Abstract
Antidepressants increase adult hippocampal neurogenesis in animal models, but the underlying molecular mechanisms are unknown. In this study, we used human hippocampal progenitor cells to investigate the molecular pathways involved in the antidepressant-induced modulation of neurogenesis. Because our previous studies have shown that antidepressants regulate glucocorticoid receptor (GR) function, we specifically tested whether the GR may be involved in the effects of these drugs on neurogenesis. We found that treatment (for 3–10 days) with the antidepressant, sertraline, increased neuronal differentiation via a GR-dependent mechanism. Specifically, sertraline increased both immature, doublecortin (Dcx)-positive neuroblasts (+16%) and mature, microtubulin-associated protein-2 (MAP2)-positive neurons (+26%). This effect was abolished by the GR-antagonist, RU486. Interestingly, progenitor cell proliferation, as investigated by 5′-bromodeoxyuridine (BrdU) incorporation, was only increased when cells were co-treated with sertraline and the GR-agonist, dexamethasone, (+14%) an effect which was also abolished by RU486. Furthermore, the phosphodiesterase type 4 (PDE4)-inhibitor, rolipram, enhanced the effects of sertraline, whereas the protein kinase A (PKA)-inhibitor, H89, suppressed the effects of sertraline. Indeed, sertraline increased GR transactivation, modified GR phosphorylation and increased expression of the GR-regulated cyclin-dependent kinase-2 (CDK2) inhibitors, p27Kip1 and p57Kip2. In conclusion, our data suggest that the antidepressant, sertraline, increases human hippocampal neurogenesis via a GR-dependent mechanism that requires PKA signaling, GR phosphorylation and activation of a specific set of genes. Our data point toward an important role for the GR in the antidepressant-induced modulation of neurogenesis in humans.
Keywords: adult hippocampal neurogenesis, depression, glucocorticoids, neuroplasticity, stem cells
Introduction
Several studies have shown that antidepressants increase hippocampal neurogenesis in both animals and humans,1, 2, 3, 4, 5 and whether this increase in neurogenesis is necessary to overcome behavioral deficits in animal models of depression is being intensely debated.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 However, the molecular pathways underlying such effects have not been described to date. The effects of antidepressants on hippocampal neurogenesis have been demonstrated for different, chemically unrelated classes of antidepressants,1 suggesting that a common molecular mechanism may underlie their neurogenic potential. Identifying such a mechanism would allow us to modulate neurogenesis, and thereby possibly counteract some of the neurobiological disturbances in depression.
Notably, recent studies have suggested that glucocorticoids are involved in the neurogenic action of antidepressants.12, 17 For example, one study has observed that antidepressants increase hippocampal neurogenesis only in mice that are co-treated with glucocorticoid hormones, but not in control mice.12 The potential role of glucocorticoids in antidepressant-induced neurogenesis is also consistent with an extensive literature, some from our own group, which shows that antidepressants directly regulate the function of the glucocorticoid receptor (GR).18, 19, 20, 21, 22, 23, 24 The GR is a ligand-activated nuclear transcription factor. Upon ligand binding, the GR translocates into the nucleus, binds to glucocorticoid response elements on the DNA and subsequently activates gene transcription (so called transactivation). Multiple kinases have been reported to differentially phosphorylate the GR at its serine residues, S203, S211 and S226, an effect which regulates GR nuclear translocation and GR-dependent gene transcription.25, 26, 27, 28, 29 Studies by us and others have shown that antidepressants also induce GR nuclear translocation and transactivation, which is, at least in part, mediated by the cyclic AMP (cAMP)/protein kinase A (PKA) cascade.21, 22, 23, 24, 30, 31, 32, 33 Moreover, the intracellular availability of cAMP is tightly regulated by phosphodiesterase type 4 (PDE4); and, consistent with PKA-dependent regulation of the GR, the PDE4 inhibitor, rolipram, enhances GR activation by antidepressants.30
Considering this body of evidence, we hypothesized that antidepressants modulate adult hippocampal neurogenesis via a GR-dependent mechanism that requires PKA signaling and GR phosphorylation. To test this hypothesis in a clinically relevant model, we have used hippocampal progenitor cells from humans. Using this in vitro model, we have been able to separate the direct effects of antidepressants on progenitor cells from any potential indirect effects, which would be present in an in vivo model. Moreover, we have examined the differential effects of antidepressants (and glucocorticoid hormones) on progenitor cell proliferation and differentiation, as well as on GR phosphorylation, transactivation and subsequent changes in gene expression. In all our experiments, we have used the selective serotonin reuptake inhibitor, sertraline, which has recently been described as one of the most clinically effective antidepressants.34 Confirmatory replication experiments have been conducted also using the tricyclic antidepressants, amitriptyline and clomipramine.
Materials and methods
Cell culture
The multipotent, human hippocampal progenitor cell line HPC03A/07 (provided by ReNeuron, Surrey, UK) was used for all experiments. Further information on this cell line and media components are available in the Supplementary Materials.
Differentiation assay
To assess changes in neuronal differentiation, HPC03A/07 cells were plated on black-sided 96-well plates (Nunclon, Roskilde, Denmark) at a density of 1.1 × 104 cells per well. HPC03A/07 cells were cultured in the presence of epidermal growth factor (EGF), basic fibroblast growth factor (bFGF) and 4-hydroxytamoxifen (4-OHT) for 72 h, and then washed and cultured in media without growth factors and 4-OHT for subsequent 7 days. Cells were treated with the antidepressant sertraline (1 μ), the GR-agonists, dexamethasone (1 μ) and cortisol (100 μ), the GR-antagonist RU486 (50 n), the PDE4-inhibitor rolipram (100 n) or the PKA-inhibitor H89 (50 n). For these experiments, cells were treated during the initial proliferation phase and the subsequent differentiation phase (total treatment of 10 days), or only during the proliferation phase (72 h), or only during the differentiation phase (7 days). At the end of the total incubation time (10 days), cells were fixed with 4% paraformaldehyde (PFA) for 20 min at room temperature.
Proliferation assay
To assess progenitor cell proliferation, HPC03A/07 cells were plated as described above, and cultured for 72 h in the presence of growth factors and 4-OHT. The synthetic nucleotide 5′-bromodeoxyuridine (BrdU, 10 μ) was added to the culture media 4 h before the end of the incubation, and cells were fixed as described above.
