simple technique that can offer a direct visualization of the individual nano-hybrids with high resolutions. Therefore, it has become one of the most commonly used techniques for the characterization of nanomaterials.
It is easy to visualize inorganic nanomaterials such as gold nanoparticles under TEM without any staining due to the high contrast between the inorganic materials and the underlying supporting film such as carbon. Biological molecules are mainly composed of carbon, hydrogen, and nitrogen atoms, and thus are only recognized under TEM with heavy metal ion staining. Bio-inorganic nano-hybrids are composites of biological molecules with functional inorganic nanomaterials. If biological molecules are densely packed by inorganic materials, the structures of the hybrids can be observed without staining. Our previous results have shown that in a nano-composite of silica-coated bacterial flagella, both silica and flagella could be recognized without staining (Wang and others, 2009). However, if inorganic materials are loosely arranged around biological molecules, only inorganic materials having higher molecular weights can be visualized. In such characterization, direct evidence showing the close proximity between inorganic and biological components, which can help us understand the interactions between both components, is missing. Therefore, negative staining is needed to visualize the biological components in such nano-hybrids so that both components can be observed under TEM.
Negative staining is based on the principle that heavy staining ions are repelled by the charged groups of the specimen and sit along biological molecules (Hayat and Miller, 1990). However, the conventional negative-staining procedure for regular biological samples cannot be directly applied to such bio-inorganic nanohybrids. For a specific bio-inorganic nanohybrid, some negative-staining factors should be modified, such as the type of negative stain, working pH, staining time, and drying method. In this work, bacteriophage-gold nanoparticle nanohybrid was used as a model to systematically study the effects of each factor on the negative staining of the nanohybrids (Figure 1 b). The optimum negative-staining condition for bacteriophage-gold nanoparticle hybrid was obtained and the effects of each factor on the negative staining of the nanohybrids are discussed.
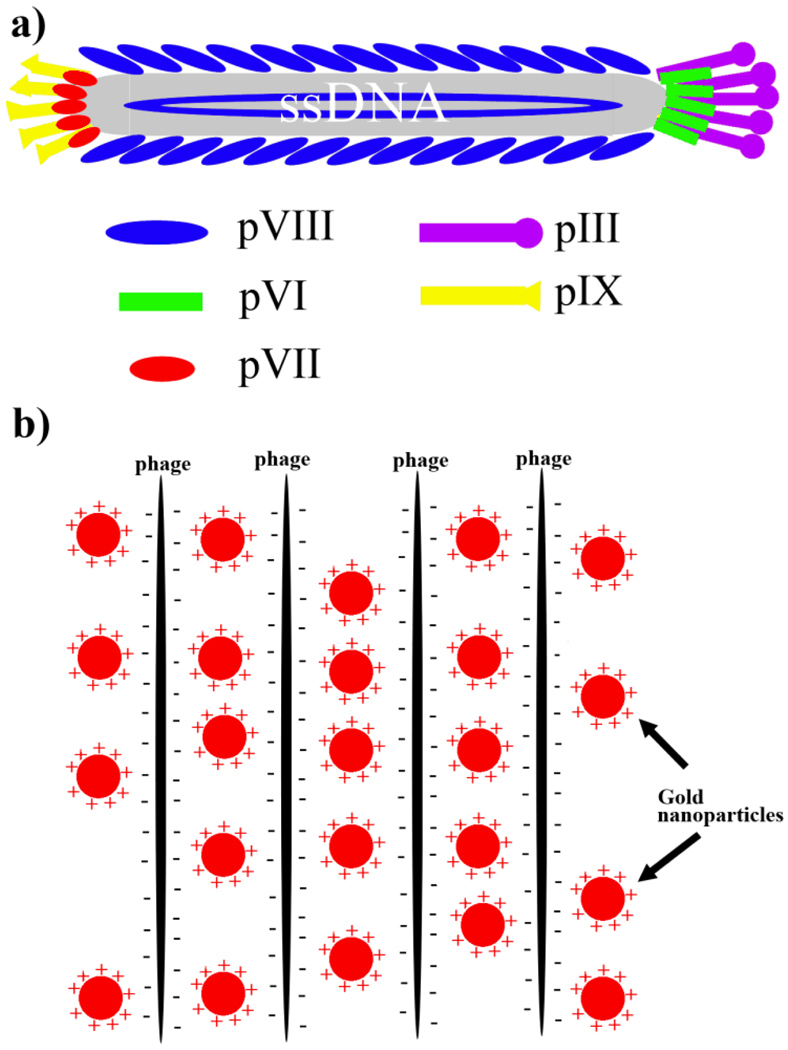
Figure 1.
a) M13 wild type phage particles composed of ~2700 copies of major coat proteins (pVIII) and 5 copies of each minor coat protein (including pIII, pVI, pVII, and pIX); b) Positively charged Au-NPs interacted with anionic phage particles into bundle structures.
Materials and Methods
Materials
Uranyl acetate (UA) powder was purchased from Ted Pella Inc. Phosphotungstic acid (PTA) was purchased from Ted Pella Inc. Trehalose was purchased from Sigma Inc. Gold chloride solution was purchased from Sigma Inc. Polyethylenimine (branched, average MW=~25000) was purchased from Sigma Inc. Sodium borohydride powder was purchased from Sigma Inc. TEM grids were purchased from Ted Pella Inc. M13 wild type bacteriophage was purchased from New England Biolabs Inc.
