Abstract
Atrial fibrillation is a significant public health burden, with clinically, epidemiologically and economically significant repercussions. In the last decade, catheter ablation has provided an improvement in morbidity and quality of life, significantly reducing long-term healthcare costs and avoiding recurrences compared with drug therapy. Despite recent progress in techniques, current catheter ablation success rates fall short of expectations. Late gadolinium-enhancement cardiovascular MRI (LGE-MRI) is a well-established tool to image myocardium and most specifically the left atrium. Unique imaging protocols allow for left atrial structural remodeling and fibrosis assessment, which has been demonstrated to correlate with clinical outcomes after catheter ablation, assessment of the individual’s risks of thromboembolic events, and effective imaging of patients with left atrial appendage thrombus. LGE-MRI aids in the individualized treatment of atrial fibrillation, stratifying recurrence risk and guiding specific ablation strategies. Real-time MRI offers significant safety and effectiveness profiles that would optimize the invasive treatment of atrial fibrillation.
Keywords: ablation, atrial, fibrillation, fibrosis, MRI, outcomes, stroke
Atrial fibrillation (AF) is a disorder affecting 20 million people of all ages worldwide, with the incidence increasing with age. It is estimated that 15.9 million people in the USA alone will have the disease by 2050 [1]. AF is the leading cause of stroke, which is the third leading cause of death in the USA. The increased risk of stroke, heart failure, morbidity from anticoagulation therapy and complications of AF treatment increases the rate of hospitalizations. AF, as a principal diagnosis, comprises over 400,000 hospital admissions and an estimated US$6–7 billion in direct medical expenditure annually in the USA. This in turn makes AF an extreme public health burden – clinically, epidemiologically and economically.
In the last decade, catheter ablation has provided an improvement in morbidity and quality of life, and has significantly reduced long-term healthcare costs by decreasing hospital readmissions and avoiding repeat episodes of care compared with drug therapy [2]. Despite recent progress in techniques, current catheter ablation strategies fall short of expectations [3,4]. In patients with paroxysmal and persistent AF, recurrence rates after 1 year following discontinuation of antiarrhythmic treatment are 70–80% and 50%, respectively. Furthermore, the incidence of significant complications with catheter ablation is approximately 6%. One of these complications, left atrioesophageal fistula, carries with it a very high mortality rate and can be subtle and unrecognizable. With occurrences greater than 0.05%, new approaches to prevent left atrioesophageal fistula are needed [5]. In addition, extensive ablation strategies can be be proarrhythmic, cause collateral damage to surrounding structures, and decrease atrial transport function and coronary sinus patency. Furthermore, current unreliable methods to ascertain transmural lesion production are being replaced as technology advances.
This inadequate qualification of transmurality could be a contributing factor to recurrence rates. Another consideration should be the use of nontranscending standard lesion sets rather than the use of a tailored approach. A multidisciplinary approach is needed to drive the engine of translation of investigational research into highly patient-personalized treatment plans and procedures. Identification of individual factors that contribute to the development and progression of AF, the success of treatment, and the risk of stroke and death are within reach.
Late gadolinium-enhancement cardiovascular MRI (LGE-MRI) is a well-established tool to image myocardium. The behavior of left atrial (LA) tissue following AF ablation can easily be visualized with the current spatial and temporal resolution of cardiac MRI [6,7]. Moreover, new computational and mathematical tools now allow us to deal with data dimensionality that is utterly staggering. By developing a new scan sequence at our institution, postablation scarring is quantified based on the distribution of pixel intensities between normal and radio frequency (RF)-induced scarred LA wall tissue. [7]. The scan sequence takes advantage of gadolinium’s ability to shorten the T1 relaxation time in its surroundings, thus changing signal intensities [8].
