Abstract
Multiple extracellular factors have been shown to modulate adult hippocampal neural progenitor cell (NPC) proliferation and self-renewal, and we have previously shown that Akt is an important mediator of the effects of these extracellular factors on NPC proliferation and differentiation. However, very little work has investigated how and whether Akt is involved in maintaining the multipotency of these cells. Here we demonstrate that Akt promotes expression of Sox2, a core transcription factor important for the self-renewal of NPCs. Retroviral-mediated overexpression of wild-type Akt increased Sox2 protein expression, particularly under conditions that promote cell differentiation, whereas Akt inhibition decreased Sox2. Similarly, quantitative reverse transcription (RT)–PCR in differentiating cultures indicated that Akt rescued Sox2 mRNA to levels present under conditions that promote cell proliferation. Additionally, pharmacological inhibition of Akt did not affect Sox2 protein levels in cells constitutively expressing Sox2 from a retroviral vector, indicating that Akt does not affect Sox2 protein stability. Further, in contrast to Akt overexpression, Sox2 overexpression does not increase NPC viable cell number or proliferation yet does inhibit differentiation. Collectively, these results indicate that Akt promotes cell proliferation and maintenance of a multipotent state via two downstream paths.
Introduction
Neural progenitor cells (NPCs) from the adult hippocampus have the potential to maintain their population, a process called self-renewal, as well as to undergo lineage commitment and differentiation into the three major cell types of the mammalian brain: neurons, astrocytes, and oligodendrocytes [1]. The regulation of these processes is central to adult neurogenesis [2,3], which in turn may be important for learning, memory, and mood regulation [4–10]. A number of extracellular factors have been shown to modulate NPC proliferation and self-renewal, including basic fibroblast growth factor (FGF-2) [11], epidermal growth factor [12], and Sonic hedgehog [13]. However, the intracellular signaling cascades that functionally mediate these extracellular signals in NPCs have only recently been explored. The importance of Akt has been demonstrated in many stem cell types, including mouse embryonic stem (ES) cells [14,15], primate ES cells [16], rabbit ES cells [17], mesenchymal stem cells [18], and hematopoietic stem cells [19,20]. Additionally, we have previously reported that Akt is important for NPC proliferation and inhibition of differentiation [21]. Others have shown the importance of signaling events that lie downstream of Akt to NPC maintenance, including mTOR [22], FoxO [23], and GSK-3 [24]. However, very little work has investigated whether and how this signaling pathway may be involved in maintaining the multipotency of these cells.
The SRY-related HMG-box 2 (Sox2) transcription factor is important in the self-renewal of ES cells [25] and is a critical factor that can contribute to reprogramming and generation of induced pluripotent stem (iPS) cells [26]. Within the central nervous system, lineage tracing studies have shown that Sox2-positive cells in the hippocampus can self-renew and generate differentiated progeny [27]. It is also involved in NPC maintenance [28] and has increasingly been utilized as an NPC marker [29]. Further, in chick embryos, Sox2 overexpression prevents differentiation [30], and it is also known to repress the transcription of glial fibrillary acidic protein (GFAP), an important astrocytic marker [31], consistent with its promotion of an immature cell state.
Control of Sox2 expression and activity in NPCs is not well understood, though some Sox2 control mechanisms have been studied in other cell types. Post-translational modifications, including sumoylation [32], phosphorylation [33], and poly(ADP-ribosyl)ation [34], as well as heterodimer formation with Oct4 [35], can regulate Sox2 activity. Transcriptional control of Sox2 is promoted by the Sox2 regulatory region 2, a Sox2 enhancer known to regulate its expression in the telencephalon [36]. Despite these advances in our understanding of Sox2 regulation, little is known about the signaling pathways that drive Sox2 expression in general and particularly in NPCs. The importance of both Akt and Sox2 to pluripotency [14–17] and multipotency [18–20] raises the question whether Sox2 expression is linked to Akt activity.
Here we extend our previous work with Akt [21] to demonstrate that it both enhances cell proliferation via a Sox2-independent mechanism, as well as promotes the expression of the transcription factor Sox2 to support multipotency. Retroviral-mediated overexpression reveals that Akt activity promotes increased Sox2 expression. We also find that increased Sox2 protein levels, while inhibiting differentiation, do not increase proliferation. These results indicate that Akt serves as an important master regulator in NPC maintenance by independently promoting downstream cell proliferation and Sox2-dependent self-renewal.
Materials and Methods
Cell culture
Adult NPCs isolated from the hippocampi of 6-week-old female Fischer 344 rats as described [37] were cultured on tissue culture polystyrene coated with poly-ornithine and 5 μg/mL of laminin (Invitrogen). Cells were grown in Dulbecco's modified Eagle's medium (DMEM)/F-12 (1:1) high-glucose medium (Invitrogen) containing N-2 supplement (Invitrogen) and 20 ng/mL recombinant human FGF-2 (Peprotech).
Mutant cell lines
Progenitor cells constitutively expressing wild-type Akt or Sox2 were generated by retroviral infection. Wild-type murine Akt1 cDNA (Akt) was a kind gift from S. Ferguson (Robarts Research Institute, London, ON, Canada), and wild-type murine Sox2 cDNA was obtained from Stemgent. Neither cDNA included untranslated regions. Both cDNAs were subcloned into the MLV retroviral vector CLGPIT, a variant of CLPIT [38] in which the puromycin resistance gene puromycin N-acetyl transferase is replaced with a gene encoding a GFP-puromycin N-acetyl transferase fusion [39] (a kind gift from M. McVoy, Governor's School for Government and International Studies, Richmond, VA). An empty control vector was also produced, and correct products were confirmed by sequencing. Retroviral vectors were packaged using CMV gag-pol and CMV VSV-G envelope helper plasmids by calcium phosphate transfection as described [38]. Vectors were harvested, concentrated by ultracentrifugation, and titered on HEK 293Ts. Progenitor cells were infected at a multiplicity of infection of 1 IU/cell and subsequently selected with 0.6 μg/mL puromycin (Sigma) for 4 days.