M13 Amplification
200 µL overnight ER2738 E.coli culture and 1 µL phage suspension were added into a 20 mL LB culture in a 250 ml flask. The flask was incubated at 37 °C with shaking for 4–5 h. After incubation, the culture was centrifuged at 4500 g for 20 min to remove E. coli cells. Then the top 16 mL of the supernatant was transferred into a new tube and 4 mL of 2.5 M NaCl/20% PEG-8000(w/v) was added to precipitate the phage. After 4 hours of precipitation at 4 °C, the phage was pelleted by centrifugation at 12000 g for 15 min. Supernatant was discarded and the phage pellet was re-suspended in 1 mL TBS buffer. 200 µL 2.5 M NaCl/20% PEG-8000 was added into the re-suspended phage solution and the mixed solution was allowed to incubate on ice for 1 h. The phage was pelleted again by centrifugation at 14000 g for 10 min. Supernatant was discarded and the phage pellet was re-suspended in 200 µL TBS buffer. The quantification of phage was achieved by monitoring its OD269nm. Concentrations of phage solutions were determined from absorbance measurements and using published extinction coefficients in unit of mg−1cm2 of 3.84 at 269 nm for M13 phages (Berkowitz and Day, 1976). An OD269nm of 1 means the concentration of the phage in solution is about 1×1013 pfu/mL (See supporting information).
Synthesis of Gold Nanoparticles
AuNPs were synthesized by using a reported method (Note and others, 2006). Specifically, 15 µL 5% (w/w) polyethylenimine (PEI) (branched, average MW=~25000) was added into 1 mL 0.2% gold chloride (HAuCl4) solution with stirring. The color of the solution changed from yellow to orange. Then 20 µL of 5M NaBH4 solution was added into the PEI/HAuCl4 solution with violent stirring. Immediately, the solution color turned from orange to dark red, the color of nano-sized gold particles.
Preparation of Phage-Gold Nanoparticle Hybrid
100 µL phage solution (1.5×1013 pfu/ml) was added into 1 mL gold nanoparticle solution and mixed well. The mixture was incubated at room temperature for 4 hours. Gold-phage hybrids were formed and slowly precipitated to the bottom of the tube. Hybrids were pelleted by centrifugation at 10000 g for 1 min and re-suspended in 1 mL water.
Negative Staining
In a typical UA staining, 10 µL Au-NPs-phage hybrid solution was applied onto a formvar-coated TEM grid for 3 min During this process, the Au-NPs-phage nanohybrids was absorbed to the supporting film. The rest of the solution was wicked from the edge of the grid by the wedge of the filter paper. Immediately, 10 µL 0.5% UA solution (pH=4.5) was applied to the specimen for 10 s. Then, the stain solution was wicked from the edge of the grid by the wedge of the filter paper. Lastly, the TEM grid was dried by a hair drier and characterized under TEM (Zeiss 10). In this way the effects of each factor on the negative staining of the nanohybrids could be analyzed.
Pre-fixation with 1% Glutaraldehyde (GA)
10 µL Au-NPs-phage solution was applied onto a formvar-coated TEM grid for 3 min while the specimen was absorbed to the supporting film. The rest of the solution was wicked from the edge of the grid by the wedge of the filter paper. 20 µL 1% GA solution was applied onto the specimen for 20 min. After the incubation, the GA solution was wicked from the edge of the grid by the wedge of the filter paper and the specimen was washed 3 times by water. Then 10 µL 0.5% UA solution (pH=4.5) was applied on the specimen for 10 s. Then, the stain solution was wicked from the edge of the grid by the wedge of the filter paper. Lastly, the TEM grid was dried by a hair drier and characterized under TEM (Zeiss 10).
Results
Preparation of Gold Nanoparticles
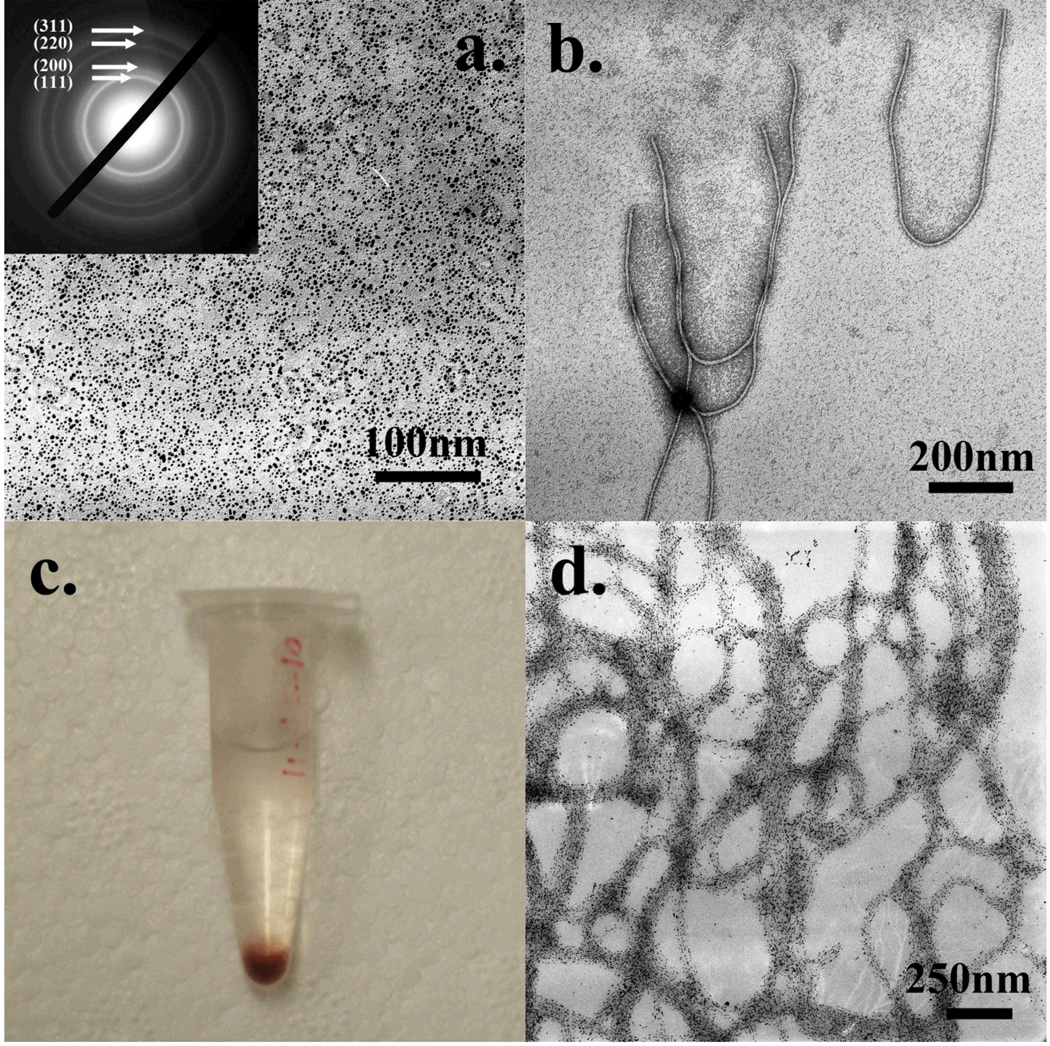
HAuCl4 was reduced by NaBH4 into gold nanoparticles (Au-NPs) and stabilized by polyethylenimine (PEI) molecules. After the reaction, the Au-NP solution became transparent and its color turned to dark red. Then the solution was diluted and characterized under a TEM. Over large areas, only well-dispersed nanoparticles could be observed with a uniform size of ~5–7 nm (Figure 2 a). In addition, a typical electron diffraction pattern corresponding to gold crystals was found from the nanoparticles, suggesting the nanoparticles in the image are gold crystals. Since the Au-NPs were stabilized by highly-branched PEI molecules, the synthesized Au-NPs should be positively-charged due to the presence of the amino groups from the surrounding PEI molecules.