LGE-MRI imaging protocols
In our protocol, scans are acquired approximately 15 min following gadolinium injection (0.1 mmol/kg; MultiHance®, Bracco Diagnostic Inc., NJ, USA). A contrast-enhanced 3D fast low-angle shot angiography sequence and a cine true-fast imaging with steady-state precession sequence are used to define the anatomy of the LA and the pulmonary veins. A 1.5-T Avanto or 3-T Trio clinical scanner (Siemens Medical Solutions, Erlangen, Germany) with a total imaging matrix phased-array receiver coil are used. The sequence for both scanners is a 3D IR prepared fast spoiled gradient recalled sequence that is respiratory triggered and navigated, ECG-gated, gradient-echo and fat suppressed. ECG gating is used to acquire a small subset of phase encoding views during the diastolic phase of the LA cardiac cycle. The time interval between the R- peak of the ECG and the start of data acquisition is defined using the cine images of the LA. A transverse imaging volume with voxel size of 1.25 × 1.25 × 2.5 mm (reconstructed to 0.625 × 0.625 × 1.25 mm) and free breathing using navigator gating is used for both types of scanners.
Flip angles of 20° and 13° are used for the 1.5- and 3-T scanners, respectively. The inversion time (TI) is identified with a scout scan and is between 270 and 310 ms for a 1.5-T scanner and usually from 280 to 330 ms with a 3-T scanner. Other acquisition parameters include the repetition time (TR), which is the time between successive RF pulses, and the echo time (TE), which is the time between RF pulse and measurement; both determine the strength of the signal. TE is chosen such that fat and water are out of phase and the signal intensity of partial volume fat-tissue voxels is reduced, allowing improved delineation of the LA wall boundary (Figure 1A). Although it can vary, TR/TE are 5.4/2.3 and 3.3/1.4 for a 1.5- and 3-T scanner, respectively. In addition, Generalized Auto-Calibrating Partially Parallel Acquisitions (GRAPPA), a parallel imaging technique, relies on undersampling and reconstructs the Fourier plane of the image from the frequency signals of each coil to speed up the MRI pulse sequence (Figure 1B). Typical scan times for 1.5- and 3-T delayed-enhancement MRI studies are expected to be between 5–10 and 4–7 min, respectively, depending on subject respiration and heart rate.
Figure 1.
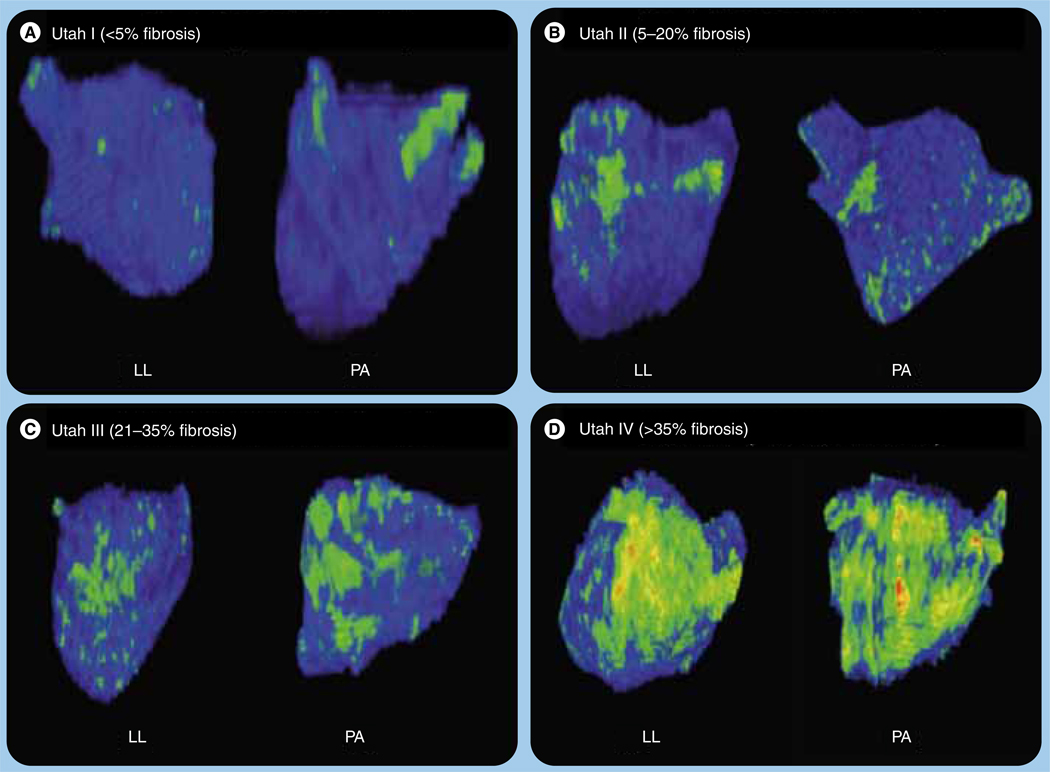
Left lateral and posteroanterior views of left atrium using late gadolinium-enhancement MRI demonstrating the different structural remodeling stages based on the amount of enhancement preablation (green/yellow).
