Abstract
Tetrahydrobiopterin (BH4) is a required cofactor for the synthesis of NO by endothelial nitric oxide synthase (eNOS), and endothelial BH4 bioavailability is a critical factor in regulating the balance between NO and superoxide production (eNOS coupling). Biosynthesis of BH4 is determined by the activity of GTP-cyclohydrolase I (GTPCH). However, BH4 levels may also be influenced by oxidation, forming 7,8-dihydrobiopterin (BH2), which promotes eNOS uncoupling. Conversely, dihydrofolate reductase (DHFR) can regenerate BH4 from BH2, but whether DHFR is functionally important in maintaining eNOS coupling remains unclear. To investigate the mechanism by which DHFR might regulate eNOS coupling in vivo, we treated wild-type, BH4-deficient (hph-1), and GTPCH-overexpressing (GCH-Tg) mice with methotrexate (MTX), to inhibit BH4 recycling by DHFR. MTX treatment resulted in a striking elevation in BH2 and a decreased BH4:BH2 ratio in the aortas of wild-type mice. These effects were magnified in hph-1 but diminished in GCH-Tg mice. Attenuated eNOS activity was observed in MTX-treated hph-1 but not wild-type or GCH-Tg mouse lung, suggesting that inhibition of DHFR in BH4-deficient states leads to eNOS uncoupling. Taken together, these data reveal a key role for DHFR in regulating the BH4 vs BH2 ratio and eNOS coupling under conditions of low total biopterin availability in vivo.
Keywords: Tetrahydrobiopterin, eNOS uncoupling, Superoxide, Dihydrofolate reductase, hph-1, Methotrexate, Free radicals
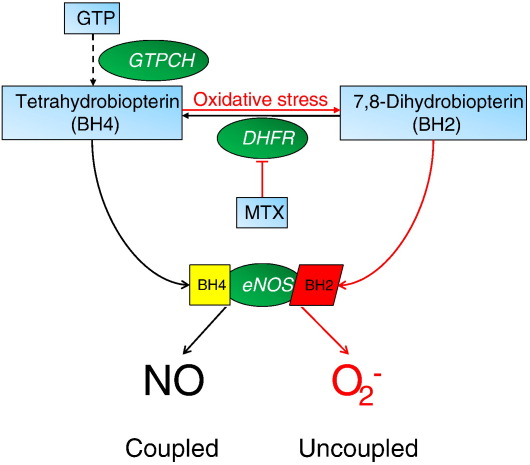
In vascular disease states such as atherosclerosis and diabetes, endothelial nitric oxide (NO) bioactivity is diminished and oxidative stress is increased, resulting in endothelial dysfunction. It has become apparent that enzymatic “coupling” of endothelial NO synthase (eNOS) by the cofactor tetrahydrobiopterin (BH4) plays a key role in maintaining endothelial function. Indeed, the balance between NO and superoxide production by eNOS seems to be determined by the availability of BH4, whereas BH4 oxidation forms 7,8-dihydrobiopterin (BH2), which is inactive for NOS cofactor function and may compete with BH4 for NOS binding and increase eNOS uncoupling [1]. Intracellular biopterin levels are regulated principally by the activity of the de novo biosynthetic pathway (Fig. 1). Guanosine triphosphate cyclohydrolase I (GTPCH; EC 3.5.4.16) catalyzes the formation of dihydroneopterin triphosphate from GTP, and BH4 is generated by two further steps through 6-pyruvoyltetrahydropterin synthase and sepiapterin reductase. GTPCH seems to be the rate-limiting enzyme in BH4 biosynthesis, and overexpression of GTPCH is sufficient to augment BH4 levels in cultured endothelial cells [2]. Electron paramagnetic resonance spectroscopy studies have shown that BH4 both stabilizes and donates electrons to the ferrous–dioxygen complex in the oxygenase domain, as the initiating step of l-arginine oxidation [3–5]. In this reaction BH4 forms the protonated trihydrobiopterin cation radical, which is subsequently reduced by electron transfer from NOS flavins. When BH4 availability is limiting, electron transfer from NOS flavins becomes uncoupled from l-arginine oxidation, eNOS generates superoxide rather than NO, and BH4 becomes oxidized to catalytically incompetent BH2, resulting in a cascade of BH4 loss [1]. Recent studies reveal that BH4 and BH2 bind eNOS with similar affinity and that BH2 can efficiently replace eNOS-bound BH4, resulting in eNOS uncoupling [6]. Indeed, we have previously shown that the relative abundance of eNOS vs BH4, together with the intracellular BH4:BH2 ratio, rather than absolute concentrations of BH4, is the key determinant of eNOS uncoupling [7]. Thus, mechanisms that regulate the BH4:BH2 ratio, independent of overall biopterin levels, may play a role in controlling eNOS coupling that is equally as important as the well-established role of GTPCH in de novo BH4 biosynthesis.
Fig. 1.
Schematic representation of the BH4 recycling pathway and eNOS coupling. BH4 is synthesized de novo from GTP via a series of reactions involving GTPCH, 6-pyruvoyltetrahydropterin synthase, and sepiapterin reductase (− − −). DHFR can regenerate BH4 from BH2 as part of the recycling pathway. Both BH4 and BH2 bind eNOS with equal affinity, however, BH4-bound eNOS produces NO, whereas BH2-bound eNOS promotes uncoupling and eNOS-derived superoxide rather than NO.