Figure 2.
a) Synthesized gold nanoparticles and the electron diffraction pattern; b) wild type M13 phages stained with 1% UA; c) gold-nanoparticle-phage hybrids precipitation; d) TEM image of the hybrid precipitation from c.
Wild-Type M13 Bacteriophage
Wild-type M13 phage is a flexible nanofiber with its single stranded DNA (ssDNA) genome packed into a protein coat which is composed of 2700 copies of major coat proteins (pVIII) and 5 copies of each minor coat protein (including pIII, pVI, pVII, and pIX) (Smith and Petrenko, 1997). 2700 copies of pVIII proteins form its side wall while 5 copies of pIII and pVI are located at one tip and 5 copies of pIX and pVII at the other tip (Figure 1 a). The ssDNA genome encodes a total of 11 proteins including 5 structural coat proteins. From its surface chemistry, the amino terminus of each pVIII protein is exposed to the surface with the first five residues (AEGDD) extending away from the phage. So it can be easily figured out that on the phage surface there are 2700 copies of Glu and 5400 copies of Asp both of which have carboxyl groups, leading to a negatively charged surface. The experimental isoelectric point (pI) value of 4.2 also confirmed the negative phage surface charge (Monjezi and others, 2010). With a high concentration, phage particles tend to form bundle structures if polycationic molecules are added (Wang and others, 2010). It was believed that the addition of polycationic molecules can trigger the assembly of neighboring phage particles via electrostatic interactions. In this work, the wild type M13 phages after the amplification were quantified by monitoring its OD269nm. Roughly, 1 OD269nm means the concentration of the phage in solution is about 1×1013 pfu/mL. The detailed calculation could be found in supporting information. The phage was stocked and used at a concentration of 1.5×1013 pfu/mL (OD269nm=1.5). Usually, up to 1×1014 phages could be harvested from 1 liter of LB culture. In order to know the morphology of the wild type M13 phage, the viruses were stained with 0.5% UA and observed under a TEM (Figure 2 b). Every single M13 phage particle has a flexible rod-like shape with a diameter of ~6.5 nm and a length of ~930 nm (Figure 2 b), which is consistent with previous literatures (Smith and Petrenko, 1997).
Gold Nanoparticle-Phage Hybrids
After a phage solution was injected into the Au-NP solution, immediately, Au-NP-phage hybrids were formed and slowly precipitated to the bottom of the tube. Free Au-NPs were then removed by centrifugation and the hybrids were re-suspended in water. Again, the gold-phage hybrids slowly precipitated to the bottom of the tube, leaving the supernatant clear and colorless (Figure 2 c). A small quantity of the hybrids was characterized under TEM without staining. Most Au-NPs were aligned into large belts, forming a net-like structure (Figure 2 d). Only a few free Au-NPs were observed away from the belts, indicating almost all of the free Au-NPs were removed during washing. We believe positively-charged Au-NPs electrostatically interacted with M13 phages to induce the formation of bundle-like Au-NP-phage hybrids. However, without any staining (Figure 2 d), this hypothesis cannot be experimentally verified because we could not see any phage particle or any phage-Au-NP connections directly. In order to visualize the structural details of such hybrids, a negative staining was applied to the hybrid specimen.
Best Negative Staining Conditions
In order to obtain the best staining condition to image the phage-gold nanohybrids, the effects of each factor on the negative staining of the nanohybrids were systemically studied. Specifically, 10 µL hybrid solution was applied onto a formvar-coated TEM grid for 3 min. During this time, the specimen was absorbed to the supporting film. The rest of the solution was wicked from the edge of the grid by the wedge of the filter paper. Immediately, 10 µL 0.5% UA solution (pH=4.5) was applied on the specimen for 10 s. Then, the stain solution was wicked from the edge of the grid by the wedge of the filter paper. Finally, the TEM grid was dried by a hair drier and characterized under TEM. With the help of UA, phage particles were negatively-stained so that both Au-NPs and phage particles could be observed under TEM (Figure 3 a&b). As a result, it is clearly seen that the M13 phage particles are interacted with positively-charged Au-NPs, which could be a direct evidence to prove our initial hypothesis.