LL: Left lateral; PA: Posteroanterior.
Patient contraindications for this specific imaging protocol include cardiac rhythm devices, renal dysfunction or a glomerular filtration rate less than 60 ml/min, severe claustrophobia, and other standard contraindications for MRI. Respiratory gating using navigator echoes does not completely suppress motion artifacts, and these as well other image-quality disturbances due to noise may lead to inappropriate interpretation of fibrosis. To reduce these negative effects, the navigator is positioned on the right hemi-diaphragm and data acquisition occurs during the end of the expiration phase. In addition, to resolve the effect of the LA motion, data are acquired during the diastolic phase of the LA and the time interval with minimal LA motion (identified with cine images). It is then further restricted to approximately 120 ms per heartbeat. As expected, MRIs performed with a 1.5-T scanner have decreased spatial resolution and a lower signal-to-noise ratio than with a higher magnetic field, leading to inferior LA wall images.
Custom software (OsiriX 2.7.5) allows 3D visualization and segmentation of the MRI images. Segments of the LA and pulmonary tree are constructed and verified manually with original image stacks before rendering. Initial visualization is done using a maximum intensity projection (MIP) to assess contrast consistency followed by raycast volume rendering with an opacity-weighted linear table. A color look-up table (CLUT) mask is applied to the rendered images to optimize differentiation between enhanced and nonenhanced tissue. Healthy tissue is then depicted as blue, whereas any tissue with delayed enhancement is depicted as green/yellow. Analysis software written in MATLAB (The Mathworks Inc, MA, USA) allows manual contouring of epicardial and endocardial borders. Extent of fibrosis within the LA is qualified with a threshold-based algorithm. For this reason, the use of specific imaging protocols is limited to patients with a creatinine clearance of more than 60 ml/min.
In our experience, the majority of patients complete the protocol and are successfully imaged. Approximately 10% have an artefact sufficient to prohibit the visualization of the left atrium and any fibrosis. The most important predictor of failure to image the left atrium is poor rate control in patients presenting with AF during the scan. The specific LA fibrosis sequence adds between 10 and 15 min to the study without any specific additional costs.
One of the significant limitations of this technique is the use of full-dose gadolinium. Even though it is rare, patients with documented renal failure receiving full-dose gadolinium are at risk for developing nephrogenic systemic fibrosis, a serious condition with no specific treatment available [9,11].
Clinical applicability of LGE-MRI preablation
Stroke risk assessment
Late gadolinium-enhancement-MRI has provided insights into how to approach planning of strategies for preventative and more individualized care. We know that AF results in LA structural remodeling due to deposition of fibrous tissue that has a characteristic high impedance measurement. [12–14]. We also know that LA structural remodeling increases the risk of thromboembolism in AF patients, making it a potential parameter when assessing a patient’s risk of stroke [15]. Remodeling can now be easily qualified and quantified with the help of LGE-MRI. Furthermore, we have previously shown that LA enhancement using LGE-MRI is more indicative of LA structural remodeling than LA volume and is a better surrogate marker of remodeling [16]. Therefore, it is plausible that future stroke risk stratification schemes will be more rigorous, based, in part, on individual LA pathophysiological properties. Current schemes have proved to be poor predictors of stroke. [17] More tailored anticoagulation therapy would unquestionably spare low-risk AF patients from the cost, inconvenience and risk of warfarin therapy [17,18]. In addition, more demanding risk assessment will be useful for those with a moderate risk of stroke, a substantial portion of the AF population, because physicians rely heavily on clinical judgment when managing these individuals [17].
