Abstract
For the past 10 years, number of evidence has shown that activation of complement cascade has been associated with age-related macular degeneration (AMD). The genome wide association study in American population with dominantly dry-type AMD has revealed strong association with single nucleotide polymorphism (SNP) of complement genes. Protein composition of drusen, a deposit observed in sub-retinal space between Bruch’s membrane and retinal pigment epithelial (RPE), contains active complement molecules in human and monkey. These evidences have leaded us to consider the possibility of suppressing complement cascade in the retina to delay or reverse the onset of AMD. To test is hypothesis we used the C3 inhibitor Compstatin on primate model with early-onset macular degeneration which develop drusen in less than 2 years after birth. Our preliminary result showed drusen disappearance after 6 months of intravitreal injection.
1 AMD and Association of Complement Related Genes
The most prevalent eye disease for elderly Europeans and Americans is AMD. AMD is a blinding disorder characterized by a marked decrease in central vision associated with retinal pigment epithelial (RPE) atrophy with or without choroidal neovascularization (CNV). The non-neovascular type is called the dry-type AMD and includes more than 80% of the cases, and the neovascular type is called the wet-type AMD which is progressive with a higher probability of blindness. In some cases of CNV, the new vessels penetrate Bruch’s membrane and pass into the sub-retinal space. The progressive impairment of the RPE and damage to Bruch’s membrane and choriocapillaris results in retinal atrophy and photoreceptor dysfunction.
Genetic, behavioral, and environmental factors are believed to be involved for the onset of this disease. The prevalence of AMD differs considerably among the different ethnic groups, but the incidence increases with age in all groups. Epidemiological studies have shown that genetic factor play critical role for AMD. However, only a small proportion of the families with AMD show Mendelian inheritance, and the majority of the individuals inherit AMD in a complex multi-gene pattern. With the help of the haplotype marker project (HapMap Project), genome wide scanning has identified at least 13 loci linked to AMD on different chromosomes (Iyengar et al. 2004; Schick et al. 2003; Majewski et al. 2003). Other risk factors such as cigarette smoking, obesity, hypertension, and atherosclerosis are also associated with the disease.
Recently, a polymorphism of complement factor H (CFH) gene (Y402H) was shown to be associated with an increased risk for AMD (Klein et al. 2005; Edwards et al. 2005; Haines et al. 2005; Hageman et al. 2005). These results were confirmed in many of the countries with large Caucasian populations but not in Japan (Okamoto et al. 2006; Gotoh et al. 2006). This gene is located on chromosome 1q25–31 where one of the candidate loci was identified by whole genome association studies by linkage markers. Another recent study reported that a haplotype association of tandemly located complement 2 and factor B (Gold et al. 2006) was protective and C3 (Yates et al. 2007) as risk for AMD. HTRA1, a serine protease 11 was recently discovered to be strongly associated with AMD (Yang et al. 2006; Dewan et al. 2006). Unlike the CFH, our study shows strongly association with this gene for Japanese AMD patients (Yoshida et al. 2007). This difference of gene association is probably related to the difference of AMD type dominant in each country. Our genome wide association study on Japanese population with typical wet-type AMD and polypoidal choroidal vasculopathy (PCV) shows significant association at p-value of 10−14 and 10−7 respectively for ARMS2/Htra1 locus. However when much lower associated SNPs of CFH or C3 or combined the odds ratio significantly increased (Goto et al. 2009)
2 Activated Complement Component in Drusen
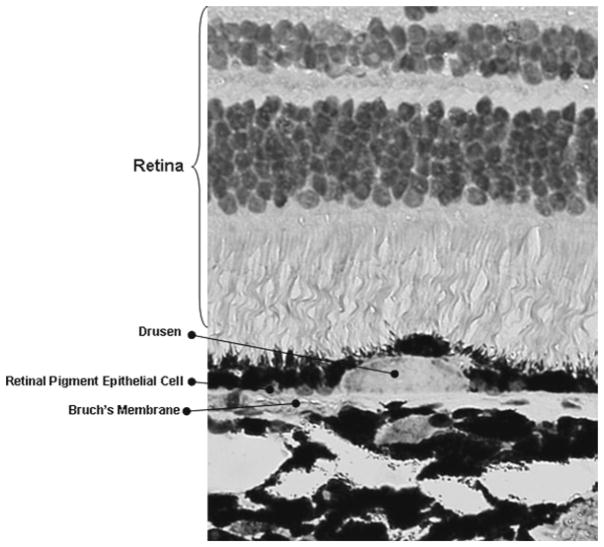
The early stage of the dry-type AMD is characterized by thickening of Bruch’s membrane, aggregation of pigment granules, and increasing numbers of drusen. Drusen are small yellowish-white deposits that are composed of lipids, proteins, glycoproteins, and glycosaminoglycans. They accumulate in the extracellular space and the inner aspects of Bruch’s membrane. Drusen are not directly associated with visual loss but represent a risk factor for dry-type AMD. The classification of hard and soft drusen is based on their size, shape, and color; hard drusen are yellowish with diameters <50 μm and are found in eyes that are less likely to progress to advanced stages of the disease, while soft drusen are darker yellow and larger in size, and are found in eyes more likely to progress to more advanced stages of AMD.