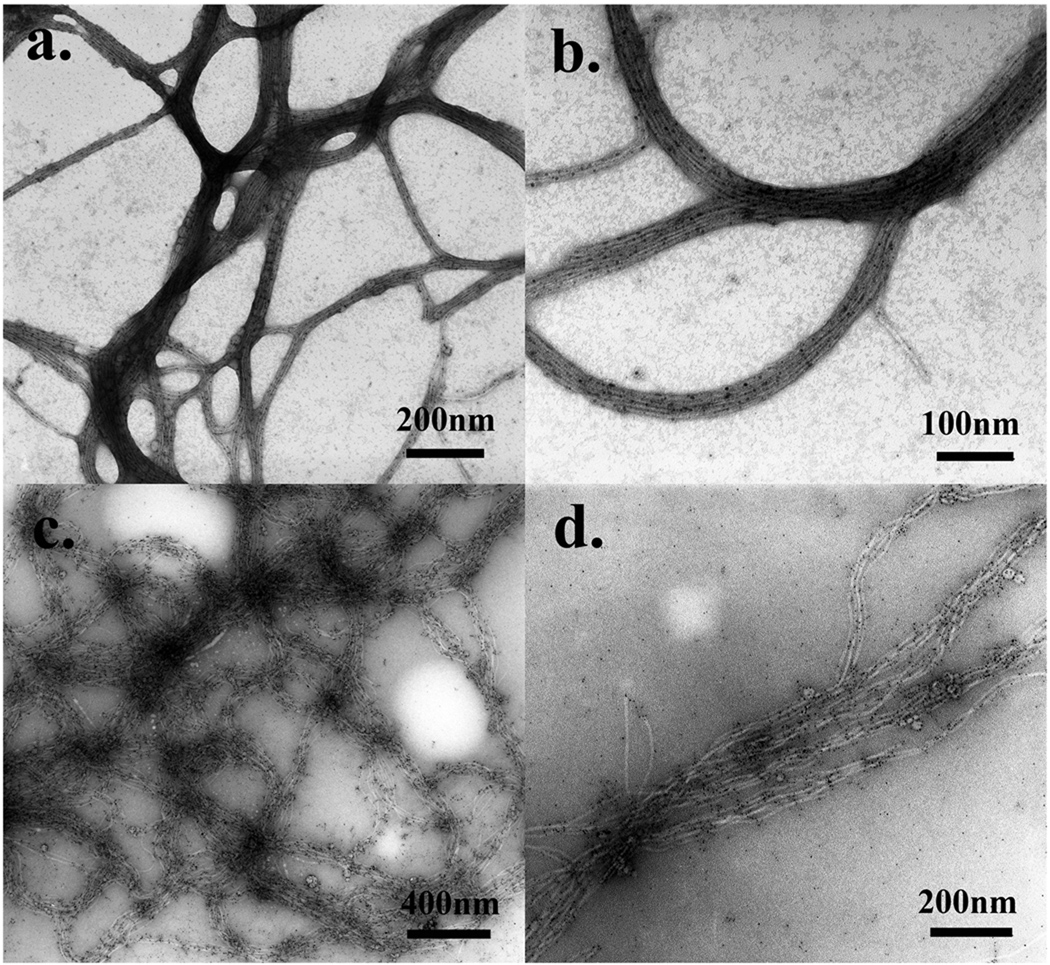
Figure 3.
a) Au-NP-phage hybrids were stained with 0.5% UA solution (pH=4.5, 10 s of staining, rapid drying); b) high resolution image of a); c) Au-NP-phage hybrids were stained with 0.5% PTA solution (pH=7, 10s of staining, rapid drying); d) high resolution image of c).
One problem was in some areas the Au-NPs were covered by the heavy stain-salts so that some of the nanoparticles were not easily recognized. This situation resulted from the pH of the staining solution. As previously mentioned, the isoelectric point of M13 phages is about 4.2 which is lower than the pH value of the staining solution (4.5). Hence, the staining may contain some positive-staining effects which darkened the biological structures in certain areas. Decreasing the pH of the stain solution could solve this problem. However, a stain solution with a low pH will cause other problems to the nanohybrids which will be mentioned later in more detail. In fact, the synthesized Au-NPs have an excellent contrast and could still be recognizable in a light staining. Therefore, when we reduced the concentration of the stain to 0.5%, this problem was reduced (Figure 3 b). We carefully highlighted the Au-NPs in the negatively-stained sample (Figure 3 b) and compared it with the non-stained sample (Figure 2 d). The results clearly showed that the nanohybrids were nicely stained and most Au-NPs were still on the hybrids after negative staining (Figure S1).
Choice of Stains
Besides UA, phosphotungstic acid (PTA) is another popular stain for negative staining. Usually, the working concentration is about 0.5%–2% at a neutral pH. Here, we tried to use 0.5% PTA solution (pH=7.0) for the staining to check if PTA is better than UA for this system. Under similar condition using 10s of the staining solution and rapid drying, the structure of Au-NP-phage hybrid was greatly changed as a result of the staining process. Specifically, individual phage particles within bundles were detached from each other and there was no dense packing of the Au-NPs with phage particles could be found (Figure 3 c&d). Moreover, a lot of Au-NPs were detached from phage bodies during the process of staining (Figure 3 d). Such detachment could not be found from the non-stained specimen (Figure 2 d). These results suggest that the staining ions from the PTA solution interrupted the interactions between Au-NPs and phage particles, resulting in the detachment of Au-NPs from phage particles. Without the help of positively charged Au-NPs, phage particles could not be attracted any further so they repelled each other and finally broke the densely packed bundle structures. Therefore, PTA is not a suitable stain for this nanohybrid because it interrupts the interactions between the two components and greatly changes the hybrid structures. From this PTA-stained sample only inaccurate structural details were obtained.