Immunocytochemistry
Neuronal differentiation was assessed by doublecortin (Dcx), microtubulin-associated protein-2 (MAP2) and neuron-specific class III ß-tubulin (TuJ1) immunocytochemistry (Figure 1a, Supplementary Figure 2a). Specificity of the MAP2 antibody for mature neurons in our culture was confirmed by co-labeling experiments and Western Blotting (Supplementary Figure 11). Briefly, PFA-fixed cells were incubated in blocking solution (5% normal goat serum, Alpha Diagnostics, San Antonio, TX, USA) in phosphate-buffered saline containing 0.3% Triton-X for 2 h at room temperature, and with primary antibodies (rabbit anti-Dcx, 1:1000; mouse anti-MAP-2 (HM), 1:500, Abcam, Cambridge, UK; rabbit anti-TuJ1, 1:500, Sigma, St-Louis, MO, USA) at 4 °C over night. Cells were incubated sequentially in blocking solution for 30 min, secondary antibodies (Alexa 594 goat anti-rabbit, 1:1000 and Alexa 488 goat anti-mouse, 1:500, Invitrogen, Paisley, UK) for 1 h and Hoechst 3 3342 dye (0.01 mg ml−1, Invitrogen) for 5 min at room temperature. The number of Dcx, MAP2 and TuJ1 positive cells over total Hoechst 33 342 positive cells was counted in an unbiased setup with an inverted microscope (IX70, Olympus, Hamburg, Germany) and ImageJ 1.41 software (http://rsbweb.nih.gov). To assess progenitor cell proliferation, BrdU-containing cells were incubated with hydrochloric acid (HCl, 2 ) for 15 min at room temperature, blocking solution for 60 min at room temperature, primary antibody (rat anti-BrdU, Serotec, Oxford, UK. 1:500) at 4 °C over night and secondary antibody (Alexa 488 goat anti-rat, 1:500, Invitrogen) for 2 h at room temperature. The number of BrdU-positive cells over total Hoechst 33 342 positive cells was determined as described above (see Figure 1a). Negative controls were incubated with unspecific mouse IgGs (1:500, control for MAP-2), rabbit IgGs (1:500, control for Dcx and TuJ1) or rat IgGs (1:500, control for BrdU) in place of the specific primary antibody (see Figure 1a).
Figure 1.
Antidepressants induce differentiation and neuronal maturation of HPC03A/07 human hippocampal progenitor cells. Immunocytochemistry (ICC) for doublecortin (Dcx) and microtubulin-associated protein-2 (MAP2) was used to assess neuronal differentiation and maturation, respectively. 5′-bromodeoxyuridine (BrdU) incorporation and immunocytochemistry were used to assess progenitor cell proliferation (a). When HPC03A/07 cells were treated during the proliferation phase (72 h) and the subsequent differentiation phase (7 days), drug treatment had a significant effect on MAP2-positive neurons (one-way analysis of variance, P<0.0001, F1,4=62.22, R2=0.9120, n=5) and on Dcx-positive neuroblasts (one-way analysis of variance, P=0.0007, F1,4=13.28, R2=0.6713, n=5). Sertraline (SERT, 1 μ) increased the number of MAP2-positive neurons but did not alter the number of Dcx-positive neuroblasts, whereas dexamethasone (DEX, 1 μ) decreased both. No effect was observed upon co-treatment with SERT and DEX (b). When HPC03A/07 cells were treated only during the proliferation phase, drug treatment had a significant effect on MAP2-positive neurons (one-way analysis of variance, P=0.0022, F1,4=9.764, R2=0.5824, n=5) and on Dcx-positive neuroblasts (one-way analysis of variance, P<0.0001, F1,4=44.44, R2=0.8724, n=5). SERT increased the number of Dcx-positive neuroblasts, without an effect on MAP2-positive neurons (c). Treatment only during the differentiation phase did not have an effect on MAP2-positive neurons (one-way analysis of variance, P=0.5699, F1,4=0.6184, R2=0.1709, n=5) or on Dcx-positive neuroblasts (one-way analysis of variance, P=0.2146, F1,4=2.127, R2=0.4596, n=5) (d). Five independent experiments were conducted on five independent cultures (n=5), four wells were analyzed per treatment condition in each experiment and three random, non-overlapping pictures were analyzed for each well. All data are mean±s.e.m. *P<0.05, **P<0.01 and ***P<0.001 compared with the corresponding vehicle-treated control.
Western blot analysis of GR phosphorylation
To assess changes in GR phosphorylation, HPC03A/07 cells were treated for 1, 6 and 12 h. Cells were washed with ice cold phosphate-buffered saline containing phosphatase inhibitors (Pierce, Rockford, IL, USA), scraped carefully from the flask and centrifuged in a pre-cooled centrifuge for 10 min at 3000 rpm at 4 °C. Cell pellets were resuspendend in protein extraction buffer (20 m Tris-HCl, 150 m NaCl, 1 m EDTA, 1 m ethylene glycol tetraacetic acid, 1% TritonX-100, 2 n calyculin A (Cell Signaling, Danvers, MA, USA), 1 × protease and phosphatase inhibitors (Pierce)), and incubated for 15 min at 4 °C. Protein lysates were centrifuged for 15 min at 14 000 g at 4 °C. Protein concentrations were quantified using a bicinchoninic acid colorimetric assay system (Merck, Nottingham, UK) (see Supplementary Materials). Protein samples containing 50 μg of total protein were boiled for 10 min at 72 °C in 1 × NuPAGE LDS sample buffer (Invitrogen) and 1 × NuPAGE sample-reducing agent (Invitrogen), and subjected to reducing SDS-polyacrylamide gel electrophoresis on 10% NuPAGE Bis–Tris gels for 1 h at 200 V. Proteins were electrophoretically transferred to Immuno- Blot PVDF membranes (Bio-Rad laboratories, Hercules, CA, USA) at 110 V for 1.5 h at 4 °C. Transfer efficiency was controlled by Ponceau S staining and by pre-stained protein standards. Unspecific binding sites were blocked for 1 h in 5% bovine serum albumin in Tris-buffered saline, and membranes were immunoprobed with the polyclonal rabbit anti-P-S203 (1:10000) and anti-P-S226 (1:1000) antibodies (both from Dr Michael J Garabedian), anti-P-S211 antibody (1:500; abcam), anti-GR59 antibody (1:500, Fisher) and anti-beta-actin antibody (1:500, Biolegend, San Diego, CA, USA) in blocking solution at 4 °C over night. Membranes were washed with Tris-buffered saline containing 0.1% Tween-20, and incubated with a horseradish peroxidase-conjugated swine anti-rabbit secondary antibody (1:2000, DAKO, Glostrup, Denmark) in 5% non-fat dry milk in Tris-buffered saline, for 1 h at room temperature. Membranes were washed in Tris-buffered saline containing 0.1% Tween-20 and proteins were visualized with enhanced chemiluminescence detection system (GE Healthcare, UK). Data are expressed as fold change from the vehicle-treated control condition.
GR transactivation assay
To determine changes in GR transactivation, HPC03A/07 cells were treated with sertraline for 1, 6, 12, 24 and 72 h. Nuclear protein extracts were obtained using a commercially available nuclear extraction kit (Active Motif, Rixensart, Belgium). GR transactivation was analyzed using the enzyme-linked immunosorbent assay-based TransAM GR method (Active Motif), according to the manufacturer's instructions (see Supplementary Methods).
Gene expression analysis
RNA was isolated using RNeasy mini kit (Qiagen, Crawley, UK) and processed for gene expression analysis by quantitative real-time PCR using the SYBR® Green method (see Supplementary Methods). Each sample was assayed in duplicate and each target gene (GR, GRα, p27Kip1, p57Kip2, p21Cip1, cyclin-dependent kinase2 (CDK2), cyclin A2 (CCNA2), cyclin D1 (CCND1), p53, human murine double minute2 (HDM2), FK506-binding protein 5 (FKBP5), serum/glucocorticoid-regulated kinase 1 (SGK1), forkhead box O1 (FOXO1), growth-arrest and DNA-damage-inducible ß (GADD45B), brain-derived neurotrophic factor (BDNF), p11 and ß-arrestin-2) was normalized to the geometric mean of the three reference genes glyceraldehyde 3-phosphate dehydrogenase (GAPDH), beta-actin (ACTB) and beta-2-microglobulin (B2M). Pfaffl Method was used to determine relative target gene expression. Data are expressed as fold change from the vehicle-treated control condition. Primer sequences are available upon request.