We have taken advantage of LGE-MRI to further study the relationship between LA structural remodeling and thromboembolism. In one study, we assigned participants to one of four groups based on their LGE-MRI LA structural remodeling distribution quartiles (Q), expressed as percentage of LA wall enhancement [16]. In doing so, we found that participants at high risk (CHADS2 ≥2) had a significantly larger amount of LA structural remodeling compared with those with a moderate and low risk. Those with mild remodeling (Q1 <8.5% enhancement) experienced low rates of thromboembolism (2.8%), while over half of those with severe enhancement (Q4 >21.1%) had experienced an ischemic event. We also found that those who had a prior stroke had a significantly higher percentage of remodeling compared with those with no history (24.4 ± 12.4 vs 16.1 ± 9.8%; p < 0.001).
Structural remodeling staging system
In addition to releasing collagen-1 and fibronectin-1 inducing factors, evidence exists that atrial myocytes during sustained AF show features resembling myocytes during heart development, which is interpreted as phenotypic changes characteristic of less differentiated cells [19]. It is also suggestive that causality between fibrillation and fibrosis is bidirectional. Various studies have illustrated that extensive structural remodeling correlates to recurrence and worse phenotypic AF. This validates the phenotypic link between fibrosis and disease severity in AF. In addition, those with more pronounced structural remodeling tend to have persistent rather than paroxysmal AF. In a previous study, it was found that the greatest degree of variance for ablation outcome and response to medical therapy was explained by the degree of fibrotic enhancement in the LA wall [16]. The association of enhancement with stroke risk and phenotype of AF offers a means for earlier intervention.
We have developed a specific staging system based on the degree of structural remodeling. The Utah stage system assigns patients to four stages based on the preprocedure LGE-MRI enhancement: Utah I: less than 5%; Utah II: 5–20%; Utah III: 21–35%; and Utah IV: 35% or more (Figure 1) [20]. We found a significant association between the different stages and the clinical outcomes at 1 year (Figure 2). Moreover, this staging system has allowed ablation technologies and strategies to be individualized and better distributed. In those with mild remodeling stages (Utah I and II), we have found that producing lasso-guided antral isolation of all four veins is sufficient to maximize clinical outcomes. On the other hand, on those with more advanced stages of structural remodeling (Utah II and IV), it is imperative further substrate modification is targeted, including posterior wall and septal LA region debulking. Given the extremely poor response rates and higher risk for stroke, one could argue that for patients with severe enhancement (Utah IV >35%) catheter ablation is probably an unnecessary risk to undertake and perhaps a more conservative and palliative therapy for the AF should be pursued.
Figure 2.
Freedom from atrial fibrillation Kaplan-Meier survival curves following catheter ablation according to the structural remodeling stating system.
p denotes the significance level from Log Rank test.