In addition to key roles in folate metabolism, dihydrofolate reductase (DHFR; EC 1.5.1.3) can reduce BH2, regenerating BH4 [8,9]. Thus, it is likely that net BH4 cellular bioavailability reflects the balance between de novo BH4 synthesis, loss of BH4 by oxidation to BH2, and regeneration of BH4 by DHFR. In human liver extracts, DHFR has been shown to reduce BH2 to BH4 as part of the salvage pathway for biopterin synthesis [10]. Recent studies have investigated the recycling function of DHFR in cultured endothelial cells. Exposure to angiotensin II down-regulated DHFR expression, decreased BH4 levels, and increased eNOS uncoupling, which was restored by overexpression of DHFR [2]. Pharmacological inhibition of DHFR activity by methotrexate, or genetic knockdown of DHFR by RNA interference, diminished intracellular BH4 and increased BH2 levels resulting in enzymatic uncoupling of eNOS in endothelial cells. In cells expressing eNOS with low biopterin levels, DHFR inhibition or knockdown further diminished the BH4:BH2 ratio and exacerbated eNOS uncoupling [11,12].
Despite these insights from studies in cultured endothelial cells, the extent to which DHFR regulates intracellular BH4 levels and eNOS uncoupling in vivo remains unknown. We previously demonstrated that DHFR activity is critical in regulating BH4:BH2 ratio and hence eNOS coupling in vitro, particularly at low biopterin levels. Accordingly, we compared the effect of DHFR inhibition by methotrexate (MTX) treatment on BH4 levels and eNOS coupling in vivo, using mice with either BH4 deficiency (hph-1) or elevated BH4 levels due to GTPCH overexpression (GCH-Tg) in comparison with wild-type mice. We report that DHFR activity is required to maintain BH4 levels in vivo and that DHFR protein activity is required to maintain efficient coupling of eNOS in the hph-1 mouse in which BH4 levels are deficient. BH4 augmentation, as in the endothelium of the GCH-Tg mouse, protects against these deleterious effects of DHFR inhibition by MTX.
Methods
Experimental animals
The hph-1 mouse, generated by ENU mutagenesis, was used as a model of BH4 deficiency [13]. In these animals, tissue BH4 levels are low because of constitutively reduced expression of GTPCH. GCH-Tg mice, in which human GTPCH transgene overexpression is targeted to the endothelium under control of the murine Tie-2 promoter, were generated in a C57BL/6 background as described previously [14]. Sixteen- to 20-week-old mice were housed in temperature-controlled cages (20 to 22 °C) with a 12-h light–dark cycle and were given free access to water and formulated diets.
Treatment of experimental animals with methotrexate
To avoid nonspecific effects of MTX treatment, three low doses of MTX (2 mg/kg) were injected intraperitoneally (ip) into wild-type, hph-1, or GCH-Tg mice. A gap of 48 h was left between each injection. Control animals were administered phosphate-buffered saline (PBS), again by ip injection at 48-h intervals. MTX was freshly dissolved in sterile phosphate-buffered saline and used within 10 min. All studies involving laboratory animals were conducted in accordance with the UK Home Office Animals (Scientific Procedures) Act 1986.
Western blotting
Tissue lysates were prepared by homogenization in RIPA lysis buffer (20 mmol/L Tris–HCl, 150 mmol/L NaCl, 1 mmol/L Na2EDTA, 1 mmol/L EGTA, 1% Triton, 0.1% SDS, 0.1 sodium deoxycholate, pH 7.4) including a cocktail of protease inhibitors (Roche, West Sussex, UK) and subjected to three freeze–thaw cycles in liquid nitrogen. Western blotting was carried out using standard techniques and anti-eNOS (BD Transduction Laboratories, UK), -DHFR, -GTPCH, and -GAPDH antibodies.
Quantitative real-time RT-PCR
Total RNA was extracted from snap-frozen tissue in 1 ml of Trizol solution by homogenization. Total RNA was quantified with RiboGreen RNA reagent (Invitrogen, UK). RT-PCR was completed with SuperScript II (Invitrogen, USA) using 1 μg total RNA and the TaqMan gene expression system. Quantitative PCR was performed with 50 ng/μl cDNA on an iCycler IQ real-time detection system (Bio-Rad Laboratories, USA) using gene expression assays (Applied Biosystems, USA). Gene expression levels of mouse GCH1 and mouse DHFR were normalized to the housekeeping gene GAPDH.
Measurement of dihydrofolate reductase activity by HPLC
To determine DHFR activity in cells and tissues, we adapted a highly sensitive HPLC method [15]. Briefly, cell lysates or tissue homogenates were incubated with dihydrofolate (50 μmol/L) for 20 min at 37 °C, in a 0.1 mol/L potassium phosphate assay buffer (pH 7.4) containing 200 μmol/L NADPH, 1 mmol/L dithiothreitol (DTT), 0.5 mmol/L KCl, 1 mmol/L EDTA, and 20 mmol/L sodium ascorbate. After 30 min at 37 °C, the reaction was terminated by the addition of 0.2 mol/L trichloroacetic acid. A stabilization solution (200 mg of sodium ascorbate and 30 mg of DTT in 1 ml of water) was then added and samples were stored at − 20 °C until analysis.
The accumulation of the reaction products, tetrahydrofolate (THF) and methyltetrahydrofolate (MeTHF), was then quantified by HPLC using fluorescence detection (295 nm for excitation and 365 nm for emission). Dihydrofolate (DHF) was detectable only at concentrations over 1000 times more than those of both THF and MeTHF.
DHFR knockdown by RNA interference
DHFR “ON-TARGETplus SMARTpool” small interfering RNA (siRNA) was purchased from Dharmacon Thermo Scientific. The siRNAs were used as a pool of four specific siRNA duplexes with the following sequences, as previously described [11]: DHFR duplex 1—AGUUUGAAGUCUACGAGAA, DHFR duplex 2—AGAAAGCACAGUUGGGAUA, DHFR duplex 3—GCCUGUAGGUUGUCUAAUA, and DHFR duplex 4—GCAAGUAAAUGUGUUGUAA.