Drugs
All drugs and reagents were purchased from Sigma–Aldrich (St Louis, MO, USA), unless otherwise stated. Growth factors EGF and bFGF were purchased from Peprotech (London, UK). Sertraline hydrochloride and rolipram were dissolved in 100% dimethyl sulfoxide dimethyl sulfoxide (DMSO); dexamethasone, hydrocortisone and RU486 were dissolved in 100% ethanol (EtOH); H89 dihydrochloride hydrate, amitriptyline hydrochloride and clomipramine hydrochloride were dissolved in sterile, deionized water. BrdU was dissolved in phosphate-buffered saline fresh before use.
Statistical analysis
Data are presented as mean±s.e.m. All statistical analyses were performed with GraphPad Prism 4.03 (Graph Pad Inc, La Jolla, CA, USA) on independent biological replicates (indicated as n). One-way analysis of variance with Newman–Keuls post-hoc test was used for multiple comparisons among treatment groups. Student's t-test was used to compare means of two independent treatment groups. P-values <0.05 were considered significant.
Results
Antidepressants and glucocorticoids regulate neuronal differentiation of human hippocampal progenitor cells
To investigate the effects of antidepressants on neuronal differentiation, we treated human hippocampal progenitor cells (HPC03A/07) with sertraline for 72 h during proliferation, and for subsequent 7 days of differentiation. Treatment with sertraline (1 μ) increased the number of MAP2-positive neurons (by 28% Figure 1b, left white column), but did not significantly alter the number of Dcx-positive neuroblasts (Figure 1b, right white column). The specific GR-agonist dexamethasone (1 μ) decreased the number of MAP2-positive neurons and of Dcx-positive neuroblasts (by 27 and 25%, respectively; Figure 1b, striped columns). Treatment with cortisol induced the same effects of dexamethasone (Supplementary Figure 1a). Co-treatment of sertraline (1 μ) and dexamethasone (1 μ) abolished the reduction in MAP2 and Dcx-positive cells (Figure 1b, squared columns).
We also wanted to test whether treatment with sertraline during progenitor cell proliferation is sufficient to induce neuronal differentiation. We thus treated cells only during the proliferation phase, but not during the subsequent differentiation phase. Under these conditions, sertraline did not alter the number of MAP2-positive neurons (Figure 1c, left white column) but increased the number of Dcx-positive neuroblasts (by 16% Figure 1c, right white column), indicating that sertraline initiates neuronal differentiation by an effect on progenitor cells, and only induces neuronal maturation if continuously present during neuronal differentiation (as shown in Figure 1b). Dexamethasone reduced the number of MAP2-positive neurons and of Dcx-positive neuroblasts (by 28 and 27%, respectively; Figure 1c, striped column), an effect that was abolished by sertraline co-treatment (Figure 1c, squared columns). Again, cortisol had the same effects of dexamethasone (Supplementary Figure 1b).
Interestingly, when we treated progenitor cells only during the differentiation phase (7 days), but not during the preceding proliferation phase (72 h), neither sertraline nor dexamethasone (or cortisol) altered the number of MAP2-positive neurons or of Dcx-positive neuroblasts (see Figure 1d and Supplementary Figure 1c). Taken together, these data indicate that the effects of antidepressants on proliferating progenitor cells are essential to induce neuronal differentiation into Dcx-positive neuroblasts, but not sufficient to promote their maturation into MAP2-positive neurons. Additional experiments in which cells were immunostained for the pan-neuronal marker TuJ1, confirmed these findings (see Supplementary Results and Supplementary Figure 2).
The effects of antidepressants on neurogenesis are dependent on the GR
We specifically wanted to test whether the GR is involved in the antidepressant-induced changes in neurogenesis. We, therefore, co-treated progenitor cells with sertraline and the GR-antagonist RU486 (50 n), during both proliferation and differentiation. RU486 abolished the sertraline-induced increase in the number of MAP2-positive neurons (Figure 2a, white columns), thus confirming a role for the GR in the neurogenic action of sertraline. As expected, RU486 also eliminated the dexamethasone- (and the cortisol-) induced decrease in MAP2-positive neurons (Figure 2a, striped columns, Supplementary Figure 1). Furthermore, and consistent with the data described above, RU486 abolished the sertraline-induced increase in Dcx-positive neuroblasts, when cells were treated only during the proliferation phase but not during the subsequent differentiation phase (Figure 2b, white columns). Treatment with RU486 alone showed no effect on neuronal differentiation at the concentration used (50 nM) (data not shown). Taken together, these data support the notion that sertraline induces neuronal differentiation and promotes neuronal maturation via a GR-dependent effect.
Figure 2.
The effects of sertraline on neuronal differentiation are dependent on the glucocorticoid receptor (GR). The GR-antagonist RU486 (50 n) abolished the effect of SERT (1 μ) and of DEX (1 μ) on the number of microtubulin-associated protein-2 (MAP2)-positive neurons (a) and on the number of doublecortin (Dcx)-positive neuroblasts (b). Three independent experiments were conducted on three independent cultures (n=3), four wells were analyzed per treatment condition in each experiment, and three non-overlapping pictures were analyzed per well. All data are mean±s.e.m. *P<0.05, **P<0.01 compared with SERT or DEX-treatment alone.
The effects of antidepressants on neurogenesis are mediated by PKA signaling
Considering the role of PKA signaling in antidepressant drug action, we then wanted to examine whether the effects of sertraline on neurogenesis are mediated by the PDE4/PKA signaling cascade. Therefore, we co-treated cells with sertraline and the PKA-inhibitor, H89 (50 n) or the PDE4-inhibitor, rolipram (100 n), which enhances PKA activity by increasing cAMP levels. If treated during proliferation and differentiation, rolipram enhanced the effect of sertraline on MAP2-positive neurons (+43% vs sertraline alone), whereas H89 abolished it (Figure 3a, white columns). Interestingly, rolipram also counteracted the dexamethasone-induced reduction in MAP2-positive neurons, an effect that resembles the action of sertraline (Figure 3a, striped columns). Likewise, if cells were treated only during the proliferation phase, rolipram enhanced the sertraline-induced increase in Dcx-positive neuroblasts (+105% vs sertraline alone) while H89 abolished this effect (Figure 3b, white columns). Rolipram also counteracted the dexamethasone-induced reduction in Dcx-positive neuroblasts, which again resembles the action of sertraline (Figure 3b, striped columns).
Figure 3.