WST-1 assay
NPCs overexpressing wild-type Sox2 or an empty vector control were plated at 1,000 cells/well on 96-well poly-ornithine/laminin-coated tissue culture plates with either 0 or 1 ng/mL FGF-2 in DMEM/F-12 + N-2 medium. Each condition was cultured in biological quintuplicate, and 50% medium changes were conducted daily. After 5 days in culture, cell number was quantified using the WST-1 assay following the manufacturer's instructions (Roche Applied Science) and utilizing a standard curve generated with known cell numbers, according to hemocytometer.
Bromodeoxyuridine proliferation assay
NPCs transduced with retrovirus to overexpress wild-type Sox2 or the corresponding empty vector control were seeded in triplicate at 15,000 cells/well onto Falcon 8-well chamber slides coated with poly-ornithine and 10 μg/mL laminin. Cells were cultured overnight in either 0 or 1 ng/mL FGF-2, pulsed with 10 μM bromodeoxyuridine (BrdU) for 12 or 24 h, and then fixed with 4% paraformaldehyde for 10 min. Cells were rinsed with phosphate-buffered saline (PBS) 3 times and then incubated in 2 N HCl at 37°C for 30 min to expose the BrdU antigen. The HCl was removed, and residual HCl was neutralized with 0.1 M borate buffer followed by 3 PBS washes. Cells were then blocked for 1 h in PBS containing 5% goat serum + 0.3% Triton X-100 followed by staining with rat anti-BrdU (Abcam, ab6326, 1:250) in blocking buffer overnight at 4°C. After 3 PBS washes, slides were stained with Cy3-conjugated, goat anti-rat secondary antibody (Jackson ImmunoResearch, 1:250) for 2 h in blocking buffer. Slides were washed 3 more times, and the final wash contained 5 μg/mL of the nuclear stain 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen, D21490). Slides were mounted with Cytoseal 60 (Fisher Scientific), and the percentage of cells staining positive for BrdU was quantified manually.
Quantitative reverse transcription–PCR
NPCs were seeded at 200,000 cells/well in 6-well poly-ornithine/laminin-coated culture plates in DMEM/F-12 + N-2 medium containing either 1 ng/mL FGF-2 to sustain multipotency or 1% fetal bovine serum (FBS) plus 1 μM retinoic acid (RA) (Biomol) to induce differentiation. Eight replicates were performed for each condition. The medium was replenished daily, and on day 5 RNA was isolated by TRIzol (Invitrogen) according to manufacturer's instructions. cDNA was then generated using Invitrogen's Thermoscript RT-PCR kit according to manufacturer's instructions. Using a BioRad iCycler, TaqMan probe QPCR was performed for the astrocytic marker GFAP, the neuronal marker β-tubulin III, or Sox2 with the 18S ribosomal subunit as an internal control. Quantitative RT-PCR for GFAP and β-tubulin III has been shown to be a reliable measure of NPC differentiation [40]. GFAP, β-tubulin III, and Sox2 probes from Biosearch Technologies contained the FAM490 fluorophore with Black Hole Quencher, whereas the 18S rRNA probe contained the CAL610 fluorophore with Black Hole Quencher. Table 1 lists primer and probe sequences.
Table 1.
Primer and Probe Sequences for Quantitative RT-PCR
| Sequence | |
|---|---|
| β-Tubulin III | |
| Forward primer | 5′-GCATGGATGAGATGGAGTTCACC-3′ |
| Reverse primer | 5′-CGACTCCTCGTCGTCATCTTCATAC-3′ |
| Probe | FAM490-TGAACGACCTGGTGTCTGAG-BHQ |
| GFAP | |
| Forward primer | 5′-GACCTGCGACCTTGAGTCCT-3′ |
| Reverse primer | 5′-TCTCCTCCTTGAGGCTTTGG-3′ |
| Probe | FAM490-TCCTTGGAGAGGCAAATGCGC-BHQ |
| Sox2 | |
| Forward primer | 5′-CGAGTGGAAACTTTTGTCGGAGAC-3′ |
| Reverse primer | 5′-CGGGAAGCGTGTACTTATCCTTCTT-3′ |
| Probe | FAM490-CTCTGCACATGAAGGAGCACC-BHQ |
| 18S | |
| Forward primer | 5′-GTAACCCGTTGAACCCCATTC-3′ |
| Reverse primer | 5′-CCATCCAATCGGTAGTAGCGA-3′ |
| Probe | CAL610-AAGTGCGGGTCATAAGCTTGCG-BHQ |
BHQ, Black Hole Quencher; GFAP, glial fibrillary acidic protein.