LA Doppler & thrombus evaluation
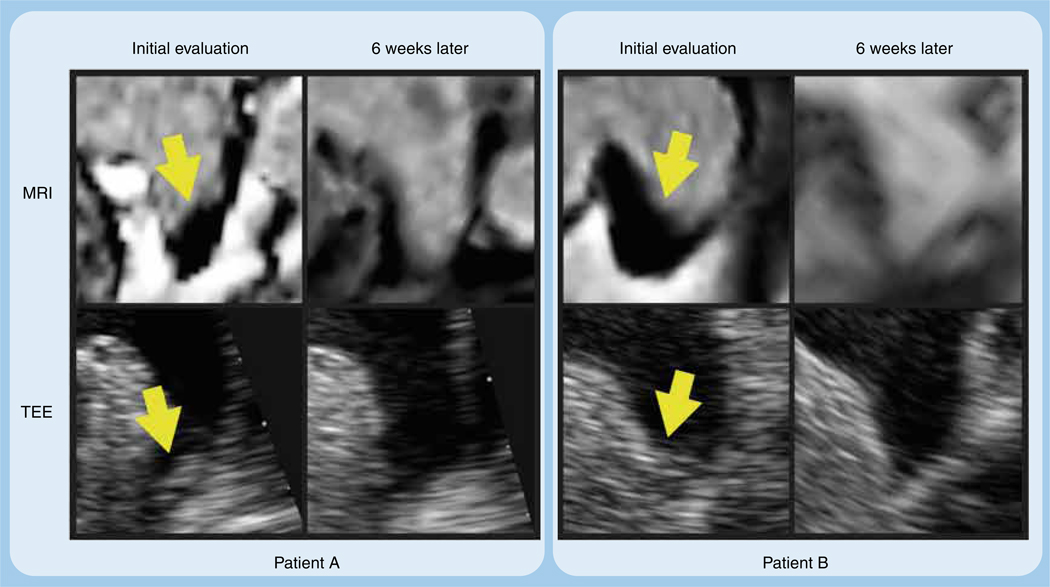
Transesophageal echocardiogram (TEE) is the accepted standard for evaluation of LA appendage thrombus with high diagnostic accuracy. However, TEE is invasive and, even though the risks are low, can result in complications due to conscious sedation, bleeding and aspiration. Recently, MRI has emerged as a powerful tool to detect thrombus and is significantly less invasive. When imaging for thrombus, MRI has advantages over TEE in the evaluation of soft tissue with multiple imaging sequences that can be employed to help characterize and distinguish between tissue types. Visualization of appendage thrombus has been demonstrated on MRI using bright blood, dark blood and perfusion imaging techniques, with detection enhanced when a combination of these imaging sequences are used [21,22]. In addition, delayed contrast-enhanced T1-weighted (T1w) IR can be used to visualize intracardiac thrombi and has been well validated [23,24]. IR imaging utilizes differences in T1 relaxation to create tissue contrast by nulling or enhancing signal. When optimizing the IR sequence and increasing the inversion time, thrombus will appear dark and is differentiated from other tissue (Figure 3). In patients with AF, we have applied T1w IR MRI to detect LAA thrombus and demonstrated its utility in this patient population [22]. Specifically, we have shown LAA thrombus on IR MRI confirmed on TEE with resolution over time after warfarin treatment (Figure 4).
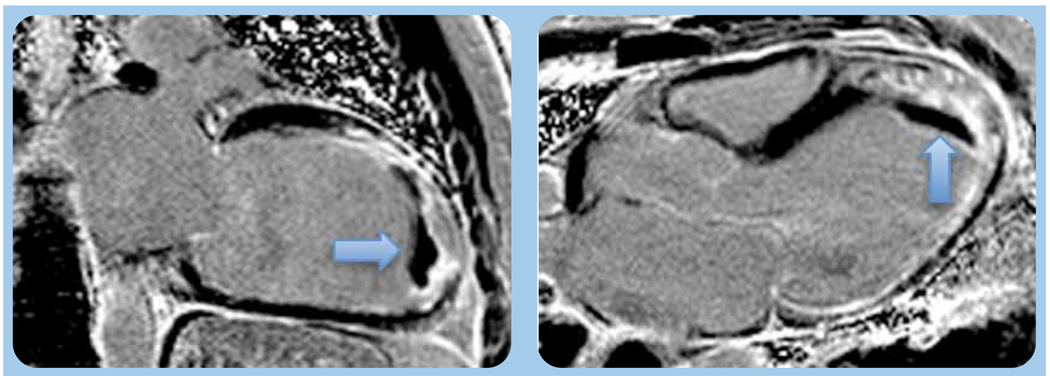
Figure 3.
Left ventricular apical mural thrombus (arrow) appears distinctly dark on contrast-enhanced T1-weighted inversion recovery magnetic resonance image.