The effects of sertraline on neuronal differentiation are mediated by phosphodiesterase type 4 (PDE4)/protein kinase A (PKA) signaling. Rolipram (100 n) increased the effect of SERT (1 μ) on the number of microtubulin-associated protein-2 (MAP2)-positive neurons, whereas H89 abolished it. Rolipram and H89 also counteracted the effect of DEX (1 μ) on MAP2-positive neurons (a). Rolipram increased the effect of SERT on the number of doublecortin (Dcx)-positive neuroblasts, whereas H89 abolished it. Rolipram also counteracted the effect of DEX (1 μ) on Dcx-positive neuroblasts (b). Five independent experiments were conducted on five independent cultures (n=5), four wells were analyzed per treatment condition in each experiment and three random, non-overlapping pictures were analyzed for each well. All data are mean±s.e.m. *P<0.05, **P<0.01 compared with SERT or DEX alone.
Antidepressants regulate human hippocampal progenitor cell proliferation
In all previous experiments, we examined the effects of sertraline on neuronal differentiation. In order to study the effects on cell proliferation, we treated progenitor cells with sertraline for 72 h, and incorporated BrdU (10 μ) during the last 4 h of incubation. Treatment with sertraline decreased the number of BrdU-positive cells (by 16% Figure 4a, left white column), which supports the notion that progenitor cells stop proliferating in order to start to develop into neurons (as displayed in Figure 1). Again, as for the effects on neuronal differentiation, the effect of sertraline on progenitor proliferation was abolished by RU486 co-treatment (Figure 4a, right white column). Dexamethasone also reduced the number of BrdU-positive cells (by 20%), an effect that was again abolished by RU486 (Figure 4a, striped columns). A similar effect was observed using cortisol (Supplementary Figure 3a, black columns). Interestingly, proliferation was significantly increased only when progenitor cells were co-treated with sertraline and dexamethasone (+14% Figure 4a, squared columns) (or sertraline and cortisol, Supplementary Figure 3a, squared columns). This effect was again abolished by RU486 (Figure 4a, squared columns). Treatment with RU486 alone showed no effect on proliferation at the concentration used (50 nM) (data not shown).
Figure 4.
Effects of sertraline on progenitor cell proliferation. SERT (1 μ) and DEX (1 μ) decreased the number of 5′-bromodeoxyuridine (BrdU)-positive cells. These effects were abolished by the glucocorticoid receptor (GR)-antagonist RU486 (50 n). Co-treatment of DEX and SERT increased the number of BrdU-positive cells. This effect was also abolished by RU486 (*P<0.05, **P<0.01) (a). Rolipram (100 n) enhanced the effect of SERT (b, white columns), and of SERT and DEX co-treatment (b, squared columns), whereas H89 (50 n) abolished both effects. The number of BrdU-positive cells was significantly increased when cells were co-treated with rolipram and DEX (b, striped columns). Six independent experiments were conducted on six independent cultures (n=6), four wells were analyzed per treatment condition in each experiment and three random, non-overlapping pictures were analyzed for each well. All data are mean±s.e.m. *P<0.05, **P<0.01 and ***P<0.001 compared with the respective vehicle or as indicated.
The same results were obtained using lower concentrations of BrdU (1 μ) (Supplementary Figure 4), using Ki67 immunocytochemistry (Supplementary Methods and Supplementary Figure 12), or using the tricyclic antidepressants, amitriptyline (1 μ) and clomipramine (1 μ) (Supplementary Figure 5). Cell death, as measured by propidium iodide live-staining, was not affected by dexamethasone, cortisol, sertraline, or dexamethasone and sertraline co-treatment in the concentrations used in this study (see Supplementary Materials and Supplementary Figure 10).
Finally, we wanted to investigate whether the effects of sertraline on cell proliferation also require PKA signaling. Indeed, rolipram enhanced the sertraline-induced reduction in cell proliferation (+37% vs sertraline alone), whereas H89 inhibited it (−41% vs sertraline alone; Figure 4b, white columns). Moreover, co-treatment with rolipram and dexamethasone significantly increased proliferation (+17% vs vehicle; Figure 4b, striped columns), which again resembles the effect of sertraline and dexamethasone co-treatment. Finally, rolipram further increased proliferation upon co-treatment with sertraline and dexamethasone (+59% vs dexamethasone + sertraline), and this effect was abolished by H89 (Figure 4c, squared columns). These results support the notion that the effects of sertraline on cell proliferation, as those on neuronal differentiation, are mediated by PDE4/PKA signaling. It is important to note that rolipram did not change cell proliferation at the concentration used in these experiments (that is, 100 n). However, higher concentrations of rolipram (10 μ) did indeed decrease cell proliferation via a GR-dependent effect (Supplementary Figure 6), further supporting a role for the interaction of PDE4/PKA and GR on neurogenesis.
Antidepressants modulate GR phosphorylation via PKA signaling
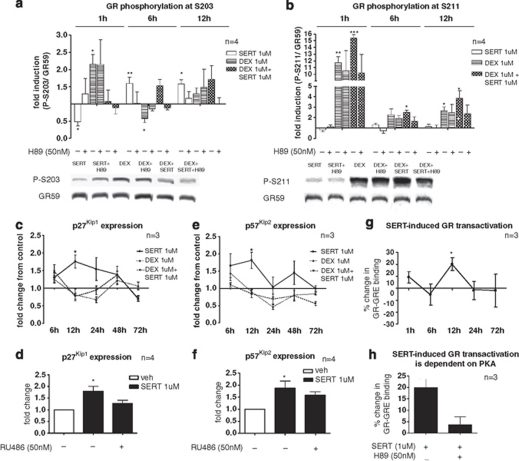
In the experiments above, we have demonstrated that sertraline, dexamethasone, and sertraline and dexamethasone co-treatment, have profoundly different effects on neurogenesis, but all these effects are mediated by the activation of the GR. One potential explanation for these results is differential regulation of GR phosphorylation by these three different treatment conditions. Therefore, we investigated GR phosphorylation at its serine residues S203, S211 and S226 at 1, 6 and 12 h of treatment. Our data indeed show that sertraline, dexamethasone, and sertraline and dexamethasone co-treatment, activate distinct patterns of GR phosphorylation.
At the S203 residue, sertraline decreases GR phosphorylation after 1 h of treatment (0.5-fold compared with control; Figure 5a, first white column), whereas it increases phosphorylation at 6 and 12 h (both by 1.6-fold, white columns). In line with our data showing that the antidepressant effect on neurogenesis is dependent on PKA, inhibition of PKA by H89 abolishes all sertraline-induced changes in S203 phosphorylation of the GR (Figure 5a). In contrast, treatment with dexamethasone has the opposite effect: it induces S203 phosphorylation after 1 h of treatment (1.6-fold; Figure 5a, first striped column), and it decreases phosphorylation after 6 h (by 0.4-fold). Sertraline and dexamethasone co-treatment shows yet a third time-dependent phosphorylation profile: Although no changes in S203 phosphorylation are observed after 1 h (Figure 5a; first squared column), phosphorylation is increased at 6 and 12 h (by 1.5-fold and 1.7-fold, respectively; squared columns). These co-treatment effects are again blocked by H89. H89 alone did not show any effect at the concentration used (data not shown).
Figure 5.