Western blotting
NPCs were cultured in 6-well poly-ornithine/laminin-coated plates for 5 days, unless otherwise indicated. Cells were lysed by adding lysis solution directly to the culture plate. Lysis solution contained IGEPAL (1%; Sigma), sodium dodecyl sulfate (SDS) (0.1%), phenylmethanesulfonylfluoride (0.1 mg/mL; Sigma), aprotinin (0.03 mg/mL; Sigma), and sodium orthovanadate (1 mM; Sigma) in PBS. Lysate protein concentrations were quantified by BCA Protein Assay Kit (Pierce) according to manufacturer's instructions. Equal amounts of protein from each lysate were electrophoretically separated by SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad Laboratories). In cases where blots were stripped and re-probed, phosphorylated epitopes were probed first, as previously described [41]. Primary antibodies included rabbit anti-Sox2 (Abcam, ab15830, 1:2000), rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Abcam, ab9485, 1:2000), rabbit anti-phosphoT308 Akt (Cell Signaling, 4056, 1:1,000), rabbit anti-phosphoS473 Akt (Cell Signaling, 9271, 1:1,000), and rabbit anti-total Akt (Cell Signaling, 9272, 1:2,000). Horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Pierce, 31460, 1:10,000) was used to develop the blots.
Immunofluorescence
NPCs were seeded onto Falcon 8-well chamber slides coated with poly-ornithine and 10 μg/mL laminin. Cultures grown in 1 ng/mL FGF-2 were seeded with 20,000 cells/well, whereas cultures grown with 1% FBS + 1 μM RA or with 1 μM API-2/triciribine (Akt inhibitor, dissolved in DMSO; EMD Biosciences) were seeded with 40,000 cells/well. Cells were grown for 5 days with 50% media replenishment daily then fixed for 10 min with 4% paraformaldehyde. After 4 PBS washes, cells were blocked/permeabilized for 2 h with blocking buffer containing 0.3% Triton X-100 + 5% donkey serum in PBS. Cells were then stained with goat anti-Sox2 (Santa Cruz, sc-17320, 1:200) or IgG isotype control in blocking buffer for ∼72 h at 4°C. After 4 PBS washes, slides were stained with Cy3-conjugated donkey anti-goat secondary antibody (Jackson ImmunoResearch, 705-165-147, 1:250) for 2 h in blocking buffer. Slides were washed 4 more times, and the final wash contained 5 μg/mL of the nuclear stain DAPI (Invitrogen, D21490). Slides were mounted with Cytoseal 60 (Fisher Scientific). Four random fields from each well were used for quantification. Using the freely available image analysis software CellProfiler [42], primary objects/nuclei were identified using the DAPI stain, and the pixel intensity of Sox2 staining within each nucleus was then measured. In all cases, Sox2 staining remained localized to the nucleus. The average intensity of the control condition was arbitrarily set to unity, and all other experimental conditions were normalized accordingly. At least 400 cells were quantified for each experimental condition.
Results
Sox2 expression and Akt activation increase under proliferating conditions
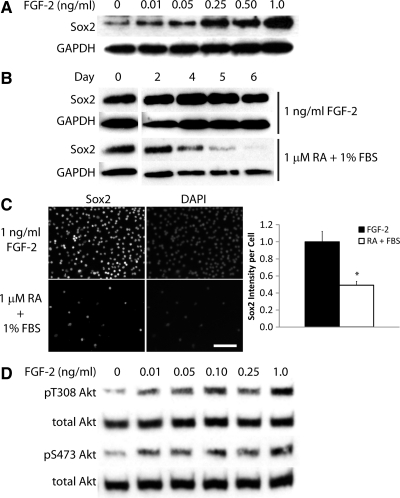
Given the importance of Sox2 in numerous stem cell processes, we sought to determine how its expression is regulated in NPCs. We first analyzed Sox2 protein levels under culture conditions known to support NPC self-renewal and proliferation. Specifically, we cultured NPCs with varying FGF-2 concentrations for 5 days, lysed the cultures, and probed for Sox2 via immunoblotting (Fig. 1A). Sox2 expression increases with increasing FGF-2 concentration. We also performed the converse experiment in which cells were differentiated for 6 days with 1 μM RA + 1% FBS, which also halts proliferation, and Sox2 protein expression was measured by immunoblotting (Fig. 1B). Consistent with its role as a NPC marker, Sox2 expression decreases upon differentiation, a result confirmed quantitatively by immunostaining (Fig. 1C).
FIG. 1.
Sox2 expression and Akt activation both increase under proliferating conditions. (A) Cells were cultured in various fibroblast growth factor 2 (FGF-2) concentrations. Lysates were probed for Sox2, using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a loading control. (B) Cells were cultured for the indicated number of days in media containing either 1 ng/mL FGF-2 or 1 μM retinoic acid (RA) + 1% fetal bovine serum (FBS). All bands are from the same blot; however, intervening lanes have been removed for clarity. (C) Left panel: cells were cultured in the indicated media and stained for Sox2. Counterstain: DAPI. Scale bar: 200 μm. Right panel: quantification of images. Error bars are 95% confidence intervals, and *P < 0.05 according to Student's t-test. (D) Cells were FGF-2-starved overnight, then stimulated with indicated concentrations of FGF-2 for 10 min. Lysates were probed for Akt phosphorylated at S473 and T308. Loading control: total Akt.
Our previous results showed that Akt stimulates NPC proliferation and inhibits differentiation [21]; therefore, we sought to determine whether Akt activation and Sox2 expression acted in concert. To analyze Akt activation as a function of FGF-2 dosage, analogous to the experiment with Sox2 (Fig. 1A), NPCs were starved of FGF-2 overnight, stimulated with various FGF-2 concentrations, lysed, and probed for phosphorylated, active Akt (Fig. 1D). Increasing FGF-2 concentrations resulted in higher Akt phosphorylation at both the S473 and T308 sites, which are critical for full activation of the molecule. These data indicate a correlation between Akt activation and Sox2 expression within NPCs.