Both immunohistochemistry and proteomic techniques have shown that drusen are composed of molecules that mediate inflammatory and immune processes (Russell et al. 2000; Mullins et al. 2000). These molecules include components of the complement pathway and modulators of complement activation, viz., vitronectin, clusterin, membrane cofactor protein, and complement receptor-1. In addition, molecules triggering inflammation, amyloid P component, α1-antitrypsin, and apolipo-protein E, were identified in drusen. Cellular debris from macrophages, RPE cells, and choroidal dendritic cells has been also identified in drusen. Additional proteins such as crystallins, EEFMP1, and amyloid-beta have been found in drusen. The presence of immunoreactive proteins and the oxidative modifications of many proteins in drusen imply that both oxidation and immune functions are involved in the pathogenesis of AMD. Finding of these molecules suggest that complement activation triggers innate immune responses in the subretinal space.
3 Cynomolgus Monkey with Early-Onset Macular Degeneration
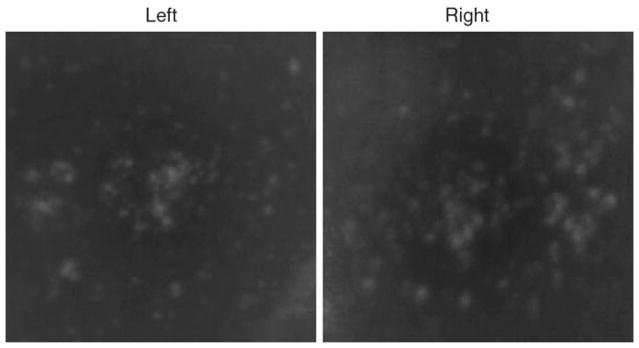
Over the past years non-human primates with well-defined fovea has been the target for AMD research. A monkey with macular degeneration was first described by Stafford et al. in 1974. They reported that 6.6% of the elderly monkeys they examined showed pigmentary disorders and drusen-like spots (Stafford et al. 1984). We also observed at approximately the same rate of disorder in elderly cynomolgus monkeys in the Philippines primate facility (SICONBREC) (Umeda et al. 2005a, b). El-Mofty et al. (1978) reported that the incidence of maculopathy was 50% in a colony of rhesus monkeys at the Caribbean Primate Research Center of the University of Puerto Rico. In 1986, a single cynomolgus monkey (Macaca fascicularis) with large number of small drusen in the macula was found in Tsukuba Primate Research Center at Tsukuba City, Japan (Nicolas et al. 1996a, b; Suzuki et al. 2003). This single affected monkey has been bred to a large pedigree of more than 300 monkeys (Fig. 1). Drusen are observed in the macula as early as 2 years after birth, and the number increase and spread toward the peripheral retina throughout life (Figs. 2–3). Histological abnormalities of the retina and abnormal electroretinogram (ERG) were observed in sever case showing physiological dysfunction of the macula.
Fig. 1.
Fundus photography of affected monkey at TPRC
Fig. 2.
Fundus photograph of affected monkey showing accumulation of drusen in macula of both eyes
Fig. 3.