Murine sEnd.1 endothelial cells were grown in Dulbecco's modified Eagle medium (Gibco Life Technologies, USA) supplemented with glutamine (2 mmol/L), penicillin (100 U/ml), and streptomycin (0.1 mg/ml). Cells were seeded into six-well plates, cultured for 24 h, and then transfected with DHFR-specific siRNA (100 nmol/L) or GAPDH-positive (100 nmol/L) or nonspecific pooled duplex negative control siRNA (100 nmol/L). After 72 h gene silencing was detected by analysis of DHFR protein by Western blotting using DHFR-specific antibodies.
Biopterin quantification by HPLC with electrochemical detection
BH4, BH2, and biopterin (B) levels in tissue homogenates or intact mouse aorta were determined by HPLC followed by electrochemical and fluorescence detection, as previously described [16]. Briefly, either a small piece of lung tissue (approximately 20 mg) or a whole intact aorta was resuspended in 300 μl of PBS (50 mmol/L; pH 7.4), containing dithioerythritol (DTE; 1 mmol/L) and EDTA (100 μmol/L), and either homogenized (for lung tissue) or subjected to three freeze–thaw cycles (for aorta). After centrifugation (15 min at 13,000 rpm and 4 °C), samples were transferred to new, cooled microtubes and precipitated with cold phosphoric acid (1 mol/L), trichloroacetic acid (2 mol/L), and DTE (1 mmol/L). Samples were vigorously mixed and then centrifuged for 15 min at 13,000 rpm and 4 °C. Samples were injected onto an isocratic HPLC system and quantified using sequential electrochemical (Coulochem III; ESA Inc., MA, USA) and fluorescence (Jasco, UK) detection. HPLC separation was performed using a 250-mm, ACE C-18 column (Hichrom, UK) and mobile phase comprising sodium acetate (50 mmol/L), citric acid (5 mmol/L), EDTA (48 μmol/L), and DTE (160 μmol/L) (pH 5.2) (all ultrapure electrochemical HPLC grade), at a flow rate of 1.3 ml/min. Background currents of + 500 and − 50 μA were used for the detection of BH4 on electrochemical cells E1 and E2, respectively. BH2 and biopterin were measured using a Jasco FP2020 fluorescence detector. Quantification of BH4, BH2, and B was done by comparison with authentic external standards and normalized to sample protein content. Total biopterin levels are expressed as the sum of detectable BH4, BH2, and B.
GTPCH activity assay
GTPCH activity was measured in tissue extracts by HPLC analysis after iodine oxidation as described previously [17]. In brief, snap-frozen tissue samples were homogenized and cell pellets were freeze–thawed in lysis buffer (0.1 M Tris, 0.3 M potassium chloride, 2.5 mM EDTA, 100 μM phenylmethylsulfonyl fluoride, pH 7.8). Lysates were incubated for 1 h (37 °C) with 10 mM GTP in the absence of light and then oxidized with potassium iodide/iodine (0.1 mol/L). After deproteination with HCl (1 mol/L), the reaction was stopped by the addition of ascorbic acid (0.1 mol/L) and alkaline phosphatase (16 U/ml). Neopterin content was quantified by isocratic HPLC and fluorescence detection (Jasco, UK). Quantitation of neopterin was carried out by comparison with external standards and normalized for sample protein content. The protein concentration of each sample was measured using the BCA protein assay (Pierce, USA).
Analysis of NO synthesis by eNOS
Cellular NO synthesis by eNOS was assessed in lung homogenates by measuring the conversion of l-[14C]arginine to citrulline with HPLC detection, in the presence and absence of L-NMMA, as previously described [18]. Samples were homogenized in Krebs–Hepes buffer in the presence of the arginase inhibitor Nor-NOHA (5 μmol/L) and the accumulation of [14C]citrulline was assessed after a 30-min incubation with calcium ionophore (1 μmol/L).
Quantification of superoxide production by HPLC
Measurement of 2-hydroxyethidium formation by HPLC was used to quantify superoxide production by methods adapted from those previously described [19,20]. Quantification of the “ethidium” peak was used as an indicator of total reactive oxygen species production [21]. Lung homogenates were prepared in Krebs–Hepes buffer in the presence and absence of the NOS inhibitor L-NAME (100 μmol/L). After 30 min incubation at 37 °C, dihydroethidium (50 μmol/L) was added. After a further 20 min, cells were lysed in ice-cold methanol (1:1 v/v). Hydrochloric acid (100 mmol/L) was added (1:1 v/v) before loading into the autosampler for analysis. All samples were stored in darkened tubes and protected from light at all times. Separation of dihydroethidium, 2-hydroxyethidium, and ethidium was performed using a gradient HPLC system (Jasco) with an ODS3 reverse-phase column (250 mm, 4.5 mm; Hichrom, UK) and quantified using a fluorescence detector set at 510 nm (excitation) and 595 nm (emission). A linear gradient was applied from mobile phase A (0.1% trifluoroacetate (TFA)) to mobile phase B (0.085% TFA in acetonitrile) over 23 min (30% acetonitrile to 50% acetonitrile).
Statistical analysis
Data are presented as means ± SEM. Data were subjected to the Kolmogorov–Smirnov test to determine distribution. Groups were compared using the Mann–Whitney U test for nonparametric data or the Student t test for parametric data. When comparing multiple groups, data were analyzed by analysis of variance with a Bonferroni posttest. A value of P < 0.05 was considered statistically significant.