Akt promotes Sox2 expression
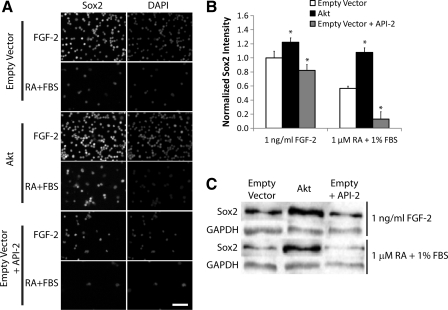
To begin to analyze whether the parallel between Akt activity and Sox2 expression is correlative or causal, we overexpressed wild-type Akt in NPCs via retroviral transduction. Cells were cultured under either proliferative (1 ng/mL FGF-2) or differentiation (1 μM RA + 1% FBS) conditions. After 5 days in culture, the intensity of Sox2 expression was measured via immunofluorescence and compared with empty vector infected control cells and control cells cultured with the Akt inhibitor API-2/triciribine (1 μM) (Fig. 2A, B). Cells overexpressing Akt had only slightly increased Sox2 expression under proliferation conditions, likely due to the already strong mitogenic signal provided by the FGF-2 present in the culture. However, despite the finding that differentiation induces a considerable loss of Sox2 expression (Fig. 1B), cells overexpressing Akt cultured under differentiation conditions maintained Sox2 expression levels comparable to control cells cultured in proliferation conditions, indicating a complete rescue of Sox2 expression.
FIG. 2.
Akt promotes Sox2 expression. (A) Cells overexpressing Akt, vector control cells, or control cells cultured with Akt inhibitor (1 μM API-2/triciribine) were seeded in media containing either 1 ng/mL FGF-2 or 1 μM RA + 1% FBS. Cells were stained for Sox2. Counterstain: DAPI. Scale bar: 200 μm. (B) Quantification of images. Results are normalized to the vector control + FGF-2 condition. Error bars are 95% confidence intervals, and *P < 0.05 according to Student's t-test. (C) Same cells and conditions as above. Lysates were probed for Sox2, using GAPDH as loading control.
Additionally, empty vector control cells cultured with API-2 had decreased Sox2 expression under proliferation conditions. This partial but not complete elimination of Sox2 expression in the presence of API-2 indicates that Akt is important but likely not the sole mediator of FGF-2 upregulation of Sox2 expression. That said, Akt inhibition under differentiation conditions almost completely eliminated Sox2 expression. To further confirm the immunofluorescence results, we repeated the experiment and probed cell lysates for Sox2 levels via Western blotting (Fig. 2C). Akt overexpression again increased Sox2 protein expression, whereas Akt inhibition decreased expression, particularly under differentiation conditions.
Akt effects on Sox2 protein stability and mRNA concentration
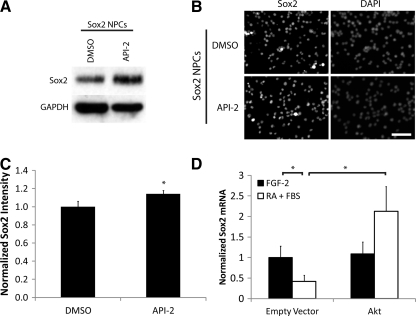
Akt could modulate Sox2 levels by acting at the protein and/or mRNA level. To analyze whether Akt promotes Sox2 protein stability, we stably overexpressed wild-type Sox2 in NPCs using a retroviral vector, where Sox2 is constitutively transcribed and expressed. These cells were cultured for 5 days with 1 ng/mL FGF-2 and 1 μM API-2 and analyzed for Sox2 protein expression by western blot (Fig. 3A) and immunofluorescence (Fig. 3B, C). Compared with carrier control (DMSO), API-2 did not decrease Sox2 protein concentration. In fact, immunofluorescence quantification revealed a slight but statistically significant increase in Sox2 intensity per cell when cultured with the Akt inhibitor (Fig. 3C). However, the fact that Akt inhibition did not decrease Sox2 protein in cells constitutively expressing Sox2 indicates that Akt is unlikely to promote Sox2 protein stability.
FIG. 3.
(A) Sox2-overexpressing neural progenitor cells (NPCs) were cultured with API-2 (Akt inhibitor) or dimethyl sulfoxide (DMSO) carrier control. Lysates were analyzed by immunoblotting for Sox2, using GAPDH as a loading control. (B) Similar to above, Sox2-overexpressing cells were cultured with API-2 or DMSO and stained for Sox2, with DAPI counterstain. Scale bar: 200 μm. (C) Quantification of images. (D) Akt-overexpressing NPCs along with control were cultured with either 1 ng/mL FGF-2 or 1 μM RA + 1% FBS. Quantitative RT-PCR was performed to detect Sox2. Samples were normalized to the 18S ribosomal subunit as an internal control. Error bars are 95% confidence intervals, and *P < 0.05 according to Student's t-test.
To assess alternatively whether Akt increases Sox2 protein concentration by modulating mRNA levels, we again cultured Akt-overexpressing NPCs and empty vector control cells for 5 days under proliferation or differentiation conditions. RNA was isolated, and quantitative RT-PCR for Sox2 was performed on the resulting cDNA (Fig. 3D). As expected, Sox2 transcript concentration decreased upon differentiation of the control cells; however, Sox2 mRNA did not decrease in Akt-overexpressing cells under the same conditions, demonstrating rescue of Sox2 by Akt, similar to the rescue of Sox2 protein expression (Fig. 2). Taken together, these results indicate that Akt may drive Sox2 expression by increasing the levels of its mRNA, rather than promoting Sox2 protein stability; however, more study is required to elucidate the precise mechanism.