Sertraline regulates glucocorticoid receptor (GR) phosphorylation, GR-mediated expression of p27Kip1 and p57Kip2 and GR transactivation. During progenitor cell proliferation, SERT (1 μ) decreases GR phosphorylation at S203 after 1 h of treatment, but increases S203 phosphorylation after 6 h and 12 h (a, white columns). These effects are counteracted by H89. DEX increases S203 phosphorylation after 1 h and decreases S203 phosphorylation after 6 h (a, striped columns). DEX+SERT co-treatment does not change S203 phosphorylation at 1 h, but increases S203 phosphorylation at 6 h and 12 h (a, squared columns). These effects of DEX+SERT are also abolished by H89. SERT does not change GR phosphorylation at S211 (b, white columns). DEX increases S211 phosphorylation after 1, 6 and 12 h (b, striped columns). DEX+SERT co-treatment induces hyperphosphorylation at S211, which exceeds the phosphorylation induced by DEX alone, and this effect is also abolished by H89 (b, squared columns). Western blots for the GR-phosphoisoforms S203 and S211 are shown after 1 h of treatment (a,b). SERT increased the expression of p27Kip1 (c) and p57Kip2 (e). The increased expression of p27Kip1 and p57Kip2 after 12 h of sertraline treatment was abolished by RU486 (d,f). SERT increased GR transactivation after 12 h of treatment (g), an effect which was abolished by H89 (h). S203 and S211 phosphorylation are normalized to the expression of the unphosphorylated, total GR protein. Gene expression of p27Kip1 and p57Kip2 is normalized to the housekeeping genes ACTB, GAPDH and B2M. All data are mean±s.e.m. *P<0.05, **P<0.01 and ***P<0.001 compared with the respective vehicle.
Similar differential effects of the three treatment conditions are present at the S211 phosphosite. Sertraline alone does not induce changes in S211 phosphorylation at any time point (Figure 5b; white columns). In contrast, dexamethasone strongly induces phosphorylation at S211 at 1, 6 and 12 h of treatment (by ninefold, twofold and threefold, respectively; striped columns). Interestingly, co-treatment with sertraline and dexamethasone induces hyperphosphorylation at S211 to levels higher than those induced by dexamethasone alone (12-fold at 1 h, 2.5-fold at 6 h, 4-fold at 12 h; squared columns). This hyperphosphorylation at S211 is also counteracted by H89, confirming again that the effect of sertraline on GR phosphorylation is indeed mediated by PKA signaling (Figure 5b).
No significant treatment effects were observed for phosphorylation at the S226 phosphosite (Supplementary Figure 9).
Antidepressants induce GR transactivation and enhance expression of the GR-dependent genes p27Kip1 and p57Kip2
To further elucidate the molecular mechanisms underlying the differential effects of sertraline, dexamethasone, and sertraline and dexamethasone co-treatment on neurogenesis, we compared the effects of these three treatments on gene expression of: the CDK2 inhibitors, p27Kip1, p57Kip2 and p21Cip1; the cell cycle-regulating genes p53, CCNA2, CCND1 and HDM2; and the glucocorticoid- and antidepressant-responsive genes, FKBP5, SGK1, FOXO1, GADD45B, GR, GRα, p11 and ß-arrestin-2. These genes were chosen because they have all recently been implicated in antidepressant drug action and neurogenesis.35, 36, 37, 38, 39, 12, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49 We conducted these experiments during cell proliferation, because, as described above, all effects (including those on differentiation) were initiated during the proliferation phase.
Gene expression analysis revealed that the three treatment conditions indeed activate distinct gene expression profiles. Details of the gene expression profiles and gene interactions for the three treatment conditions (at 6, 12, 24, 48 and 72 h) are described in Figure 5, Supplementary Results and Supplementary Figures 7 and 8. Here, we summarize the main findings.
Sertraline increased expression of the CDK2 inhibitors, p27Kip1 and p57Kip2 (Figures 5c and e), an effect that was particularly prominent after 12 h of treatment. Indeed, at this 12 h time point, sertraline also induced GR transactivation (+20%, Figure 5g; an effect, which was abolished by H89, Figure 5h) and reduced GR-expression (GRα mRNA by −30%, GR mRNA by −40%, GR protein by −50% also blocked by H89; Supplementary Figure 7). The increased expression of p27Kip1 and p57Kip2 was also blocked by RU486 after 12 h of treatment (Figures 5d and f). Moreover, sertraline increased p11 and ß-arrestin-2 (Supplementary Figure 8l,m), and reduced GADD45B gene expression (Supplementary Figure 8j).
In contrast, dexamethasone reduced p27Kip1 and p57Kip2 (Figures 5c and e) and upregulated GADD45B, FKBP5, SGK1 and FOXO1 (Supplementary Figure 8g-j).
The only genes, which were regulated only by sertraline and dexamethasone co-treatment were the cell cycle-promoting genes, CCND1 and HDM2, which were both upregulated by this co-treatment (Supplementary Figure 8e,f).
Discussion
In this study, we identify for the first time that the antidepressant-induced changes in neurogenesis are dependent on the GR. Specifically, the selective serotonin reuptake inhibitor antidepressant, sertraline, increases neuronal differentiation and promotes neuronal maturation of human hippocampal progenitor cells via a GR-dependent mechanism that is associated with GR phosphorylation via PKA signaling. Interestingly, this effect is only observed when sertraline is present during the proliferation phase, and it is accompanied by exit of cells from the cell cycle, as shown by reduced proliferation and increased GR-dependent expression of the CDK2 inhibitors, p27Kip1 and p57Kip2.
We and others have previously shown that antidepressants, including selective serotonin reuptake inhibitors, induce GR nuclear translocation,50, 21, 22 modulate GR-dependent gene transcription,21, 24, 50, 30 and change GR expression in cell culture,51, 52, 53, 54, 55, 19 animals19, 52, 56, 57, 58, 59, 60, 61, 36 and humans.19, 62, 63, 64 In this study, we identify for the first time a critical role for the GR in the antidepressant effects on neurogenesis: first, the sertraline-induced changes in neuronal differentiation and progenitor cell proliferation are abolished by the GR antagonist, RU486; and second, sertraline modulates GR phosphorylation, induces GR transactivation and changes the expression of GR-regulated genes relevant to neurogenesis (see below).