Results
Characterization of wild-type, hph-1, and GCH-Tg mice
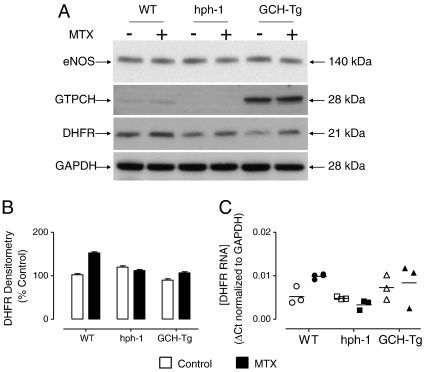
We first set out to characterize the respective mouse models of BH4 deficiency (hph-1) and BH4 augmentation (GCH-Tg), by the determination of the relative expression levels of eNOS, GTPCH, and DHFR proteins in the presence and absence of MTX. Whereas eNOS protein levels were equal, GTPCH protein was increased by over 20-fold in GCH-Tg compared to wild-type mice, and GTPCH protein in the hph-1 mouse was barely detectable. DHFR protein levels were variable in wild-type, hph-1, and GCH-Tg mice, but no significant difference was observed (Figs. 2A and B). Moreover, wild-type, but not hph-1 or GCH-Tg mice, exhibited a small but significant increase in DHFR mRNA levels in lung tissue after exposure to MTX compared to control PBS-treated mice (Fig. 2C). These observations of variable increases in both DHFR protein and mRNA levels after treatment of wild-type, hph-1, and GCH-Tg mice with MTX have been previously demonstrated. DHFR levels are increased in mouse lung and kidney after treatment with MTX by subcutaneous infusion [22] and in Chinese hamster ovary cells [23,24], human lymphoblasts [25], and astrocytes [26] after exposure to MTX in culture. Importantly, potentially confounding nonspecific effects of MTX were not demonstrated on the levels of either eNOS or GTPCH protein.
Fig. 2.
Characterization of wild-type, hph-1, and GCH-Tg mice. 16- to 20-week-old mice were treated with three ip injections of either PBS (control) or MTX as detailed under Methods. The animals were sacrificed and lung tissue was harvested for analysis by Western blotting using eNOS-, GTPCH-, and DHFR-specific antibodies. Representative blots are shown; n = 3. (A) eNOS protein levels were comparable in all three groups. GTPCH protein levels were diminished in hph-1 and elevated by 20-fold in GCH-Tg mouse lung homogenates, compared to wild-type controls. MTX treatment did not alter the protein levels of either eNOS or GTPCH protein. (B) DHFR protein was elevated in wild-type, but not hph-1 or GCH-Tg mouse lung tissue in response to MTX treatment, as measured by densitometric analysis of the DHFR band. (C) DHFR mRNA was increased by MTX in wild-type (P < 0.05) but not hph-1 or GCH-Tg animals (n = 3).
Effects of methotrexate on DHFR activity
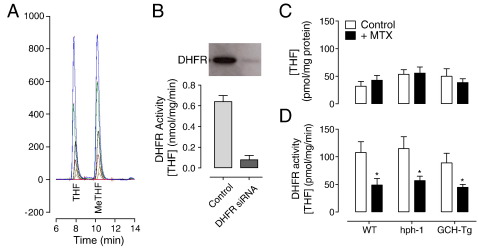
We next sought to investigate the effects of systemic inhibition of DHFR activity in lung tissue from wild-type, hph-1, and GCH-Tg animals treated with either MTX or placebo. To demonstrate that our in vivo ip MTX treatment was sufficient to induce inhibition of DHFR protein activity, we adapted a previously published HPLC-based method [15]. After incubation of lung tissue homogenates with the substrate dihydrofolate, the accumulation of tetrahydrofolate was quantified as an indicator of DHFR activity. Importantly, THF and MeTHF were easily separated by HPLC (Fig. 3A). Approximately 1000-fold more DHF than either THF or MeTHF was required to be detectable by fluorescence (data not shown). To test the dependence of THF accumulation on DHFR activity, we exposed murine endothelial cells to DHFR-specific siRNA. Subsequently, DHFR protein was diminished by over 95%, which resulted in a 90% reduction in measurable THF and therefore DHFR activity (Fig. 3B). As it was important to eliminate any confounding effects of our MTX-dosing regimen on intracellular THF levels within lung tissue, THF concentration in lung homogenates was quantified by HPLC. Endogenous basal levels of THF were comparable in wild-type, hph-1, and GCH-Tg mouse lung tissue and remained unaffected by MTX (Fig. 3C). In contrast, MTX treatment of wild-type, hph-1, and GCH-Tg mice was sufficient to significantly inhibit lung DHFR activity in all genotypes (Fig. 3D).
Fig. 3.
DHFR protein activity is inhibited by MTX treatment. 16- to 20-week-old mice were treated with three ip injections of either PBS or MTX as detailed under Methods. Lung tissue was harvested and DHFR activity measured by HPLC. For cell culture siRNA experiments, sEnd.1 murine endothelial cells were transfected with DHFR-specific or control scrambled nonspecific siRNA as described. (A) Example chromatogram showing THF and MeTHF standards at a range of concentrations (0–100 nmol/L). (B) DHFR protein was knocked down by over 90% in endothelial cells using DHFR-specific siRNAs. Cells were incubated with DHF (50 μmol/L) and NADPH (200 μmol/L), and the accumulation of THF was measured by HPLC as an indicator of DHFR activity. DHFR siRNAs resulted in an 80% inhibition of DHFR activity (n = 6, P < 0.01). (C) MTX treatment had no effect on basal THF levels in wild-type, hph-1, or GCH-Tg mouse lung tissue (n = 6!10). (D) DHFR activity was significantly decreased after MTX but not PBS (control) treatment in lungs taken from wild-type, hph-1, and GCH-Tg mice (n = 6–10, *P < 0.05).