Sox2 inhibits differentiation but does not promote proliferation of NPCs
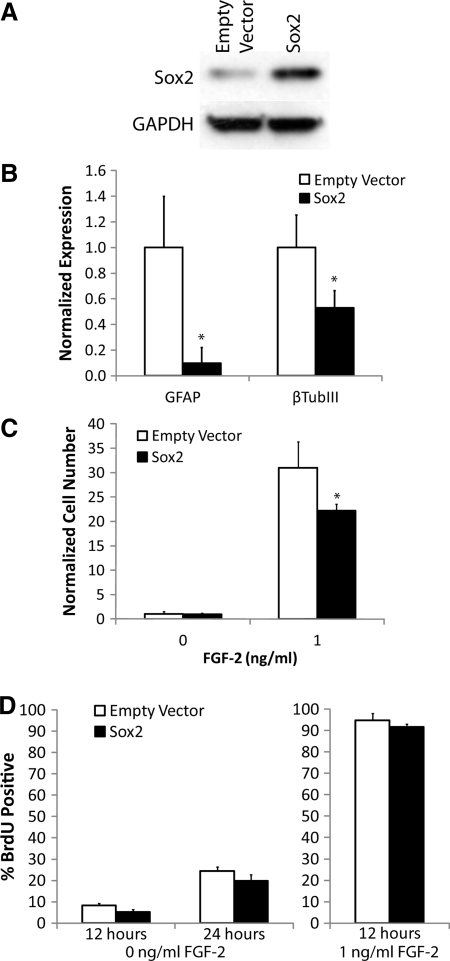
We have shown that Akt upregulates Sox2 expression (Fig. 2), as well as promotes cell proliferation and inhibits differentiation [21], but it is unclear whether Sox2 promotes proliferation, maintains NPC multipotency, or both. We again used the Sox2-overexpressing NPCs, which were confirmed by Western blot analysis (Fig. 4A). Both the Sox2 cell line and an empty vector control cell line were infected in parallel and differentiated with 1 μM RA + 1% FBS, a condition that promotes the generation of both neurons and astrocytes. After 5 days, quantitative RT-PCR of lineage markers was used to analyze cell differentiation (Fig. 4B). We have previously shown this technique to be a reliable measure of NPC differentiation [40], and it has been used in other work [21,40,43–46]. Compared with cells infected with the control vector, Sox2-overexpressing NPCs had significantly decreased expression of both the neuronal marker β-tubulin III and the astrocytic marker GFAP; however, GFAP expression was more strongly inhibited than β-tubulin III, very similar to previous results in cells overexpressing wild-type Akt [21].
FIG. 4.
Sox2 inhibits differentiation without affecting proliferation of NPCs. (A) Lysates from cells overexpressing Sox2 or vector control were probed to confirm Sox2 overexpression. Loading control: GAPDH. (B) Cells overexpressing Sox2 or vector control were cultured under differentiating conditions (1% FBS + 1 μM RA). Quantitative RT-PCR was performed for glial fibrillary acidic protein (GFAP) and β-tubulin III, and samples were normalized to the 18S ribosomal subunit as an internal control. (C) Sox2-overexpressing cells and control cells were cultured with and without FGF-2. Viable cell number was quantified by WST-1 and normalized to the control sample. (D) Cells cultured with 0 or 1 ng/mL FGF-2 were pulsed with bromodeoxyuridine (BrdU) for the indicated time period, and the percentage of BrdU-positive cells was quantified. Error bars are 95% confidence intervals, and *P < 0.05 according to Student's t-test.
We have also previously shown that Akt strongly upregulates NPC proliferation [21]. To determine whether Sox2 has a similar effect, we cultured the cells in both a WST-1 assay, measuring live cells, and a BrdU proliferation assay (Fig. 4C, D). In the WST-1 assay, Sox2 overexpression surprisingly had no effect on the number of live NPCs in the absence of FGF-2. Further, it moderately decreased viable NPCs in the presence of the growth factor. Similarly, Sox2 overexpression had no effect on overall NPC proliferation, or more specifically DNA synthesis, as determined in the BrdU assay. Taken together, these results indicate that Sox2 inhibits NPC differentiation, consistent with its known role in self-renewal [27,28], but does not promote NPCs proliferation.
Discussion
Sox2 is an important regulator of ES cell and NPC self-renewal [27–30], and it is regulated as a part of the core transcriptional circuitry controlling ES cell self-renewal [35,47,48]. However, very little is known about how it is regulated by upstream signaling pathways. One upstream pathway important for the maintenance of numerous stem cell populations is the PI3K/Akt pathway [14–20]; however, it is unknown how or even whether these two critical elements of stem cell regulatory machinery interact. Here we demonstrate that Akt promotes Sox2 protein expression; however, increased Sox2 expression did not increase NPC proliferation. Given our previous work showing that Akt drives NPC proliferation and inhibits differentiation [21], this indicates that Akt is an important regulator of both proliferation and self-renewal in NPCs, but via different downstream pathways.
Akt overexpression completely rescued differentiation-induced loss of Sox2 expression (Fig. 2). Additionally, Akt slightly increased Sox2 expression in cells cultured under proliferative conditions, though this was not a large effect, likely because the cells are already exposed to a strong, FGF-2-mediated mitogenic signal. Interestingly, though Akt inhibition led to a statistically significant reduction in Sox2 expression in cells cultured with FGF-2, the effect was not strong, indicating that Akt is not the only mediator of FGF-2-induced Sox2 expression. There is some evidence that Wnt/β-catenin signaling is important for NPC self-renewal [49,50]; however, a link between the Wnt signal and Sox2 expression was not established in either of these studies. Additional studies may reveal interesting links between Sox2 and other signaling pathways.