Surprisingly, sertraline (like amitriptyline and clomipramine) increases proliferation only in the presence of glucocorticoids (dexamethasone or cortisol), but not by itself. The strength of our in vitro model is that it separates the direct effects of antidepressants on progenitor cells from the indirect effects, which occur in vivo. Notably, increased hippocampal cell proliferation by antidepressant treatment in animals occurs always in the context of endogenous glucocorticoid production—which is not present in our in vitro system, unless when cells are co-treated. Indeed, some animal studies have reported that administration of exogenous glucocorticoids, or circadian fluctuations of endogenous glucocorticoids, are required for increased hippocampal cell proliferation upon antidepressant treatment.12, 17 Moreover, a study on human post-mortem brain tissue has found that antidepressants increase the number of neural progenitor cells in patients with major depression to levels above those present in controls, and depressed patients are generally characterized by elevated endogenous levels of glucocorticoids.4 Taken together, our in vitro findings and the above mentioned in vivo studies indicate a pivotal role of the GR in the effects of antidepressants on hippocampal progenitor cells. It is important, however, to emphasize that our findings also demonstrate that the molecular processes that lead to increased neuronal differentiation are activated directly by antidepressants alone and do not require glucocorticoids. Our data suggest that such complex GR-dependent regulation of cell fate is the result of differential GR phosphorylation and GR-dependent gene expression by antidepressants, glucocorticoids and by antidepressant and glucocorticoid co-treatment. Indeed, different GR phosphoisoforms have been reported to selectively occupy promoters of different GR target genes, which may therefore explain the diverse GR-dependent effects on gene expression and neurogenesis that we observed in our study (see below).26, 65, 66, 67, 68
Further investigation of the molecular signaling mechanisms, which underlie the effect of antidepressants on neurogenesis showed that sertraline increases GR transactivation after 12 h of treatment. At this time-point, expression of the CDK2 inhibitors, p27Kip1 and p57Kip2, was strongly increased, and this increase was blocked by RU486. p27Kip1 and p57Kip2 are GR-target genes that promote cell cycle exit and increase neuronal differentiation in the developing rat brain.39, 38, 69 Therefore, increased expression of these genes is consistent with our findings (discussed above), which show that sertraline decreases proliferation and initiates neuronal differentiation already during the proliferation phase. It is also of note that sertraline decreased GR expression (both at the mRNA and the protein level) at the same time point at which it induced GR transactivation. This is in line with reports from the literature in both cell culture and animal studies,23, 51, 61 and probably results from the increased GR transactivation.19, 23, 24 Finally, sertraline increased the expression of p11 and ß-arrestin-2, which have both recently been implicated in the antidepressant effects on neurogenesis in vivo.12, 40, 42, 43 Interestingly, the cell cycle-promoting genes, CCND1 and HDM2, were upregulated only by sertraline and dexamethasone co-treatment, the only condition which increases cell proliferation. In contrast, dexamethasone increased expression of the cell cycle-inhibiting genes, FOXO1 and GADD45B, which may explain the reduced cell proliferation and reduced neuronal differentiation with this treatment. See also Supplementary Discussion (‘Gene interactions') and Supplementary Figure 13 for further discussion and a summary model.
Previous studies have proposed that antidepressants modulate GR-function by cAMP/PKA signaling.30, 31 Our data confirm and extend this model, by showing that the GR-dependent changes in neurogenesis upon sertraline treatment are mediated by PKA. Specifically, the PDE4-inhibitor, rolipram, which increases cAMP levels and thus leads to higher PKA activity, mimics and enhances the effects of sertraline on neuronal differentiation and progenitor proliferation. Accordingly, the PKA inhibitor, H89, inhibits the effects of sertraline. Furthermore, the sertraline-induced changes in GR phosphorylation and GR transactivation are also abolished by inhibition of PKA. One potential mechanism by which sertraline may cause this PKA activation is by liberating G-protein alpha(s) subunits from membrane-associated lipid rafts.70, 71, 72, 73 The subsequent cAMP production and PKA activation may then ultimately induce the GR phosphorylation, GR transactivation and GR-dependent gene transcription, which we observed in our study.19, 66, 74, 25, 26 Interestingly, such a mechanism may be independent of monoamine reuptake systems, and could thus explain how neurogenesis is modulated by antidepressants of different pharmacological classes3 (and, in our experiments, by both the selective serotonin reuptake inhibitor, sertraline and the tricyclic antidepressants, amitriptyline and clomipramine).
In conclusion, our data suggest that anti-depressants regulate differentiation and proliferation of human hippocampal progenitor cells by a GR-dependent mechanism that requires PKA signaling. This is accompanied by changes in GR phosphorylation and GR-dependent gene transcription, including increased expression of p27Kip1 and p57Kip2. Of note, our findings point toward a complex regulation of neurogenesis by antidepressants, with different GR-dependent mechanisms that lead to enhanced cell proliferation without changes in neuronal differentiation, or enhanced neuronal differentiation in the presence of decreased cell proliferation. It will be important for future studies to elucidate the impact of progenitor proliferation vs neuronal differentiation on depression and other mental illnesses. Moreover, modulation of GR phosphorylation and GR-dependent gene transcription may represent a future strategy of antidepressant drug treatment to overcome neurogenesis-related disturbances in depression.
Acknowledgments
This work was funded by a studentship to C Anacker from the NIHR ‘Biomedical Research Centre for Mental Health', Institute of Psychiatry and South London and Maudsley NHS Foundation Trust, London, UK; a Clinician Scientist Fellowship from the Medical Research Council, UK; and a grant from the Commission of European Communities 7th Framework Programme Collaborative Project Grant Agreement n 22963 (Mood Inflame) to CM Pariante. S Thuret is supported in part by a grant from Research Councils UK. MJ Garabedian is supported in part by a Grant from the NIH (R01MH086651).
Professor Jack Price acted as a consultant and received payment from ReNeuron Group within the last 2 years.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
Supplementary Material
References
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, et al. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, van Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Bannerman D, Flint J. Chronic fluoxetine treatment alters behavior, but not adult hippocampal neurogenesis, in BALB/cJ mice. Mol Psychiatry. 2008;13:119–121. doi: 10.1038/sj.mp.4002104. [DOI] [PubMed] [Google Scholar]
- Tfilin M, Sudai E, Merenlender A, Gispan I, Yadid G, Turgeman G. Mesenchymal stem cells increase hippocampal neurogenesis and counteract depressive-like behavior. Mol Psychiatry. 2009;15:1164–1175. doi: 10.1038/mp.2009.110. [DOI] [PubMed] [Google Scholar]