DHFR inhibition by MTX leads to BH4 oxidation in vivo
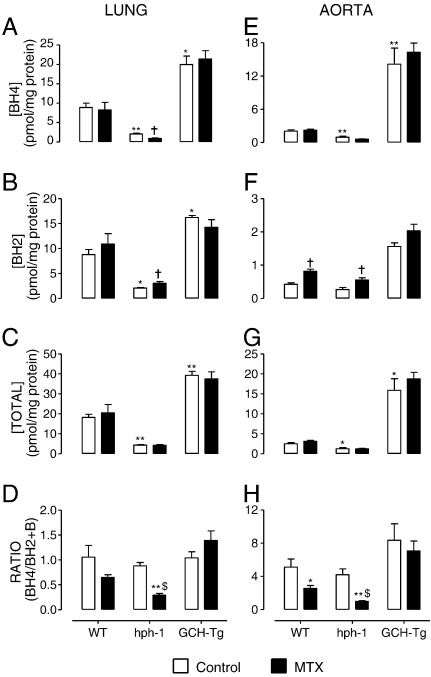
Having demonstrated that MTX treatment of mice is sufficient to induce inhibition of DHFR activity without confounding effects on basal THF levels, we reasoned that this would have important effects on biopterin homeostasis and the redox state of intracellular BH4. We compared BH4 levels in the lung and aorta of wild-type, hph-1, and GCH-Tg mice after ip treatment of mice with MTX or the placebo, PBS. No significant effect of MTX was observed in lung tissue from wild-type mice. However, striking oxidation of BH4 to BH2 occurred in the hph-1 mouse (Fig. 4): BH4 was significantly decreased (2.0 ± 0.5 vs 0.9 ± 0.2, P < 0.05) and BH2 elevated (2.0 ± 0.3 vs 3.0 ± 0.7, P < 0.05), resulting in a marked reduction in the BH4:BH2 ratio, a key determinant of eNOS coupling (0.8 ± 0.1 vs 0.3 ± 0.1, P < 0.05). This was probably due to the abrogation of the protective, reductive effect of DHFR by the MTX treatment. Similarly, MTX treatment allowed for increased oxidation of BH4 observed in the aorta of hph-1 mice. Moreover, BH2 accumulation was also seen in the wild-type mouse (0.4 ± 0.1 vs 0.8 ± 0.2, P < 0.05). Importantly, the reduction in BH4:BH2 ratio induced by MTX in hph-1 lung homogenates was greater than that observed in wild-type controls (2.6 ± 1.1 vs 1.0 ± 0.2, P < 0.05), illustrating that the relative magnitude of the effect of DHFR inhibition was greater at low vs high total biopterin levels. Indeed, these MTX-induced changes did not occur in either the lung or the aorta of GCH-Tg mice, in which total biopterin levels were significantly elevated. Because nonspecific effects of MTX treatment on GTPCH activity could explain these changes in biopterin levels, we eliminated this possibility by comparison of GTPCH enzymatic activity measured by HPLC (Fig. 5). These findings suggest that DHFR is critical in regulating BH4 redox state at low, but not high, levels of total biopterin, at which de novo biosynthesis alone is probably sufficient to maintain saturating levels of BH4.
Fig. 4.
MTX treatment leads to evidence of BH4 oxidation in lung and aorta. Wild-type, hph-1, and GCH-Tg mice were treated with MTX or PBS as outlined under Methods. Lung and aorta were harvested and processed for biopterin analysis by HPLC. (A) BH4 was significantly oxidized († P < 0.05), (B) BH2 was markedly elevated († P < 0.05), and (C) total biopterins remained unchanged, which resulted in (D) a striking reduction in the BH4:BH2 ratio in hph-1 ($P < 0.05), but not wild-type or GCH-Tg lung tissue in response to MTX treatment (n = 6–10). (E, F, and G) Similarly, BH4 oxidation was observed in the aorta of hph-1 mice. BH2 accumulation was also seen in the wild-type mouse (F, † P < 0.05). An exacerbation of the BH4:BH2 ratio was revealed after ip MTX treatment in hph-1 lung homogenates and aorta compared to wild-type controls (D and H, $P < 0.05). Total biopterin levels are expressed as the sum of detectable BH4, BH2, and B (n = 6 – 10, *P < 0.05, **P < 0.01).
Fig. 5.
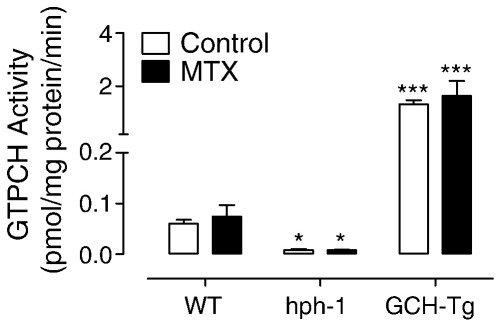
GTPCH activity remains unchanged after treatment of wild-type, hph-1, and GCH-Tg mice with MTX. Wild-type, hph-1, and GCH-Tg mice were treated with MTX or PBS (control) as outlined under Methods. GTPCH activity in lung tissue was measured by HPLC. GTPCH activity was considerably diminished in hph-1 (*P < 0.05) and dramatically increased in GCH-Tg (***P < 0.001) mouse lung tissue compared to wild type. No difference was detected between PBS- and MTX-treated mice (n = 6–10).