It is well known that Akt promotes protein translation by activating its downstream effector mTORC1 [51]. However, one interesting result of our work is that Akt rescues Sox2 transcript levels in cells cultured under differentiating conditions (Fig. 3D). Additionally, Akt inhibition did not affect Sox2 protein levels in a constitutively expressing Sox2 mutant (Fig. 3A–C), where the exogenous Sox2 cDNA did not contain untranslated regions, indicating that Akt inhibition did not modify protein levels by increasing protein degradation. Overall, these results indicate that Akt activation may modulate sox2 transcription. One potential mechanism for this is through stabilization of c-Myc [52,53], a transcription factor shown to modulate Sox2 expression in mouse ES cells [48]. Further experimentation is required to determine the precise effects of Akt on Sox2 transcription and translation in addition to any potential intermediate steps between Akt activity and Sox2 expression.
In addition to its role in NPCs, Akt is also known to promote the proliferation and self-renewal of ES cells [14–17]; therefore, investigating the effects of Akt on Sox2 expression in ES cells could have important implications for the development of more efficient ES cell culture systems and perhaps eventually ES cell-based therapies. Further, enhancement of Akt signaling may conceivably improve the efficiency of reprogramming and the generation of iPS cells (for a review of other modulators of reprogramming, see [54]). Recent work has demonstrated that pharmacological inhibition of ERK and GSK3β can generate iPS cells from neural stem cells transduced with only two of the four Yamanaka factors [55]. Other studies have used GSK3β inhibition along with ERK and ALK5 inhibition to promote reprogramming [56]. Because GSK3β activity is directly inhibited by Akt, these findings, taken together with our results, may indicate that modulation of the Akt pathway could potentially improve reprogramming efficiencies by upregulating expression of endogenous Sox2.
Our Sox2-overexpressing NPCs had a decreased ability to differentiate as measured by quantitative RT-PCR (Fig. 4B). In particular, upon exposing cells to conditions strongly favoring astrocytic and neuronal differentiation, Sox2-overexpressing cells had ∼10 × lower expression of the astrocytic marker GFAP and ∼2 × lower expression of the neuronal marker β-tubulin III. These results, similar to those seen in NPCs overexpressing Akt [21], further support observations in chick embryos constitutively expressing Sox2, which also experienced impaired neuronal differentiation [28]. Notably, however, Sox2 is still required for proper neuronal differentiation and development. Cells derived from Sox2 hypomorphic mice and cultured in vitro generated abundant β-tubulin III-positive cells, but those cells failed to mature [31]. Therefore, a minimal threshold amount of Sox2 appears to be required for proper neuronal development.
Although Sox2 overexpression inhibited differentiation, it did not increase the number of viable NPCs or their proliferation (Fig. 4C, D). This is consistent with the observation that Sox2-positive cells within the subgranular zone of the hippocampus are able to remain quiescent for extended periods [27]. Additionally, it indicates that although Sox2 is important for NPC self-renewal, it is not sufficient for their proliferation. This is not the case in other cell types. Sox2 promotes proliferation of tracheal and airway stem cells [57], and as an oncogene it is necessary for the proliferation and transformation of lung or esophageal squamous cell carcinomas [58].
In summary, this work demonstrates that in addition to being important for NPC proliferation [21], Akt is also an important promoter of Sox2 expression, thereby maintaining NPC multipotency. Importantly, however, Sox2 itself does not promote adult NPC proliferation, but it does inhibit neuronal and glial differentiation. Therefore, Akt is a key, parallel regulator of NPC proliferation and self-renewal. It may also play these roles in maintaining or even generating other stem cell types, such as ES and iPS cells.
Acknowledgments
We thank S. Ferguson and M. McVoy for the kind gift of reagents and Ashley Fritz for molecular biology assistance. This work was funded by a training grant fellowship from the California Institute for Regenerative Medicine (T1-00007) (to J.P.), a National Science Foundation Graduate Research Fellowship (to A.J.K.), and by NIH EB007295.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 2.Zhao C. Deng W. Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Suh H. Deng W. Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- 4.Duan X. Chang JH. Ge S. Faulkner RL. Kim JY. Kitabatake Y. Liu XB. Yang CH. Jordan JD. Ma DK. Liu CY. Ganesan S. Cheng HJ. Ming GL. Lu B. Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130:1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santarelli L. Saxe M. Gross C. Surget A. Battaglia F. Dulawa S. Weisstaub N. Lee J. Duman R. Arancio O. Belzung C. Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 6.Aimone JB. Wiles J. Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9:723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 7.Clelland CD. Choi M. Romberg C. Clemenson GD., Jr. Fragniere A. Tyers P. Jessberger S. Saksida LM. Barker RA. Gage FH. Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snyder JS. Hong NS. McDonald RJ. Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–852. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Imayoshi I. Sakamoto M. Ohtsuka T. Takao K. Miyakawa T. Yamaguchi M. Mori K. Ikeda T. Itohara S. Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- 10.Saxe MD. Battaglia F. Wang JW. Malleret G. David DJ. Monckton JE. Garcia AD. Sofroniew MV. Kandel ER. Santarelli L. Hen R. Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer TD. Markakis EA. Willhoite AR. Safar F. Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palmer TD. Schwartz PH. Taupin P. Kaspar B. Stein SA. Gage FH. Cell culture. Progenitor cells from human brain after death. Nature. 2001;411:42–43. doi: 10.1038/35075141. [DOI] [PubMed] [Google Scholar]
- 13.Lai K. Kaspar BK. Gage FH. Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 14.Lee MY. Lim HW. Lee SH. Han HJ. Smad, PI3K/Akt, and Wnt-dependent signaling pathways are involved in BMP-4-induced ESC self-renewal. Stem Cells. 2009;27:1858–1868. doi: 10.1002/stem.124. [DOI] [PubMed] [Google Scholar]
- 15.Chen L. Khillan JS. A novel signaling by vitamin A/retinol promotes self renewal of mouse embryonic stem cells by activating PI3K/Akt signaling pathway via insulin-like growth factor-1 receptor. Stem Cells. 2010;28:57–63. doi: 10.1002/stem.251. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe S. Umehara H. Murayama K. Okabe M. Kimura T. Nakano T. Activation of Akt signaling is sufficient to maintain pluripotency in mouse and primate embryonic stem cells. Oncogene. 2006;25:2697–2707. doi: 10.1038/sj.onc.1209307. [DOI] [PubMed] [Google Scholar]
- 17.Wang S. Shen Y. Yuan X. Chen K. Guo X. Chen Y. Niu Y. Li J. Xu RH. Yan X. Zhou Q. Ji W. Dissecting signaling pathways that govern self-renewal of rabbit embryonic stem cells. J Biol Chem. 2008;283:35929–35940. doi: 10.1074/jbc.M804091200. [DOI] [PubMed] [Google Scholar]
- 18.Mangi AA. Noiseux N. Kong D. He H. Rezvani M. Ingwall JS. Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J. Grindley JC. Yin T. Jayasinghe S. He XC. Ross JT. Haug JS. Rupp D. Porter-Westpfahl KS. Wiedemann LM. Wu H. Li L. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto K. Araki KY. Naka K. Arai F. Takubo K. Yamazaki S. Matsuoka S. Miyamoto T. Ito K. Ohmura M. Chen C. Hosokawa K. Nakauchi H. Nakayama K. Nakayama KI. Harada M. Motoyama N. Suda T. Hirao A. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Peltier J. O'Neill A. Schaffer DV. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol. 2007;67:1348–1361. doi: 10.1002/dneu.20506. [DOI] [PubMed] [Google Scholar]
- 22.Sinor AD. Lillien L. Akt-1 expression level regulates CNS precursors. J Neurosci. 2004;24:8531–8541. doi: 10.1523/JNEUROSCI.1470-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paik JH. Ding Z. Narurkar R. Ramkissoon S. Muller F. Kamoun WS. Chae SS. Zheng H. Ying H. Mahoney J. Hiller D. Jiang S. Protopopov A. Wong WH. Chin L. Ligon KL. DePinho RA. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–553. doi: 10.1016/j.stem.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim WY. Wang X. Wu Y. Doble BW. Patel S. Woodgett JR. Snider WD. GSK-3 is a master regulator of neural progenitor homeostasis. Nat Neurosci. 2009;12:1390–1397. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avilion AA. Nicolis SK. Pevny LH. Perez L. Vivian N. Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi K. Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Suh H. Consiglio A. Ray J. Sawai T. D'Amour KA. Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2(+) neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graham V. Khudyakov J. Ellis P. Pevny L. SOX2 functions to maintain neural progenitor identity. Neuron. 2003;39:749–765. doi: 10.1016/s0896-6273(03)00497-5. [DOI] [PubMed] [Google Scholar]
- 29.Ellis P. Fagan BM. Magness ST. Hutton S. Taranova O. Hayashi S. McMahon A. Rao M. Pevny L. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Dev Neurosci. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- 30.Bylund M. Andersson E. Novitch BG. Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- 31.Cavallaro M. Mariani J. Lancini C. Latorre E. Caccia R. Gullo F. Valotta M. DeBiasi S. Spinardi L. Ronchi A. Wanke E. Brunelli S. Favaro R. Ottolenghi S. Nicolis SK. Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development. 2008;135:541–557. doi: 10.1242/dev.010801. [DOI] [PubMed] [Google Scholar]
- 32.Tsuruzoe S. Ishihara K. Uchimura Y. Watanabe S. Sekita Y. Aoto T. Saitoh H. Yuasa Y. Niwa H. Kawasuji M. Baba H. Nakao M. Inhibition of DNA binding of Sox2 by the SUMO conjugation. Biochem Biophys Res Commun. 2006;351:920–926. doi: 10.1016/j.bbrc.2006.10.130. [DOI] [PubMed] [Google Scholar]
- 33.Van Hoof D. Munoz J. Braam SR. Pinkse MW. Linding R. Heck AJ. Mummery CL. Krijgsveld J. Phosphorylation dynamics during early differentiation of human embryonic stem cells. Cell Stem Cell. 2009;5:214–226. doi: 10.1016/j.stem.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 34.Gao F. Kwon SW. Zhao Y. Jin Y. PARP1 poly(ADP-ribosyl)ates Sox2 to control Sox2 protein levels and FGF4 expression during embryonic stem cell differentiation. J Biol Chem. 2009;284:22263–22273. doi: 10.1074/jbc.M109.033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyer LA. Lee TI. Cole MF. Johnstone SE. Levine SS. Zucker JP. Guenther MG. Kumar RM. Murray HL. Jenner RG. Gifford DK. Melton DA. Jaenisch R. Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyagi S. Nishimoto M. Saito T. Ninomiya M. Sawamoto K. Okano H. Muramatsu M. Oguro H. Iwama A. Okuda A. The Sox2 regulatory region 2 functions as a neural stem cell-specific enhancer in the telencephalon. J Biol Chem. 2006;281:13374–13381. doi: 10.1074/jbc.M512669200. [DOI] [PubMed] [Google Scholar]