Choice of the Concentrations of the Stain
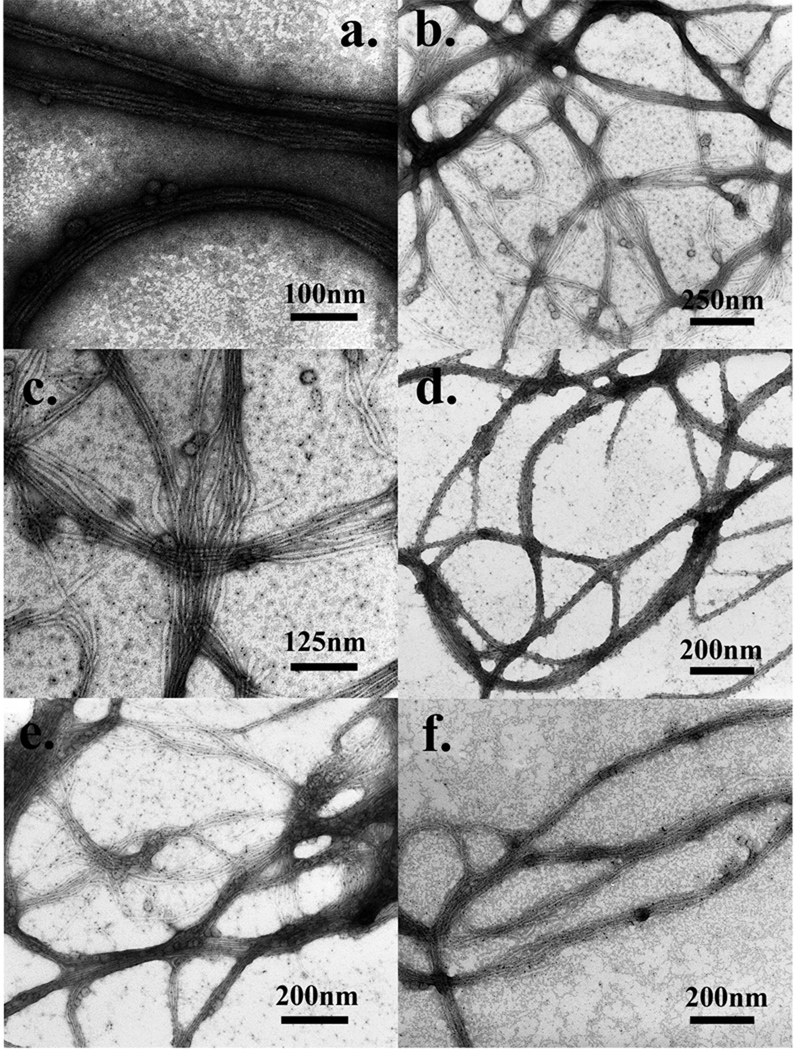
In order to test if a higher concentration of the stain is better in this system, we increased the concentration of the UA solution from 0.5% up to 2% and kept other factors the same as in the optimal condition (10s of staining, rapid drying, pH=4.5). When stained with 2% UA, the Au-NP-phage hybrids were heavily darkened (Figure 4 a). Densely packed phage bundles were still recognizable with careful identification, but the Au-NPs within Au-NP-phage hybrids were totally covered by the heavy stain and became unrecognizable (Figure 4 a). As previously mentioned, the isoelectric point of M13 phages is about 4.2 which is lower than the pH of the staining solution (pH=4.5). So such staining by the 2% UA solution caused an obvious positive-staining effect which heavily darkened the Au-NP-phage hybrids. This test suggested that a lower concentration (0.5%) of UA solution brought fewer positive-staining effects to the specimen and worked better on the Au-NP-phage nanohybrids than a concentrated UA solution (2%).
Figure 4.
a) Au-NP-phage hybrids were stained with 2% UA solution (pH=4.5, 10s of staining, rapid drying); b) Au-NP-phage hybrids were stained with 0.5% UA solution (pH=4.5, 1 minute of staining, rapid drying); c) high resolution image of b); d) Au-NP-phage hybrids were stained with 0.5% UA solution (pH=3.5, 10s of staining, rapid drying); e) Au-NP-phage hybrids were stained with 0.5% UA solution (pH=4.5, 10s of staining, slow drying); f) Au-NP-phage hybrids were pre-fixed by 1% GA solution and stained with 0.5% UA solution (pH=3.5, 1 minute of staining, slow drying).
Staining Time
Staining time is another factor that may affect the negative staining. We increased the staining time from 10 s up to 1 min and kept all other factors the same as the optimal condition (0.5% UA, rapid drying, pH=4.5). Again, the structure of Au-NP-phage hybrid was greatly changed during the staining. Phage particles were separated from each other and most of the Au-NPs were detached from the phage bodies (Figure 4 b&c). These results suggest that the staining ions from the UA solution are more electrostatically attracted to phage particles than Au-NPs and therefore slowly replaced Au-NPs. This replacement needs a longer time to occur (1 min). Consequently, after a longer time of staining, the densely packed bundle structures were broken. Unlike the stain PTA, UA molecules needed a longer time to break up the Au-NP-phage hybrids. Therefore, we believe staining with 0.5% UA for a short time (10 s) is a better choice for this model system.
pH of the Stain
The pH of the stain solution plays a crucial role in most staining processes. It is known that in negative staining heavy staining ions are repelled by the charged groups of the specimen and sit along biological molecules whereas in positive staining heavy metals react with and attach to biological molecules. The charges on the surface of the biological molecules are mainly dependent on its isoelectric point and local pH value. Since the isoelectric point is constant for a specific biological molecule, the surface chemistry of the molecules is dependent on the local conditions.
In the Au-NP-phage hybrid system, the isoelectric point of M13 phages is about 4.2, so in theory, a lower pH of the UA solution should not cause positive staining effects and will have a better performance in staining. Therefore, we decreased the pH of the UA solution from 4.5 to 3.5 while keeping all other factors the same as the optimal condition (0.5% UA, rapid drying, 10s of staining). Densely packed phage bundles were nicely stained by the staining ions and Au-NPs could also be observed clearly (Figure 4 d). However, most Au-NPs were detached from the phage bundles, which were not found in the non-stained specimen (Figure 2 d). The possible reason for this phenomenon is the low pH of the UA solution made the M13 phage particles positively charged. Under this condition, the positive charges on phage particles made a perfect negative staining by UA, but at the same time, the positive charges also repelled positively charged Au-NPs from phage particles. Captured TEM images should ideally reflect the real structures of nanohybrids. Therefore, any stain solution with a pH lower than 4.2 is not a good choice for the visualization of Au-NP-phage hybrid.