Figure 4.
Left atrial appendage thrombus as visualized by inversion recovery MRI with an inversion time of 600 ms and by transesophageal echocardiogram in two patients (patient A and patient B) on initial evaluation and then again after 6 weeks of warfarin with international normalized ratios between 2 and 3.
TI: Inversion time.
Furthermore, LGE-MRI overcomes the limitations seen with current noninvasive imaging techniques in evaluating the intimate relationship between structure and function of the LA following ablation. In a recent study, segmentation of LA and degree of enhancement was determined using a semi-automated quantification LGE-MRI algorithm and compared with 2D echocardiography. Longitudinal LA strain and strain rate during ventricular systole with velocity vector imaging was found to be inversely related to LA wall fibrosis by LGE-MRI, which is related to the AF burden. These results indicate that echocardiographic assessment of the structure and function of the LA is feasible and helpful in predicting AF outcomes [25].
Expert commentary
Over recent years, catheter ablation of AF has evolve significantly. With the advent of newer technologies, the procedure has become safer and more effective. Unfortunately ablation failures remain a common phenomena. Newer treatment strategies are concentrating on a more individualized treatment plan for patients undergoing AF ablation. Cardiac MRI successfully allows triage of patients preablation, improving our understanding of the individual LA substrate and optimize ablation therapies, achieving better clinical outcomes. The use of cardiac LGE-MRI also provides a comprehensive examination of the patients LA structural remodeling stage, potentially predicting future embolic events and leading to a better allocation of anticoagulation therapy.
Five-year view
Current image integration systems require importation of a MRI image obtained prior to the procedure, thus providing far greater anatomic information and tissue resolution than fluoroscopy, as well as the the ability to assess scar formation and structural remodeling. However, this system cannot aid the user during the procedure when RF energy is being applied. Real-time MRI (RT-MRI)-navigated catheter ablation shows promise in terms of immediate feedback of lesion formation during RF energy delivery. Furthermore, with RT-MRI, we can monitor the esophagus and pericardial space in real time, reducing complications during ablation procedures. We also gain superior resolution of soft tissue, while sparing both the patient and the operator from the harmful effects of ionizing radiation.
Recent reports of MRI-based catheter tracking and ablation within the atrial chambers using 1.5-T scanners have shown promise, but have also demonstrated their shortcomings. In one study, the authors performed real-time catheter navigation without cardiac imaging during catheter manipulation [26]. In another study, RT-MRI navigation of the catheter was successful, but there wasn’t direct tissue visualization during RF energy delivery and lesion formation [27]. Recently, with real-time 3T MRI (RT-MRI) navigation, our team has been able to guide intracardiac catheter positioning, deliver RF energy and visualize effects on myocardium, assess lesion formation, and visualize heart motion and changes in volume [28]. In addition, we have characterized the early stages of RF lesion formation, and have correlated ablation time to lesion size [28]. Challenges to RT-MRI navigated catheter ablation are still being faced. Automatic detection of catheter tip position with accurate slice alignment is a start to overcoming some of these obstacles.
Key issues.
Atrial fibrillation (AF) is a significant public heath issue.
Catheter ablation has been proven to be an effective rhythm control tool, improving quality of life and reducing costs.
Despite improved catheter and navigation technologies, AF recurrences after catheter ablation remain problematic.
Late gadolinium-enhancement cardiovascular (LGE)-MRI allows for specific structural and metabolic imaging of the left atrium prior to ablation.
Structural remodeling and fibrosis detected by LGE-MRI allows effective triaging of the AF patient, leading to improved clinical outcomes.
Left atrial fibrosis detected by MRI is a potential independent risk factor for stroke, optimizing allocation of effective anticoagulation strategies in high-risk individuals.
Acknowledgements
This work was made possible in part by software from the NIH/NCRR Center for Integrative Biomedical Computing, P41-RR12553-10.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1. Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4(6):816–861. doi: 10.1016/j.hrthm.2007.04.005. • Describes the current recommendations and standard of care for atrial fibrillation ablation patients.