Retinal histological section of affected monkey showing the accumulation of drusen
Immunohistochemical and proteomic analyses of the drusen from these monkeys showed that the drusen were very similar to those in other monkeys with aged macular degeneration sporadically found in older monkeys and also with human drusen (Umeda et al. 2005a, b; Ambati et al. 2003). These observations have shown that TPRC monkeys produce drusen that are biochemically similar to those in human AMD patients, but the development of the drusen occurs at an accelerated rate.
More than 240 loci are being investigated to try to identify the disease causing gene and to understand the biological pathways leading to complement activation. Simultaneously, we have been studying a colony of aged monkeys in SICONBREC, which develop drusen after 15 years of birth. Drusen components of these sporadically found affected monkeys were compared with human and TPRC monkeys by immunohistochemistry and proteomic analysis using ion spray mass spectrometer. Significant finding was that drusen contained protein molecules that mediate inflammatory and immune processes. These include immunoglobulins, components of complement pathway, and modulators for complement activation (e.g., vitronectin, clusterin, membrane cofactor protein, and complement receptor-1), molecules involved in the acute-phase response to inflammation (e.g., amyloid P component, α1-antitrypsin, and apolipoprotein E), major histocompatibility complex class II antigens, and HLA-DR antigens (Umeda et al. 2005a, b). Cellular components have also been identified in drusen, including RPE debris, lipofuscin, and melanin, as well as processes of choroidal dendritic cells, which contribute to the inflammatory response. The presence of immunoreactive proteins and oxidative modified proteins implicate both oxidation and immune functions in the pathogenesis of affected monkeys.
4 Suppression and Reversal of Drusen Formation by Compstatin
To test the effect of long term suppression of complement activation in the retina, an cyclic analogue (Ac-I[CV(1MeW)QDWGAHRC]T-NH2) of the small cyclic synthetic peptide compstatin (Katragadda et al. 2006) was intravitreally injected into eight affected monkeys at different dose and intervals. Four affected monkeys were injected at 1 mg dose at 1 month interval while other four affected monkeys at 50 μg dose at 1 week interval. Both 1 mg or 50 μg dose were dissolved in 100 μl of saline solution, filtrated and intravitreally injected using 30G needle.
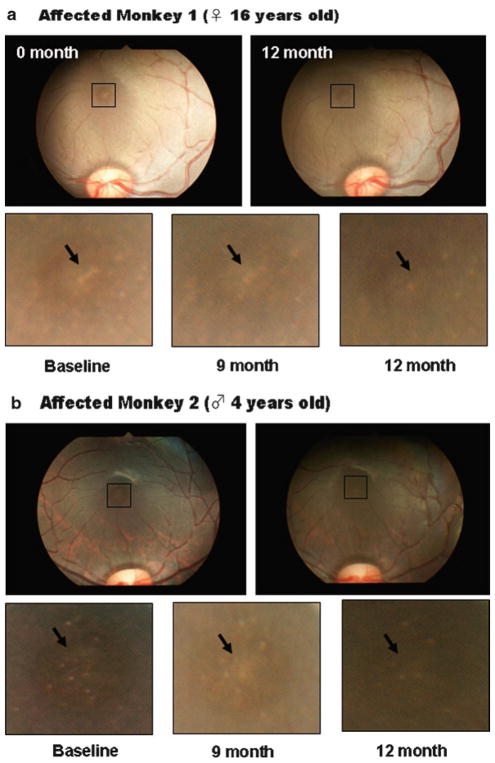
Due to the unique molecular characteristic of compstatin, immediately after injection, compstatin precipitate and form gel-like structure in the vitreous. This gel will gradually dissolve and disappear after 6 months. Four monkeys injected with 1 mg for 3 months developed significant opacity to the point where fundus observation was impossible. These monkeys were halted for further injection. On the other hand, vitreous of four monkeys with 50 μg dose were clear within 2 days. After 6 months of injection, we noticed diffusion of drusen in the macula and by 9 months partial disappearance of drusen was observed in all four monkeys (Fig. 4). This preliminary experiment has shown reversal of drusen formation by suppression of complement activation. To explain this reversal phenomenon, which has not been observed in untreated affected monkeys, will require further experiments including identification of disease causing gene and pathway leading to complement activation. The information should benefit for development of improved drug and therapy for future AMD prevention.