DHFR activity is required for eNOS coupling under conditions of BH4 deficiency
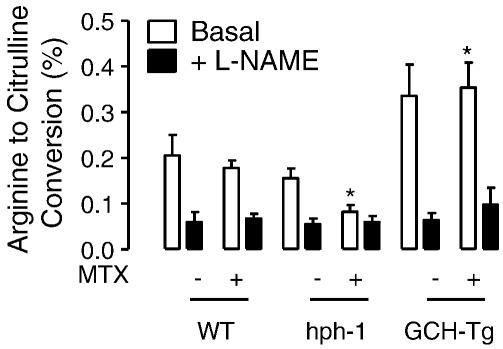
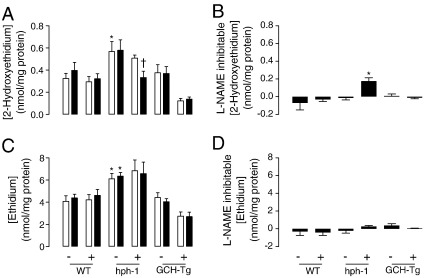
Having observed marked changes in BH4, and in particular the BH4:BH2 ratio, after MTX treatment, we reasoned that this would have detrimental effects on eNOS coupling. The activity of eNOS (as measured by quantifying the conversion of arginine to citrulline) was modestly decreased in hph-1 mouse lung tissue and significantly elevated in GCH-Tg lung (Fig. 6). There was a further, significant decrease in eNOS activity in the MTX-treated hph-1 mouse, compared to both control-treated hph-1 and wild-type animals (0.08 ± 0.03 vs 0.16 ± 0.04 and 0.21 ± 0.1, respectively, P < 0.05). In contrast, high levels of BH4 in the GCH-Tg mouse protected against the MTX-induced decrease in NO production, because we observed no difference in lung eNOS activity in MTX-treated GCH-Tg mice. Simultaneously, evidence of eNOS uncoupling was observed in the hph-1 lung after exposure to MTX (Fig. 7). Elevated accumulation of 2-hydroxyethidium (as an indicator of superoxide production) was detected in the lungs of hph-1 compared to both wild-type and GCH-Tg mice (P < 0.05). However, this superoxide production was inhibitable only with the arginine analogue L-NAME in hph-1 mice post-MTX treatment (0.51 ± 0.06 vs 0.33 ± 0.13, P < 0.05, Fig. 7A). Accordingly, marked eNOS-derived superoxide production was evident only in the MTX-treated hph-1 mouse (Fig. 7B). In further support of the protective role of BH4 against MTX-induced oxidative stress, GTPCH overexpression prevented any effect of MTX on either eNOS activity or superoxide production.
Fig. 6.
MTX-induced reduction in eNOS activity in hph-1 mice. Wild-type, hph-1, and GCH-Tg mice were treated with MTX as outlined under Methods. Lung tissue homogenates were incubated with Krebs–Hepes buffer containing calcium ionophore (1 μmol/L) in the presence and absence of L-NAME (100 μM) for 30 min. l-[14C]citrulline accumulation was then quantified by HPLC as an indicator of eNOS activity. eNOS activity was decreased in the hph-1 mouse lung (although not significantly) and elevated in the GCH-Tg (*P < 0.05). eNOS activity in the MTX-treated hph-1 mouse lung was significantly decreased compared to both PBS-treated hph-1 and wild-type animals (*P < 0.05). No effect of MTX was observed in the GCH-Tg mouse (n = 6–10).
Fig. 7.
DHFR regulates eNOS coupling in the hph-1 mouse lung. Wild-type, hph-1, and GCH-Tg mice were treated in vivo with MTX ip, as outlined under Methods. The accumulation of 2-hydroxyethidium and ethidium in lung homogenates, after exposure to dihydroethidium (50 μmol/L), was quantified by HPLC and used as an indicator of superoxide and total reactive oxygen species production, respectively. (A) Elevated levels of 2-hydroxyethidium were detected in the lungs of hph-1 compared to both wild-type and GCH-Tg mice (*P < 0.05). This superoxide production was attenuated by L-NAME (100 μmol/L) only in hph-1 mice post MTX treatment († P < 0.05). (B) eNOS-derived superoxide production is evident only in the MTX-treated hph-1 mouse (*P < 0.05). (C) Levels of total reactive oxygen species were greater in hph-1 compared to both wild-type and GCH-Tg mice (*P < 0.05). (D) L-NAME treatment had no effect on the elevation of reactive oxygen species (P not significant). Open bars, PBS; black bars, L-NAME treatment (n = 6–10).
Although the apparent switch from eNOS-independent to eNOS-dependent superoxide production in hph-1 lung tissue in response to MTX treatment is surprising, total levels of reactive oxygen species (as quantified by the accumulation of ethidium in lung homogenates after exposure to dihydroethidium) are 10-fold those of superoxide (measured by the accumulation of 2-hydroxyethidium). The lack of any inhibition of the formation of ethidium by L-NAME suggests that the contribution of eNOS to total oxidant production is small (Figs. 7C and D). The anti-inflammatory effects of MTX treatment may attenuate superoxide production from other sources, and this would mask any simultaneous increase in superoxide production from eNOS becoming detectable in the hph-1 mouse.
Taken together, these data indicate that DHFR activity regulates BH4 recycling and BH2 accumulation with simultaneous effects on eNOS coupling. These data also reveal that DHFR is required to maintain NOS coupling under conditions of low biopterin synthesis.
Discussion
In this study we tested a potential role for DHFR in regulating biopterin redox state and eNOS coupling, using pharmacological inhibition of DHFR by MTX in vivo. Advancing our previous studies in cell culture [11], we compared BH4-deficient (hph-1) and GTPCH-overexpressing (GCH-Tg) mice with wild-type controls in the presence and absence of MTX treatment. We reveal a key role for DHFR and the recycling pathway of BH4 biosynthesis in the maintenance of intracellular BH4 homeostasis in vivo and test the dependence of eNOS coupling on DHFR activity under low biopterin conditions observed in the hph-1 mouse. The major findings of this study are as follows: first, treatment of hph-1, wild-type, and GCH-Tg mice with methotrexate leads to an approximately 60% reduction in DHFR activity. Second, this diminished activity of the recycling pathway leads to BH4 oxidation as shown by a reduction in the BH4:BH2 ratio in mouse lung. Third, a striking exacerbation of these effects occurs in both aorta and lung of the hph-1 mouse model of BH4 deficiency compared to wild-type controls. Fourth, these changes in biopterin redox status do not occur when BH4 levels are high, as in the GCH-Tg animal. Finally, these changes in biopterin homeostasis result in eNOS uncoupling and the production of significant levels of eNOS-derived superoxide in the hph-1 mouse. Taken together our findings provide clear mechanistic evidence to support a key role for the recycling pathway in the regulation of cellular biopterin homeostasis in vivo and a regulatory role for DHFR on eNOS coupling under conditions of BH4 deficiency.