- 2.Reynolds MR, Zimetbaum P, Josephson ME, Ellis E, Danilov T, Cohen DJ. Cost-effectiveness of radiofrequency catheter ablation compared with antiarrhythmic drug therapy for paroxysmal atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2009;2(4):362–369. doi: 10.1161/CIRCEP.108.837294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oral H, Knight BP, Ozaydin M, et al. Segmental ostial ablation to isolate the pulmonary veins during atrial fibrillation: feasibility and mechanistic insights. Circulation. 2002;106(10):1256–1262. doi: 10.1161/01.cir.0000027821.55835.00. [DOI] [PubMed] [Google Scholar]
- 4.Kanagaratnam L, Tomassoni G, Schweikert R, et al. Empirical pulmonary vein isolation in patients with chronic atrial fibrillation using a three-dimensional nonfluoroscopic mapping system: long-term follow-up. Pacing Clin. Electrophysiol. 2001;24(12):1774–1779. doi: 10.1046/j.1460-9592.2001.01774.x. [DOI] [PubMed] [Google Scholar]
- 5.Khandhar S, Nitzschke S, Ad N. Left atrioesophageal fistula following catheter ablation for atrial fibrillation: off-bypass, primary repair using an extrapericardial approach. J. Thorac. Cardiovasc. Surg. 2010;139(2):507–509. doi: 10.1016/j.jtcvs.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 6.Peters DC, Wylie JV, Hauser TH, et al. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology. 2007;243(3):690–695. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 7. McGann CJ, Kholmovski EG, Oakes RS, et al. New magnetic resonance imaging-based method for defining the extent of left atrial wall injury after the ablation of atrial fibrillation. J. Am. Coll. Cardiol. 2008;52(15):1263–1271. doi: 10.1016/j.jacc.2008.05.062. •• Describes the specific methodology used to evaluate postablation patients using Late gadolinium-enhancement cardiovascular (LGE)-MRI.
- 8. Saeed M, Wendland MF, Watzinger N, Akbari H, Higgins CB. MR contrast media for myocardial viability, microvascular integrity and perfusion. Eur. J. Radiol. 2000;34(3):179–195. doi: 10.1016/s0720-048x(00)00198-4. • Reviews the basic principles of gadolinium-based contrast imaging in cardiology.
- 9. Cheong BY, Muthupillai R. Nephrogenic systemic fibrosis: a concise review for cardiologists. Tex. Heart Inst. J. 2010;37(5):508–515. • Reviews nephrogenic systemic fibrosis and cardiac MRI and possible prevention strategies.
- 10.Martin DR, Semelka RC, Chapman A, et al. Nephrogenic systemic fibrosis versus contrast-induced nephropathy: risks and benefits of contrast-enhanced MR and CT in renally impaired patients. J. Magn. Reson. Imaging. 2009;30(6):1350–1356. doi: 10.1002/jmri.21968. [DOI] [PubMed] [Google Scholar]
- 11.Kim KH, Fonda JR, Lawler EV, Gagnon D, Kaufman JS. Change in use of gadolinium-enhanced magnetic resonance studies in kidney disease patients after US Food and Drug Administration warnings: a cross-sectional study of Veterans Affairs Health Care System data from 2005–2008. Am. J. Kidney Dis. 2010;56(3):458–467. doi: 10.1053/j.ajkd.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Anne W, Willems R, Roskams T, et al. Matrix metalloproteinases and atrial remodeling in patients with mitral valve disease and atrial fibrillation. Cardiovasc. Res. 2005;67(4):655–666. doi: 10.1016/j.cardiores.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Spach MS, Miller WT, 3rd, Dolber PC, Kootsey JM, Sommer JR, Mosher CE., Jr The functional role of structural complexities in the propagation of depolarization in the atrium of the dog. Cardiac conduction disturbances due to discontinuities of effective axial resistivity. Circ. Res. 1982;50(2):175–191. doi: 10.1161/01.res.50.2.175. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Zlochiver S, Vikstrom KL, et al. Spatial distribution of fibrosis governs fibrillation wave dynamics in the posterior left atrium during heart failure. Circ. Res. 2007;101(8):839–847. doi: 10.1161/CIRCRESAHA.107.153858. [DOI] [PubMed] [Google Scholar]
- 15.Abhayaratna WP, Seward JB, Appleton CP, et al. Left atrial size: physiologic determinants and clinical applications. J. Am. Coll. Cardiol. 2006;47(12):2357–2363. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 16. Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119(13):1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. •• Describes the specific method to determine structural remodeling of the left atrium preablation using LGE cardiac MRI.