- Montero-Pedrazuela A, Venero C, Lavado-Autric R, Fernandez-Lamo I, Garcia-Verdugo JM, Bernal J, et al. Modulation of adult hippocampal neurogenesis by thyroid hormones: implications in depressive-like behavior. Mol Psychiatry. 2006;11:361–371. doi: 10.1038/sj.mp.4001802. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, et al. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, et al. The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling Mol Psychiatry 200914764–773.739. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, Marsteller D, Mendez I, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Kuruba R, Shuai B, Shetty AK. Predictable chronic mild stress improves mood, hippocampal neurogenesis and memory. Mol Psychiatry. 2009;16:171–183. doi: 10.1038/mp.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol Psychiatry. 2010;15:1152–1163. doi: 10.1038/mp.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Lucassen PJ, Stumpel MW, Wang Q, Aronica E. Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology. 2010;58:940–949. doi: 10.1016/j.neuropharm.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Herbert J. Stimulation of neurogenesis in the hippocampus of the adult rat by fluoxetine requires rhythmic change in corticosterone. Biol Psychiatry. 2006;59:619–624. doi: 10.1016/j.biopsych.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Anacker C, Zunszain PA, Carvalho LA, Pariante CM. The glucocorticoid receptor: pivot of depression and of antidepressant treatment. Psychoneuroendocrinology. 2010;36:415–425. doi: 10.1016/j.psyneuen.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM.Glucocorticoids, cytokines and brain abnormalities in depression Prog Neuropsychopharmacol Biol Psychiatry 2010[e-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- Pariante CM, Pearce BD, Pisell TL, Owens MJ, Miller AH. Steroid-independent translocation of the glucocorticoid receptor by the antidepressant desipramine. Mol Pharmacol. 1997;52:571–581. doi: 10.1124/mol.52.4.571. [DOI] [PubMed] [Google Scholar]
- Funato H, Kobayashi A, Watanabe Y. Differential effects of antidepressants on dexamethasone-induced nuclear translocation and expression of glucocorticoid receptor. Brain Res. 2006;1117:125–134. doi: 10.1016/j.brainres.2006.08.029. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Hye A, Williamson R, Makoff A, Lovestone S, Kerwin RW. The antidepressant clomipramine regulates cortisol intracellular concentrations and glucocorticoid receptor expression in fibroblasts and rat primary neurones. Neuropsychopharmacology. 2003;28:1553–1561. doi: 10.1038/sj.npp.1300195. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Kim RB, Makoff A, Kerwin RW. Antidepressant fluoxetine enhances glucocorticoid receptor function in vitro by modulating membrane steroid transporters. Br J Pharmacol. 2003;139:1111–1118. doi: 10.1038/sj.bjp.0705357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Frederick J, Garabedian MJ. Deciphering the phosphorylation ‘code' of the glucocorticoid receptor in vivo. J Biol Chem. 2002;277:26573–26580. doi: 10.1074/jbc.M110530200. [DOI] [PubMed] [Google Scholar]
- Galliher-Beckley AJ, Williams JG, Collins JB, Cidlowski JA. Glycogen synthase kinase 3beta-mediated serine phosphorylation of the human glucocorticoid receptor redirects gene expression profiles. Mol Cell Biol. 2008;28:7309–7322. doi: 10.1128/MCB.00808-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe S, Mochizuki K, Goda T. De-phosphorylation of GR at Ser203 in nuclei associates with GR nuclear translocation and GLUT5 gene expression in Caco-2 cells. Arch Biochem Biophys. 2008;475:1–6. doi: 10.1016/j.abb.2008.03.036. [DOI] [PubMed] [Google Scholar]
- Orti E, Hu LM, Munck A. Kinetics of glucocorticoid receptor phosphorylation in intact cells. Evidence for hormone-induced hyperphosphorylation after activation and recycling of hyperphosphorylated receptors. J Biol Chem. 1993;268:7779–7784. [PubMed] [Google Scholar]
- Mason SA, Housley PR. Site-directed mutagenesis of the phosphorylation sites in the mouse glucocorticoid receptor. J Biol Chem. 1993;268:21501–21504. [PubMed] [Google Scholar]
- Miller AH, Vogt GJ, Pearce BD. The phosphodiesterase type 4 inhibitor, rolipram, enhances glucocorticoid receptor function. Neuropsychopharmacology. 2002;27:939–948. doi: 10.1016/S0893-133X(02)00381-0. [DOI] [PubMed] [Google Scholar]
- Rangarajan PN, Umesono K, Evans RM. Modulation of glucocorticoid receptor function by protein kinase A. Mol Endocrinol. 1992;6:1451–1457. doi: 10.1210/mend.6.9.1435789. [DOI] [PubMed] [Google Scholar]
- Eickelberg O, Roth M, Lorx R, Bruce V, Rudiger J, Johnson M, et al. Ligand-independent activation of the glucocorticoid receptor by beta2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J Biol Chem. 1999;274:1005–1010. doi: 10.1074/jbc.274.2.1005. [DOI] [PubMed] [Google Scholar]
- Blendy JA. The role of CREB in depression and antidepressant treatment. Biol Psychiatry. 2006;59:1144–1150. doi: 10.1016/j.biopsych.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–758. doi: 10.1016/S0140-6736(09)60046-5. [DOI] [PubMed] [Google Scholar]
- Chesnokova V, Pechnick RN. Antidepressants and Cdk inhibitors: releasing the brake on neurogenesis. Cell Cycle. 2008;7:2321–2326. doi: 10.4161/cc.6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechnick RN, Zonis S, Wawrowsky K, Pourmorady J, Chesnokova V. p21Cip1 restricts neuronal proliferation in the subgranular zone of the dentate gyrus of the hippocampus. Proc Natl Acad Sci USA. 2008;105:1358–1363. doi: 10.1073/pnas.0711030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry. 2010;15:80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MH, Mavila N, Wang WH, Vega Alvarez S, Hall MC, Andrisani OM. Time-dependent activation of Phox2a by the cyclic AMP pathway modulates onset and duration of p27Kip1 transcription. Mol Cell Biol. 2009;29:4878–4890. doi: 10.1128/MCB.01928-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye W, Mairet-Coello G, Pasoreck E, Dicicco-Bloom E. Patterns of p57Kip2 expression in embryonic rat brain suggest roles in progenitor cell cycle exit and neuronal differentiation. Dev Neurobiol. 2009;69:1–21. doi: 10.1002/dneu.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland M, Warner-Schmidt J, Greengard P, Svenningsson P. Neurogenic effects of fluoxetine are attenuated in p11 (S100A10) knockout mice. Biol Psychiatry. 2010;67:1048–1056. doi: 10.1016/j.biopsych.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Golan M, Schreiber G, Avissar S. Antidepressants, beta-arrestins and GRKs: from regulation of signal desensitization to intracellular multifunctional adaptor functions. Curr Pharm Des. 2009;15:1699–1708. doi: 10.2174/138161209788168038. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Chergui K, Rachleff I, Flajolet M, Zhang X, El Yacoubi M, et al. Alterations in 5-HT1B receptor function by p11 in depression-like states. Science. 2006;311:77–80. doi: 10.1126/science.1117571. [DOI] [PubMed] [Google Scholar]