Biosynthesis of BH4, initially characterized by its cofactor role in reactions catalyzed by the aromatic amino acid hydroxylases, proceeds via the de novo pathway involving the enzymes GTPCH, 6-pyruvoyltetrahydropterin synthase, and sepiapterin reductase (Fig. 1) [27]. As the rate-limiting enzyme in BH4 synthesis, GTPCH regulation takes place at the transcriptional and posttranslational levels, with activity and mRNA shown to be induced by mediators such as interferon-γ, tumor necrosis factor-α, and lipopolysaccharide [28–30]. Indeed, steady-state BH4 levels in cells and tissues have previously been shown to directly correlate with GTPCH mRNA levels [31]. BH4, also a cofactor for NOS enzymes, is required for efficient oxidation of arginine and ultimately NO production. When BH4 availability is limiting, eNOS generates superoxide rather than NO, BH4 becomes oxidized to catalytically incompetent BH2, and a feed-forward cascade of BH4 destruction proceeds.
A BH2 reductase activity of DHFR was first observed in cell and tissue extracts. In a Chinese hamster ovary cell mutant lacking dihydrofolate reductase (DUKX-BII), endogenous formation of BH4 proceeds normally, but unlike the parent cells that express DHFR, extracts do not convert sepiapterin or BH2 to BH4 [32]. These studies implicate the biopterin recycling pathway in the regulation of steady-state BH4 levels. Our data suggest that this BH2 reductase activity of DHFR is crucial in determining cellular BH4 homeostasis, NO bioavailability, and ultimately eNOS coupling in vivo, particularly in BH4-deficient states such as those found in the hph-1 lung. Interestingly, some previous studies have shown that DHFR level or activity is diminished in experimental models of cardiovascular disease states, suggesting that insufficient recycling of BH2 to BH4 by DHFR is at least in part responsible for the attenuated BH4 levels and the accumulation of BH2, leading to eNOS uncoupling—the marked accumulation of BH2, resulting from DHFR inhibition, would compete with BH4 for eNOS binding, diminish NO production, and elevate the synthesis of eNOS-derived superoxide. For example, DHFR protein levels are significantly decreased in streptozotocin-induced diabetic mice and diabetes-induced impairment of cardiac myocyte function is exacerbated after treatment of the mice with the DHFR inhibitor, MTX [33]. Furthermore, decreased DHFR activity in adult cardiac myocytes underlies their limited capacity to synthesize BH4 after cytokine stimulation after treatment of rat cardiac allograft recipients with sepiapterin [34]. Insufficient DHFR activity might also explain impaired vasorelaxation in atherosclerotic vessels from hypercholesterolemic rabbits, despite exposure to sepiapterin, which increases biopterin levels through BH2, requiring DHFR to increase BH4 [35].
Having previously demonstrated that BH4:BH2 ratio and eNOS uncoupling relies more heavily on DHFR activity when total biopterin levels are very low, we sought to compare two models exhibiting differential levels of de novo biopterin synthesis, akin to our studies in cells expressing tetracycline-regulatable GTPCH. The hph-1 mouse model of BH4 deficiency and the GCH-Tg mouse enabled us to make this comparison, and treatment of these mice with MTX (three ip injections of 2 mg/kg) resulted in a dramatic attenuation of DHFR activity.
Our data show that high levels of GTPCH activity, and therefore BH4, are protective against the detrimental effects of MTX on eNOS coupling. Moreover, as the levels of BH4 are increased in the GCH-Tg mouse, the BH4:BH2 ratio remains unchanged. This would suggest that as the de novo, synthetic pathway continues to produce more BH4, BH2 also continues to accumulate. If no oxidation were to occur, one would hypothesize that the ratio would in fact be increased. Even when MTX treatment attenuates DHFR activity, no significant change in either lung or aortic BH4 homeostasis is observed in the GCH-Tg mouse, indicating that the proportion of total biopterins from the recycling pathway is very small. In contrast, in the hph-1 mouse, in which tissue BH4 levels are decreased by up to 90%, attenuation of DHFR activity leads to dramatic changes in biopterins and consequently NO and superoxide production by eNOS. Hence, the relative contribution made to total biopterins and BH4 redox status by the recycling pathway, versus the synthetic pathway, is substantial. It is well established that eNOS coupling is also determined by the relative ratio of eNOS:BH4 [7,14]. Given that the total levels of BH4 in wild-type aorta are somewhat lower than those in lung, it may be expected that eNOS may be more susceptible to smaller changes in BH4:BH2 in aorta. However, it is important to consider the possibility that the tissues of the hph-1 mouse are under relative oxidative stress and that this elevated production of reactive oxygen species, rather than the low concentrations of BH4, may make hph-1 tissues susceptible to MTX-induced eNOS uncoupling.