Drying Method
Drying is also a factor that may affect the appearance of specimens. We tried a slow air-drying of the UA stain and kept all other factors the same as the optimal condition (0.5% UA, pH=4.5, 10s of staining). We found a lot of small stain crystals appearing on the TEM grid. Besides, most Au-NPs were found detached from the phage bodies and some phage bundles were broken (Figure 4 e). Obviously, compared to fast drying achieved by using a hair dryer (Figure 3 a–b), the slow air-drying of the stain encouraged the formation of stain crystals and also interrupted the electrostatic interactions between Au-NPs and phage particles.
Choice of Pre-fixation
We performed a pre-fixation with 1% glutaraldehyde (GA) for 20 min before negative staining and then stained the specimen with 0.5% UA solution (pH=3.5, 1 minute of staining, slow drying). As previously mentioned, under these staining conditions without pre-fixation, phage bundles should be disassembled and Au-NPs should be detached from the phage particles. However, none of the expected phenomena happened. Instead, phage bundles were nicely stained and most Au-NPs were still attached to phage particles (Figure 4 f). The good attachment results from the fact that phage particles and Au-NPs were covalently cross-linked by GA molecules within the hybrids. Therefore, in the process of staining, either low pH or long staining time could not break any of these covalent bonds, leaving the integrated structures of the Au-NP-phage hybrids.
Discussion
Mechanism of Negative Staining with UA and PTA
Negative staining is achieved when heavy staining ions are repelled by the charged groups of the specimen and tracking the trace of the biological molecules. These heavy atoms increase electron scattering and improve the amplitude contrast of biological molecules. Uranyl acetate (UA, C4H6O6U) is a popular negative stain that has been most widely used since 1960. It is used at a pH ranging from 3.5 to 5.5 (the usual pH is 4.5) due to its instability at pH higher than 6.0. A molecule of uranyl acetate in water is in form of uranyl cation (UO22+). At a pH of approximately 4.5, which is below the isoelectric point of most proteins, proteins are positively charged and the charged protein residues repel positively charged UO22+, resulting in negative staining (Hayat and Miller, 1990). Phosphotungstic acid (PTA, H3PW12O40) is another typical negative stain that usually works at a neutral pH. This stain can be employed at a pH range of 5.0 to 8.0, though its optimum pH of 6.9. A molecule of PTA in water is in form of ionic PW12O403−. At a pH of approximately 7.0, which is above the isoelectric point of most proteins, proteins are negatively charged and the charged protein residues repel negatively charged PW12O403−. This condition also results in negative staining (Hayat and Miller, 1990). However, a certain degree of interactions between charged groups of biological molecule and stain ions, especially UO22+, may occur, producing positive staining. The main reason for the occurrence of positive staining with UA is the small difference between the pI of the protein (approximately 5.0) and the pH of the staining solution (usually 4.5).
Bio-Inorganic Nanohybrids and Negative Staining
The conventional negative-staining procedure for regular biological samples cannot be applied to bio-inorganic nanohybrids directly. More factors should be customized according to each specific hybrid, such as the choice of pH value, staining reagent, staining time, and drying method. First, most stain solutions contain divalent or trivalent stain ions and some of them have low pH values (e.g., for UA). These stain ions and low pH values both contribute to the interruptions of the interactions between biological molecules and inorganic nanomaterials. If biological molecules and inorganic nanomaterials are covalently linked through a chemical reaction, either low pH or stain ions can’t break the covalent bonds (Sharma and others, 2008). However, if they are linked through electrostatic interactions, pH values and stain ions may greatly influence the interactions (as shown in the Au-NP-phage system). Therefore, the choices of stains, pH values, working concentrations and staining time should be selected carefully. Second, some inorganic nanomaterials and biological molecules are not stable at low pH values. For example, hydroxyapatite nanocrystals will be dissolved at a pH lower than 4.0 (Chen and others, 2009; Pan and Darvell, 2007) and bacterial flagella are not stable at an acidic pH (low than 5.0). For these materials, UA solution is not a good choice due to its acidic working pH (3.5–5.5). Third, biological molecules have different isoelectric points, the pH of stain solutions should be chosen according to the specific pI of the biological molecules of interest. Fourth, some biological molecules only have one type of charged group so not all negative stains can be applied to these samples. For example, DNA assemblies only have negative charges on their surfaces. In this case, only PTA or neutral stains can be used to negatively stain DNA assemblies. Lastly, some inorganic nanomaterials also have low contrasts (such as Al2O3 or small nanoparticles) so that they cannot be identified when covered by heavy layers of stains. Under this condition, a light staining is expected to work better.
For our Au-NP-phage model system, the formation of the hybrids is based on the electrostatic interactions between positively charged Au-NPs and negatively charged phage particles. Therefore, the hybrids may not be stable when exposed in stain solutions. After the systematic studies of the effects of each factor on the negative staining of the hybrids, we found the electrostatic interactions between Au-NPs and phage particles could be greatly interrupted when the hybrids were exposed to one of the following staining conditions: (1) PTA stain solution (Figure 3 c&d); (2) UA stain solution for a long time (>1 min) (Figure 4 b&c); (3) UA stain solution with a pH lower than the pI of phage particles which is ~4.2 (Figure 4 d); (4) UA stain solution with a slow air-drying (Figure 4 e). The respective reasons are outlined in Table 1.
Table 1.