- 17.Fang MC, Go AS, Chang Y, Borowsky L, Pomernacki NK, Singer DE. Comparison of risk stratification schemes to predict thromboembolism in people with nonvalvular atrial fibrillation. J. Am. Coll. Cardiol. 2008;51(8):810–815. doi: 10.1016/j.jacc.2007.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gage BF, van Walraven C, Pearce L, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110(16):2287–2292. doi: 10.1161/01.CIR.0000145172.55640.93. [DOI] [PubMed] [Google Scholar]
- 19.Manasek FJ. Mitosis in developing cardiac muscle. J. Cell. Biol. 1968;37(1):191–196. doi: 10.1083/jcb.37.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akoum N, Daccarett M, McGann C, et al. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: a DE-MRI guided approach. J. Cardiovasc. Electrophysiol. 2010 doi: 10.1111/j.1540-8167.2010.01876.x. (Epub ahead of print) •• Describes the clinical applicability of left atrial fibrosis detected by LGE cardiac MRI in patients undergoing atrial fibrillation ablation.
- 21.Ohyama H, Hosomi N, Takahashi T, et al. Comparison of magnetic resonance imaging and transesophageal echocardiography in detection of thrombus in the left atrial appendage. Stroke. 2003;34(10):2436–2439. doi: 10.1161/01.STR.0000090350.73614.0F. [DOI] [PubMed] [Google Scholar]
- 22.Mohrs OK, Nowak B, Petersen SE, et al. Thrombus detection in the left atrial appendage using contrast-enhanced MRI: a pilot study. AJR Am. J. Roentgenol. 2006;186(1):198–205. doi: 10.2214/AJR.04.1504. [DOI] [PubMed] [Google Scholar]
- 23.Mollet NR, Dymarkowski S, Volders W et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation. 2002;106(23):2873–2876. doi: 10.1161/01.cir.0000044389.51236.91. [DOI] [PubMed] [Google Scholar]
- 24.Barkhausen J, Hunold P, Eggebrecht H, et al. Detection and characterization of intracardiac thrombi on MR imaging. AJR Am. J. Roentgenol. 2002;179(6):1539–1544. doi: 10.2214/ajr.179.6.1791539. [DOI] [PubMed] [Google Scholar]
- 25.Kuppahally SS, Akoum N, Burgon NS, et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ. Cardiovasc. Imaging. 2010;3(3):231–239. doi: 10.1161/CIRCIMAGING.109.865683. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt EJ, Mallozzi RP, Thiagalingam A, et al. Electroanatomic mapping and radiofrequency ablation of porcine left atria and atrioventricular nodes using magnetic resonance catheter tracking. Circ. Arrhythm. Electrophysiol. 2009;2(6):695–704. doi: 10.1161/CIRCEP.109.882472. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann BA, Koops A, Rostock T, et al. Interactive real-time mapping and catheter ablation of the cavotricuspid isthmus guided by magnetic resonance imaging in a porcine model. Eur. Heart J. 2010;31(4):450–456. doi: 10.1093/eurheartj/ehp460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vergara GR, Vijayakumar S, Kholmovski EG, et al. Real time MRI guided radiofrequency atrial ablation and visualization of lesion formation at 3-Tesla. Heart Rhythm. 2010 doi: 10.1016/j.hrthm.2010.10.032. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]