- Golan M, Schreiber G, Avissar S. Antidepressants increase beta-arrestin2 ubiquitinylation and degradation by the proteasomal pathway in C6 rat glioma cells. J Pharmacol Exp Ther. 2010;332:970–976. doi: 10.1124/jpet.109.160218. [DOI] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- van Gemert NG, Meijer OC, Morsink MC, Joels M. Effect of brief corticosterone administration on SGK1 and RGS4 mRNA expression in rat hippocampus. Stress. 2006;9:165–170. doi: 10.1080/10253890600966169. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Fernandez de Mattos S, van der Horst A, Klompmaker R, Kops GJ, Lam EW, et al. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22:7842–7852. doi: 10.1128/MCB.22.22.7842-7852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XW, Zhan Q, Coursen JD, Khan MA, Kontny HU, Yu L, et al. GADD45 induction of a G2/M cell cycle checkpoint. Proc Natl Acad Sci USA. 1999;96:3706–3711. doi: 10.1073/pnas.96.7.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Oliner JD, Zhan Q, Fornace AJ, Jr, Vogelstein B, Kastan MB. Interactions between p53 and MDM2 in a mammalian cell cycle checkpoint pathway. Proc Natl Acad Sci USA. 1994;91:2684–2688. doi: 10.1073/pnas.91.7.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Makoff A, Lovestone S, Feroli S, Heyden A, Miller AH, et al. Antidepressants enhance glucocorticoid receptor function in vitro by modulating the membrane steroid transporters. Br J Pharmacol. 2001;134:1335–1343. doi: 10.1038/sj.bjp.0704368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiske A, Jesberg J, Krieg JC, Vedder H. Differential effects of antidepressants on glucocorticoid receptors in human primary blood cells and human monocytic U-937 cells. Neuropsychopharmacology. 2003;28:807–817. doi: 10.1038/sj.npp.1300056. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Omori K, Suzukawa J, Fujiseki Y, Kinoshita T, Inagaki C. Long-term treatment with antidepressants increases glucocorticoid receptor binding and gene expression in cultured rat hippocampal neurones. J Neuroendocrinol. 1999;11:887–895. doi: 10.1046/j.1365-2826.1999.00405.x. [DOI] [PubMed] [Google Scholar]
- Vedder H, Bening-Abu-Shach U, Lanquillon S, Krieg JC. Regulation of glucocorticoid receptor-mRNA in human blood cells by amitriptyline and dexamethasone. J Psychiatr Res. 1999;33:303–308. doi: 10.1016/s0022-3956(99)00006-0. [DOI] [PubMed] [Google Scholar]
- Pepin MC, Beaulieu S, Barden N. Antidepressants regulate glucocorticoid receptor messenger RNA concentrations in primary neuronal cultures. Brain Res Mol Brain Res. 1989;6:77–83. doi: 10.1016/0169-328x(89)90031-4. [DOI] [PubMed] [Google Scholar]
- Lai M, McCormick JA, Chapman KE, Kelly PA, Seckl JR, Yau JL. Differential regulation of corticosteroid receptors by monoamine neurotransmitters and antidepressant drugs in primary hippocampal culture. Neuroscience. 2003;118:975–984. doi: 10.1016/s0306-4522(03)00038-1. [DOI] [PubMed] [Google Scholar]
- Johansson IM, Bjartmar L, Marcusson J, Ross SB, Seckl JR, Olsson T. Chronic amitriptyline treatment induces hippocampal NGFI-A, glucocorticoid receptor and mineralocorticoid receptor mRNA expression in rats. Brain Res Mol Brain Res. 1998;62:92–95. doi: 10.1016/s0169-328x(98)00243-5. [DOI] [PubMed] [Google Scholar]
- Frechilla D, Otano A, Del Rio J. Effect of chronic antidepressant treatment on transcription factor binding activity in rat hippocampus and frontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:787–802. doi: 10.1016/s0278-5846(98)00040-2. [DOI] [PubMed] [Google Scholar]
- Przegalinski E, Budziszewska B. The effect of long-term treatment with antidepressant drugs on the hippocampal mineralocorticoid and glucocorticoid receptors in rats. Neurosci Lett. 1993;161:215–218. doi: 10.1016/0304-3940(93)90297-x. [DOI] [PubMed] [Google Scholar]
- Yau JL, Noble J, Thomas S, Kerwin R, Morgan PE, Lightman S, et al. The antidepressant desipramine requires the ABCB1 (Mdr1)-type p-glycoprotein to upregulate the glucocorticoid receptor in mice. Neuropsychopharmacology. 2007;32:2520–2529. doi: 10.1038/sj.npp.1301389. [DOI] [PubMed] [Google Scholar]
- Yau JL, Noble J, Hibberd C, Rowe WB, Meaney MJ, Morris RG, et al. Chronic treatment with the antidepressant amitriptyline prevents impairments in water maze learning in aging rats. J Neurosci. 2002;22:1436–1442. doi: 10.1523/JNEUROSCI.22-04-01436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JL, Noble J, Hibberd C, Seckl JR. Short-term administration of fluoxetine and venlafaxine decreases corticosteroid receptor mRNA expression in the rat hippocampus. Neurosci Lett. 2001;306:161–164. doi: 10.1016/s0304-3940(01)01890-0. [DOI] [PubMed] [Google Scholar]
- Calfa G, Kademian S, Ceschin D, Vega G, Rabinovich GA, Volosin M. Characterization and functional significance of glucocorticoid receptors in patients with major depression: modulation by antidepressant treatment. Psychoneuroendocrinology. 2003;28:687–701. doi: 10.1016/s0306-4530(02)00051-3. [DOI] [PubMed] [Google Scholar]
- Carvalho LA, Juruena MF, Papadopoulos AS, Poon L, Kerwin R, Cleare AJ, et al. Clomipramine in vitro reduces glucocorticoid receptor function in healthy subjects but not in patients with major depression. Neuropsychopharmacology. 2008;33:3182–3189. doi: 10.1038/npp.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho LA, Garner BA, Dew T, Fazakerley H, Pariante CM. Antidepressants, but not antipsychotics, modulate GR function in human whole blood: an insight into molecular mechanisms. Eur Neuropsychopharmacol. 2010;20:379–387. doi: 10.1016/j.euroneuro.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blind RD, Garabedian MJ. Differential recruitment of glucocorticoid receptor phospho-isoforms to glucocorticoid-induced genes. J Steroid Biochem Mol Biol. 2008;109:150–157. doi: 10.1016/j.jsbmb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Dang T, Blind RD, Wang Z, Cavasotto CN, Hittelman AB, et al. Glucocorticoid receptor phosphorylation differentially affects target gene expression. Mol Endocrinol. 2008;22:1754–1766. doi: 10.1210/me.2007-0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Calhoun WJ. Differential regulation of the transcriptional activity of the glucocorticoid receptor through site-specific phosphorylation. Biologics. 2008;2:845–854. doi: 10.2147/btt.s3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JC, Jewell CM, Bodwell JE, Munck A, Sar M, Cidlowski JA. Mouse glucocorticoid receptor phosphorylation status influences multiple functions of the receptor protein. J Biol Chem. 1997;272:9287–9293. doi: 10.1074/jbc.272.14.9287. [DOI] [PubMed] [Google Scholar]
- Lee YS, Liu F, Segil N. A morphogenetic wave of p27Kip1 transcription directs cell cycle exit during organ of Corti development. Development. 2006;133:2817–2826. doi: 10.1242/dev.02453. [DOI] [PubMed] [Google Scholar]
- Donati RJ, Rasenick MM. Chronic antidepressant treatment prevents accumulation of gsalpha in cholesterol-rich, cytoskeletal-associated, plasma membrane domains (lipid rafts) Neuropsychopharmacology. 2005;30:1238–1245. doi: 10.1038/sj.npp.1300697. [DOI] [PubMed] [Google Scholar]
- Donati RJ, Dwivedi Y, Roberts RC, Conley RR, Pandey GN, Rasenick MM. Postmortem brain tissue of depressed suicides reveals increased Gs alpha localization in lipid raft domains where it is less likely to activate adenylyl cyclase. J Neurosci. 2008;28:3042–3050. doi: 10.1523/JNEUROSCI.5713-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothdurfter C, Tanasic S, Di Benedetto B, Rammes G, Wagner EM, Kirmeier T, et al. Impact of lipid raft integrity on 5-HT(3) receptor function and its modulation by antidepressants. Neuropsychopharmacology. 2010;35:1510–1519. doi: 10.1038/npp.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisensamer B, Uhr M, Meyr S, Gimpl G, Deiml T, Rammes G, et al. Antidepressants and antipsychotic drugs colocalize with 5-HT3 receptors in raft-like domains. J Neurosci. 2005;25:10198–10206. doi: 10.1523/JNEUROSCI.2460-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismaili N, Garabedian MJ. Modulation of glucocorticoid receptor function via phosphorylation. Ann N Y Acad Sci. 2004;1024:86–101. doi: 10.1196/annals.1321.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.