A summary of conditions interrupting the association of Au-NPs and phage with justification.
| Conditions interrupting the association of Au-NPs and phage |
Reasons |
|---|---|
| PTA stain solution | PW12O403− competed with the -COO− on phage particles to bind with positively charged Au-NPs |
| UA stain solution for a long time (>1 min) | UO22+ competed with the positively charged amine groups on Au-NPs for the binding with negatively charged phage particles. So the staining time should be as short as possible. |
| pH lower than the pI of phage particles (~4.2) | A low pH value of UA solution (<4.2) will make the M13 phage particles have a net positive charge and thus repel positively charged Au-NPs. |
| slow air-drying | In a process of air-drying, UA concentration will increase and the local pH will decrease. A slow air-drying sets the Au-NP-phage hybrids into the harsh local environments for a longer time and thus interrupts the electrostatic interactions between Au-NPs and phage particles. |
Choice of Stain and Working pH Based on Specific Bio-Inorganic Nanohybrids
Choosing the type of stain and its working pH value according to specific bio-inorganic nanohybrids is the most important step in the negative staining. The choice of the stain and its working pH value should be based on specific bio-inorganic hybrids. Specifically, if inorganic nanomaterials or biological molecules are not stable in acidic solutions, PTA or other neutral stains will be better for the hybrids. If both materials are not pH-sensitive, the driving forces for holding together the biological molecules and inorganic nanomaterials should be checked. If both materials are linked by covalent bonds through a chemical reaction, both UA and PTA can be used as the negative stain. But if the electrostatic interaction is the driving force to hold the biological and inorganic components, more factors should be paid attention to during the staining. PTA stain is preferred if PW12O403− ions do not greatly interrupt the electrostatic interactions between the two components. Otherwise, UA stain will be tested to see if UO22+ ions will interrupt the interactions. If yes, it means both PTA and UA stains are not suitable for the staining. If no, UA will be selected as the main negative stain for the hybrids.
Once the negative stain is selected, its working pH value needs to be determined next. For PTA, the working pH value should be within the range from 5.0 to 8.0 and also higher than the pI value of the biological molecules (Hayat and Miller, 1990). For UA, the situation is more complicated: first, the specific working pH value should be within the range from 3.5 to 5.5 (Hayat and Miller, 1990); second, if positively charged biological molecules (usually pI>7.0) are interacted with negatively charged inorganic nanomaterials through an electrostatic interaction, a regular pH of 4.5 will be good for the staining; third, if negatively charged biological molecules (usually pI<7.0) are interacted with positively charged nanomaterials through an electrostatic interaction, pH=pI+0.3<5.5 (when pI<5.2) is the best working pH value. In this case, the slightly negatively charged biological molecules and positively charged UO22+ may interact with each other and cause some positive-stain effects to specimens, but the effects are not significant. However if 5.2<pI<7.0, UA stain with any pH within 3.5 to 5.5 will change the initially anionic biological molecules to cationic, resulting in the detachment of positively charged inorganic nanomaterials from biological molecules. In this work, positively charged Au-NPs and negatively charged phage particles (pI=4.2) are stable in acidic solution, and they interact with each other through an electrostatic interaction which could be greatly interrupted by PW12O403− ions but not UO22+ ions.
With the above information, if pH < pI, the interactions between biological and inorganic components will be interrupted. If pH>pI+0.3, the pH will cause severe positive-staining effects to the sample which may darken the biological structures in certain areas. If pI<pH<pI+0.3, the biological sample may not have enough charges on their surfaces for electrostatic interactions and negative staining. Thus we can easily get the conclusion that UA solution with a pH=pI+0.3=4.5 is the optimal condition for staining Au-NP-Phage hybrids (Figure 3 a&b). A best pH value of 4.5 is reasonable because it could not only favor the electrostatic interaction and but also reduce the positive-staining problems.
Choice of Stain Concentration Based on Specific Bio-Inorganic Nanohybrids
The concentration of the stain is another important factor that may affect the appearance of the specimens. A high concentration of stain solution (>1%) is not recommended for two reasons. First, highly concentrated stain ions may accelerate the departure of inorganic nanomaterials from biological molecules. Second, heavy layers of stain may cover most inorganic nanomaterials so they will be unrecognizable under TEM (Figure 4 a). Therefore, a low concentration (0.5%) of the stain will produce a better staining for bio-inorganic nanohybrids.
Choice of Fixation Based on Specific Bio-Inorganic Nanohybrids
Pre-fixation has been used in negative staining in some cases. Its function is to covalently cross-link intra- and inter-molecules to prevent drastic alterations or damages in negative staining. However, the fixation should not form inter-particle cross-links, otherwise, artificial structures may appear. The most useful fixative in biological electron microscopy is glutaraldehyde (GA) which covalently cross-links nucleophilic -NH2 groups. For the bio-inorganic nanohybrids, fixation should not be performed if either biological molecules or inorganic nanomaterials are covered by -NH2 groups. Otherwise, inter-particle cross-linking will occur and the initial appearance of the hybrids may be changed. In our Au-NP-phage system, both phage particles and Au-NPs are covered by -NH2 groups, so GA is not suitable as a fixative for the negative staining. GA molecules may cross-link neighboring phage particles so that phage bundles can be observed after fixation even without Au-NPs. They may also cross-link NH2-covered Au-NPs with phage particles. It would be difficult for us to figure out whether the cross-linking between Au-NPs and phage particles were due to electrostatic interactions or fixations.
Why Rapid Drying is better
Slow air-drying is a popular and simple strategy for the drying of stains, but it also has two main limitations. First, since hydrated crystals tend to grow by slow evaporation, slow drying of the stain after negative staining can only encourage the formation of stain crystals instead of amorphous stain deposit which is required for the desired resolution. Second, in the process of drying, salt concentration will increase and the local pH will also decrease (e.g., for UA) or increase (e.g., for PTA). Such changes can influence the final appearance of the specimen. A slow drying will put the specimen into a harsh local environment for a longer time and has greater chances to influence the appearance of the specimen. Therefore, a rapid drying by a hair drier may improve the negative staining.