Fig. 4.
Suppression and reversal of drusen formation after 9 months of intravitreal injection of 50 μl compstatin at 1 week interval
All experimental procedures for this primate study were approved by the Animal Welfare and Animal Care Committee of the TRPC and the Experimental Animal Committee of the National Tokyo Medical Center. The facilities are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International). Monkeys were routinely examined for physical and ophthalmic conditions by veterinarians and by ophthalmologists, respectively.
Acknowledgments
This work was supported by the research grants from the Japanese Ministry of Health, Labour and Welfare (TI) and NIH grants GM-62134, and AI-068730 (JDL). The authors thank Cedric Francois and Paul Olson of Potentia Pharmaceuticals Inc., for providing us the GM grade compstatin.
References
- Ambati J, Anand A, Fernandez S, Sakurai E, Lynn BC, Kuziel WA, Rollins BJ, Ambati BK. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- Dewan A, Liu M, Hartman S, Zhang SS, Liu DT, Zhao C, Tam PO, Chan WM, Lam DS, Snyder M, Barnstable C, Pang CP, Hoh J. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314:989–992. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- El-Mofty A, Gouras P, Eisner G, Balazs EA. Macular degeneration in rhesus monkey (Macaca mulatta) Exp Eye Res. 1978;27:499–502. doi: 10.1016/0014-4835(78)90027-1. [DOI] [PubMed] [Google Scholar]
- Gold B, Merriam JE, Zernant J, Hancox LS, Taiber AJ, Gehrs K, Cramer K, Neel J, Bergeron J, Barile GR, Smith RT, Hageman GS, Dean M, Allikmets R AMD Genetics Clinical Study Group. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006;38:458–462. doi: 10.1038/ng1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto A, Akahori M, Okamoto H, Minami M, Terauchi N, Haruhata Y, Obazawa M, Noda T, Honda M, Mizota A, Tanaka M, Hayashi T, Tanito M, Ogata N, Iwata T. Genetic analysis of typical wet-type age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese population. J Biochem Dis Inform. 2009;2(4):164–175. doi: 10.1007/s12177-009-9047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh N, Yamada R, Hiratani H, Renault V, Kuroiwa S, Monet M, Toyoda S, Chida S, Mandai M, Otani A, Yoshimura N, Matsuda F. No association between complement factor H gene polymorphism and exudative age-related macular degeneration in Japanese. Hum Genet. 2006;120:139–143. doi: 10.1007/s00439-006-0187-0. [DOI] [PubMed] [Google Scholar]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- Iyengar SK, Song D, Klein BE, Klein R, Schick JH, Humphrey J, Millard C, Liptak R, Russo K, Jun G, Lee KE, Fijal B, Elston RC. Dissection of genomewide-scan data in extended families reveals a major locus and oligogenic susceptibility for age-related macular degeneration. Am J Hum Genet. 2004;74:20–39. doi: 10.1086/380912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katragadda M, Magotti P, Sfyroera G, Lambris JD. Hydrophobic effect and hydrogen bonds account for the improved activity of a complement inhibitor, compstatin. J Med Chem. 2006;49:4616–4622. doi: 10.1021/jm0603419. [DOI] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski J, Schultz DW, Weleber RG, Schain MB, Edwards AO, Matise TC, Acott TS, Ott J, Klein ML. Age-related macular degeneration – a genome scan in extended families. Am J Hum Genet. 2003;73:540–550. doi: 10.1086/377701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins RF, Russell SR, Anderson DH, Hageman GS. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000;14:835–846. [PubMed] [Google Scholar]