- 37.Palmer TD. Markakis EA. Willhoite AR. Safar F. Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu JH. Schaffer DV. Selection of novel vesicular stomatitis virus glycoprotein variants from a peptide insertion library for enhanced purification of retroviral and lentiviral vectors. J Virol. 2006;80:3285–3292. doi: 10.1128/JVI.80.7.3285-3292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abbate J. Lacayo JC. Prichard M. Pari G. McVoy MA. Bifunctional protein conferring enhanced green fluorescence and puromycin resistance. Biotechniques. 2001;31:336–340. doi: 10.2144/01312st05. [DOI] [PubMed] [Google Scholar]
- 40.Abranches E. O'Neill A. Robertson MJ. Schaffer DV. Cabral JM. Development of quantitative PCR methods to analyse neural progenitor cell culture state. Biotechnol Appl Biochem. 2006;44:1–8. doi: 10.1042/BA20050218. [DOI] [PubMed] [Google Scholar]
- 41.Funakoshi Y. Ichiki T. Takeda K. Tokuno T. Iino N. Takeshita A. Critical role of cAMP-response element-binding protein for angiotensin II-induced hypertrophy of vascular smooth muscle cells. J Biol Chem. 2002;277:18710–18717. doi: 10.1074/jbc.M110430200. [DOI] [PubMed] [Google Scholar]
- 42.Carpenter AE. Jones TR. Lamprecht MR. Clarke C. Kang IH. Friman O. Guertin DA. Chang JH. Lindquist RA. Moffat J. Golland P. Sabatini DM. CellProfiler: image analysis software for identifying and quantifying cell phenotypes. Genome Biol. 2006;7:R100. doi: 10.1186/gb-2006-7-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saha K. Keung AJ. Irwin EF. Li Y. Little L. Schaffer DV. Healy KE. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tay Y. Zhang J. Thomson AM. Lim B. Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature. 2008;455:1124–1128. doi: 10.1038/nature07299. [DOI] [PubMed] [Google Scholar]
- 45.Behfar A. Zingman LV. Hodgson DM. Rauzier JM. Kane GC. Terzic A. Puceat M. Stem cell differentiation requires a paracrine pathway in the heart. FASEB J. 2002;16:1558–1566. doi: 10.1096/fj.02-0072com. [DOI] [PubMed] [Google Scholar]
- 46.D'Amour KA. Agulnick AD. Eliazer S. Kelly OG. Kroon E. Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 47.Chen X. Xu H. Yuan P. Fang F. Huss M. Vega VB. Wong E. Orlov YL. Zhang W. Jiang J. Loh YH. Yeo HC. Yeo ZX. Narang V. Govindarajan KR. Leong B. Shahab A. Ruan Y. Bourque G. Sung WK. Clarke ND. Wei CL. Ng HH. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 48.Liu X. Huang J. Chen T. Wang Y. Xin S. Li J. Pei G. Kang J. Yamanaka factors critically regulate the developmental signaling network in mouse embryonic stem cells. Cell Res. 2008;18:1177–1189. doi: 10.1038/cr.2008.309. [DOI] [PubMed] [Google Scholar]
- 49.Qu Q. Sun G. Li W. Yang S. Ye P. Zhao C. Yu RT. Gage FH. Evans RM. Shi Y. Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat Cell Biol. 2010;12:31–40. doi: 10.1038/ncb2001. sup pp 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalani MY. Cheshier SH. Cord BJ. Bababeygy SR. Vogel H. Weissman IL. Palmer TD. Nusse R. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci U S A. 2008;105:16970–16975. doi: 10.1073/pnas.0808616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manning BD. Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sears R. Nuckolls F. Haura E. Taya Y. Tamai K. Nevins JR. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev. 2000;14:2501–2514. doi: 10.1101/gad.836800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeh E. Cunningham M. Arnold H. Chasse D. Monteith T. Ivaldi G. Hahn WC. Stukenberg PT. Shenolikar S. Uchida T. Counter CM. Nevins JR. Means AR. Sears R. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol. 2004;6:308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- 54.Feng B. Ng JH. Heng JC. Ng HH. Molecules that promote or enhance reprogramming of somatic cells to induced pluripotent stem cells. Cell Stem Cell. 2009;4:301–312. doi: 10.1016/j.stem.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 55.Silva J. Barrandon O. Nichols J. Kawaguchi J. Theunissen TW. Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li W. Wei W. Zhu S. Zhu J. Shi Y. Lin T. Hao E. Hayek A. Deng H. Ding S. Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell. 2009;4:16–19. doi: 10.1016/j.stem.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 57.Que J. Luo X. Schwartz RJ. Hogan BL. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136:1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bass AJ. Watanabe H. Mermel CH. Yu S. Perner S. Verhaak RG. Kim SY. Wardwell L. Tamayo P. Gat-Viks I. Ramos AH. Woo MS. Weir BA. Getz G. Beroukhim R. O'Kelly M. Dutt A. Rozenblatt-Rosen O. Dziunycz P. Komisarof J. Chirieac LR. Lafargue CJ. Scheble V. Wilbertz T. Ma C. Rao S. Nakagawa H. Stairs DB. Lin L. Giordano TJ. Wagner P. Minna JD. Gazdar AF. Zhu CQ. Brose MS. Cecconello I., Jr. UR Marie SK. Dahl O. Shivdasani RA. Tsao MS. Rubin MA. Wong KK. Regev A. Hahn WC. Beer DG. Rustgi AK. Meyerson M. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]