Conclusion
TEM characterization of negatively stained bio-inorganic nanohybrids is a popular and simple technology for viewing such nanostructures. We chose bacteriophage-gold nanoparticle hybrid as a model to systematically study the effects of each factor on the negative staining of the nanohybrids. The best staining conditions for this hybrid system include the use of 0.5% UA solution (pH=4.5), a staining time of 10 s, and a rapid drying of the stains. The experimental results suggested that each factor could influence the final appearance of the stained specimens and the best choice of each staining factor should be customized to the specific bio-inorganic nanohybrids. The effects of each factor on the negative staining of the nanohybrids were discussed and a general method about how to select each staining factor was summarized. This work provides useful information for the negative staining of bio-inorganic nanomaterials, which will greatly help research in the fields of bionanotechnology and nanomaterials.
Supplementary Material
Acknowledgment
We thank the National Science Foundation (DMR-0847758, CBET-0854414, CBET-0854465), National Institutes of Health (R21EB009909-01A1, R03AR056848-01, R01HL092526-01A2), Department of Defense Breast Cancer Research Program (W81XWH-07-1-0572), and Oklahoma Center for the Advancement of Science and Technology (HR06-161S) for the financial support.
References
- Belcher AM, Mao CB, Solis DJ, Reiss BD, Kottmann ST, Sweeney RY, Georgiou G, Iverson B. Virus-based genetic toolkit for the directed synthesis of magnetic and semiconducting nanowires. Abstracts of Papers of the American Chemical Society. 2004;228:U542–U542. doi: 10.1126/science.1092740. [DOI] [PubMed] [Google Scholar]
- Berkowitz SA, Day LA. Mass, Length, Composition and Structure of Filamentous Bacterial Virus Fd. Journal of Molecular Biology. 1976;102(3):531–547. doi: 10.1016/0022-2836(76)90332-6. [DOI] [PubMed] [Google Scholar]
- Cao B, Mao C. Oriented nucleation of hydroxylapatite crystals on spider dragline silks. Langmuir. 2007;23(21):10701–10705. doi: 10.1021/la7014435. [DOI] [PubMed] [Google Scholar]
- Chen ZF, Huang BX, Pan HB, Darvell BW. Solubility of Bovine-Derived Hydroxyapatite by Solid Titration, pH 3.5–5. Crystal Growth & Design. 2009;9(6):2816–2820. [Google Scholar]
- Daniel MC, Astruc D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chemical Reviews. 2004;104(1):293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- Hayat MA, Miller SE. Negative staining. New York: McGraw-Hill Pub. Co.; 1990. p. 253. x. [Google Scholar]
- Lin CX, Ke YG, Li Z, Wang JH, Liu Y, Yan H. Mirror Image DNA Nanostructures for Chiral Supramolecular Assemblies. Nano Letters. 2009;9(1):433–436. doi: 10.1021/nl803328v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu AH, Salabas EL, Schuth F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angewandte Chemie-International Edition. 2007;46(8):1222–1244. doi: 10.1002/anie.200602866. [DOI] [PubMed] [Google Scholar]
- Mao CB, Flynn CE, Hayhurst A, Sweeney R, Qi JF, Georgiou G, Iverson B, Belcher AM. Viral assembly of oriented quantum dot nanowires. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(12):6946–6951. doi: 10.1073/pnas.0832310100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao CB, Solis DJ, Reiss BD, Kottmann ST, Sweeney RY, Hayhurst A, Georgiou G, Iverson B, Belcher AM. Virus-based toolkit for the directed synthesis of magnetic and semiconducting nanowires. Science. 2004;303(5655):213–217. doi: 10.1126/science.1092740. [DOI] [PubMed] [Google Scholar]
- Monjezi R, Tey BT, Sieo CC, Tan WS. Purification of bacteriophage M13 by anion exchange chromatography. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2010;878(21):1855–1859. doi: 10.1016/j.jchromb.2010.05.028. [DOI] [PubMed] [Google Scholar]
- Note C, Kosmella S, Koetz J. Poly(ethyleneimine) as reducing and stabilizing agent for the formation of gold nanoparticles in w/o microemulsions. Colloids and Surfaces a-Physicochemical and Engineering Aspects. 2006;290(1–3):150–156. [Google Scholar]
- Pan HB, Darvell BW. Solubility of hydroxyapatite by solid titration at pH 3–4. Archives of Oral Biology. 2007;52(7):618–624. doi: 10.1016/j.archoralbio.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Sargent EH. Infrared quantum dots. Advanced Materials. 2005;17(5):515–522. [Google Scholar]
- Sharma J, Chhabra R, Andersen CS, Gothelf KV, Yan H, Liu Y. Toward reliable gold nanoparticle patterning on self-assembled DNA nanoscaffold. Journal of the American Chemical Society. 2008;130(25) doi: 10.1021/ja802853r. 7820-+ [DOI] [PubMed] [Google Scholar]
- Sharma J, Chhabra R, Cheng A, Brownell J, Liu Y, Yan H. Control of Self-Assembly of DNA Tubules Through Integration of Gold Nanoparticles. Science. 2009;323(5910):112–116. doi: 10.1126/science.1165831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GP, Petrenko VA. Phage display. Chemical Reviews. 1997;97(2):391–410. doi: 10.1021/cr960065d. [DOI] [PubMed] [Google Scholar]
- Tasis D, Tagmatarchis N, Bianco A, Prato M. Chemistry of carbon nanotubes. Chemical Reviews. 2006;106(3):1105–1136. doi: 10.1021/cr050569o. [DOI] [PubMed] [Google Scholar]
- Wang F, Li D, Newton SM, Klebba PE, Mao CB. Genetically Modifiable Flagella as Templates for Silica Fibers: From Hybrid Nanotubes to 1D Periodic Nanohole Arrays (vol 18, pg 4007, 2008) Advanced Functional Materials. 2009;19(21):3355–3355. [Google Scholar]
- Wang FK, Cao BR, Mao CB. Bacteriophage Bundles with Prealigned Ca2+ Initiate the Oriented Nucleation and Growth of Hydroxylapatite. Chemistry of Materials. 2010;22(12):3630–3636. doi: 10.1021/cm902727s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.