- Nicolas MG, Fujiki K, Murayama K, Suzuki MT, Mineki R, Hayakawa M, Yoshikawa Y, Cho F, Kanai A. Studies on the mechanism of early onset macular degeneration in cynomolgus (Macaca fascicularis) monkeys. I. Abnormal concentrations of two proteins in the retina. Exp Eye Res. 1996a;62:211–219. doi: 10.1006/exer.1996.0026. [DOI] [PubMed] [Google Scholar]
- Nicolas MG, Fujiki K, Murayama K, Suzuki MT, Shindo N, Hotta Y, Iwata F, Fujimura T, Yoshikawa Y, Cho F, Kanai A. Studies on the mechanism of early onset macular degeneration in cynomolgus monkeys. II. Suppression of metallothionein synthesis in the retina in oxidative stress. Exp Eye Res. 1996b;62:399–408. doi: 10.1006/exer.1996.0045. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Umeda S, Obazawa M, Minami M, Noda T, Mizota A, Honda M, Tanaka M, Koyama R, Takagi I, Sakamoto Y, Saito Y, Miyake Y, Iwata T. Complement factor H polymorphisms in Japanese population with age-related macular degeneration. Mol Vis. 2006;12:156–158. [PubMed] [Google Scholar]
- Russell SR, Mullins RF, Schneider BL, Hageman GS. Location, substructure, and composition of basal laminar drusen compared with drusen associated with aging and age-related macular degeneration. Am J Ophthalmol. 2000;129:205–214. doi: 10.1016/s0002-9394(99)00345-1. [DOI] [PubMed] [Google Scholar]
- Schick JH, Iyengar SK, Klein BE, Klein R, Reading K, Liptak R, Millard C, Lee KE, Tomany SC, Moore EL, Fijal BA, Elston RC. A whole-genome screen of a quantitative trait of age-related maculopathy in sibships from the Beaver Dam Eye Study. Am J Hum Genet. 2003;72:1412–1424. doi: 10.1086/375500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford TJ, Anness SH, Fine BS. Spontaneous degenerative maculopathy in the monkey. Ophthalmology. 1984;91:513–521. doi: 10.1016/s0161-6420(84)34275-0. [DOI] [PubMed] [Google Scholar]
- Suzuki MT, Terao K, Yoshikawa Y. Familial early onset macular degeneration in cynomolgus monkeys (Macaca fascicularis) Primates. 2003;44:291–294. doi: 10.1007/s10329-002-0016-6. [DOI] [PubMed] [Google Scholar]
- Umeda S, Ayyagari R, Allikmets R, Suzuki MT, Karoukis AJ, Ambasudhan R, Zernant J, Okamoto H, Ono F, Terao K, Mizota A, Yoshikawa Y, Tanaka Y, Iwata T. Early-onset macular degeneration with drusen in a cynomolgus monkey (Macaca fascicularis) pedigree: exclusion of 13 candidate genes and loci. Invest Ophthalmol Vis Sci. 2005a;46:683–691. doi: 10.1167/iovs.04-1031. [DOI] [PubMed] [Google Scholar]
- Umeda S, Suzuki MT, Okamoto H, Ono F, Mizota A, Terao K, Yoshikawa Y, Tanaka Y, Iwata T. Molecular composition of drusen and possible involvement of anti-retinal autoimmunity in two different forms of macular degeneration in cynomolgus monkey (Macaca fascicularis) FASEB J. 2005b;19:1683–1685. doi: 10.1096/fj.04-3525fje. [DOI] [PubMed] [Google Scholar]
- Yang Z, Camp NJ, Sun H, Tong Z, Gibbs D, Cameron DJ, Chen H, Zhao Y, Pearson E, Li X, Chien J, Dewan A, Harmon J, Bernstein PS, Shridhar V, Zabriskie NA, Hoh J, Howes K, Zhang K. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314:992–993. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- Yates JRW, Sepp T, Matharu BK, Khan JC, Thurlby DA, Shahid H, Clayton DG, Hayward C, Morgan J, Wright AF, Armbrecht AM, Dhillon B, Deary IJ, Redmond E, Bird AC, Moore AT. Complement C3 variant and the risk of age-related macular degeneration. N Engl J Med. 2007;357:553–561. doi: 10.1056/NEJMoa072618. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Wan AD, Zhang H, Sakamoto R, Okamoto H, Minami M, Obazawa M, Mizota A, Tanaka M, Saito Y, Takagi I, Hoh J, Iwata T. HTRA1 promoter polymorphism predisposes Japanese to AMD. Mol Vis. 2007;13:545–548. [PMC free article] [PubMed] [Google Scholar]