These results in mice support our previous findings in cultured cells in which concomitant exposure of endothelial cells to both GTPCH- and DHFR-specific siRNA, inhibiting the synthetic and recycling pathways together, significantly elevated eNOS-derived superoxide compared with either treatment alone. Similarly, although no evidence of eNOS uncoupling was observed in hph-1 lung tissue alone, striking eNOS uncoupling and BH4 oxidation were discovered when both the synthetic and the recycling pathways were diminished. We therefore hypothesize that GTPCH is the principal enzyme determining overall production of BH4, whereas DHFR maintains the BH4 pool in a reduced state, limiting the accumulation of oxidized biopterins. The absence of any effect of MTX treatment in lung tissue from wild-type mice does not rule out a role for DHFR in maintaining eNOS coupling when levels of BH4 are saturating. An important limitation of the current study is that our exposure of mice to MTX resulted in only a 60% inhibition of DHFR activity—higher doses of MTX and the resulting greater inhibition of DHFR may allow enhanced accumulation of BH2 and, potentially, uncoupling of eNOS in wild-type mice. Our dose of 2 mg/kg was carefully selected to inhibit DHFR activity without having confounding effects on intracellular THF levels. We were therefore able to dose MTX without folic acid, normally coadministered when targeting DHFR for cancer and rheumatoid therapies. This was important because of the potential nonspecific actions of folates on eNOS coupling and BH4 availability [36].
Pharmacological supplementation of BH4 improves endothelium-dependent relaxation and augments NO-mediated effects on forearm blood flow in smokers and those with diabetes and elevated cholesterol [37–39]. Inefficient utilization of the administered BH4 remains problematic [40,41]. Recent studies in cells and in vivo suggest that DHFR is also required for biopterin transport. BH4 accumulation in various tissues after supplementation with BH4, BH2, or sepiapterin can be inhibited by MTX; indeed after MTX treatment the increase in tissue biopterin was almost exclusively BH2 [42]. It was concluded that the elevation in BH4 by supplementation was mainly through a “salvage pathway” that included BH2 as the key intermediate in the production of BH4 through the action of dihydrofolate reductase. It is, therefore, likely that DHFR expression is critical to cells that do not contain the apparatus required for efficient synthesis of BH4. BH2 may be transported from cells such as endothelial cells, reduced back to BH4 by DHFR, and made available for the synthesis of NO or catecholamines.
Our data suggest a potentially harmful effect of methotrexate therapy on biopterin homeostasis and endothelial function. Given the widespread clinical use of methotrexate, both as an anti-cancer therapeutic and in inflammatory diseases such as rheumatoid arthritis, further studies are merited to investigate and characterize this impact more closely. BH4 supplementation during MTX therapy, for example, may improve BH4/BH2 ratios, maintain vascular function, and serve to diminish the risk of cardiovascular events in these patients even further.
Acknowledgments
We are grateful to Professor Steven S. Gross of Weill Medical College of Cornell University (New York, USA) for the kind gift of rat anti-mouse GTPCH antibody. Rabbit anti-human GTPCH was provided by Gabriele Werner-Felmayer of the Institute for Medical Chemistry and Biochemistry (Innsbruck, Austria). This work was supported by a British Heart Foundation Programme Grant RG/07/003/23133.
References
- 1.Vasquez-Vivar J., Kalyanaraman B., Martasek P., Hogg N., Masters B.S., Karoui H., Tordo P., Pritchard K.A., Jr. Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc. Natl Acad. Sci. U. S. A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chalupsky K., Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc. Natl Acad. Sci. U. S. A. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurshman A.R., Krebs C., Edmondson D.E., Huynh B.H., Marletta M.A. Formation of a pterin radical in the reaction of the heme domain of inducible nitric oxide synthase with oxygen. Biochemistry. 1999;38:15689–15696. doi: 10.1021/bi992026c. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt P.P., Lange R., Gorren A.C., Werner E.R., Mayer B., Andersson K.K. Formation of a protonated trihydrobiopterin radical cation in the first reaction cycle of neuronal and endothelial nitric oxide synthase detected by electron paramagnetic resonance spectroscopy. J. Biol. Inorg. Chem. 2001;6:151–158. doi: 10.1007/s007750000185. [DOI] [PubMed] [Google Scholar]
- 5.Vasquez-Vivar J., Martasek P., Whitsett J., Joseph J., Kalyanaraman B. The ratio between tetrahydrobiopterin and oxidized tetrahydrobiopterin analogues controls superoxide release from endothelial nitric oxide synthase: an EPR spin trapping study. Biochem. J. 2002;362:733–739. doi: 10.1042/0264-6021:3620733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crabtree M.J., Smith C.L., Lam G., Goligorsky M.S., Gross S.S. Ratio of 5,6,7,8-tetrahydrobiopterin to 7,8-dihydrobiopterin in endothelial cells determines glucose-elicited changes in NO vs. superoxide production by eNOS. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1530–H1540. doi: 10.1152/ajpheart.00823.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crabtree M.J., Tatham A.L., Al-Wakeel Y., Warrick N., Hale A.B., Cai S., Channon K.M., Alp N.J. Quantitative regulation of intracellular endothelial nitric oxide synthase (eNOS) coupling by both tetrahydrobiopterin–eNOS stoichiometry and biopterin redox status: insights from cells with tet-regulated GTP cyclohydrolase I expression. J. Biol. Chem. 2009;284:1136–1144. doi: 10.1074/jbc.M805403200. [DOI] [PubMed] [Google Scholar]
- 8.McGuire J.J. Anticancer antifolates: current status and future directions. Curr. Pharm. Des. 2003;9:2593–2613. doi: 10.2174/1381612033453712. [DOI] [PubMed] [Google Scholar]
- 9.Nzila A., Ward S.A., Marsh K., Sims P.F., Hyde J.E. Comparative folate metabolism in humans and malaria parasites. Part I. Pointers for malaria treatment from cancer chemotherapy. Trends Parasitol. 2005;21:292–298. doi: 10.1016/j.pt.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtius H.C., Heintel D., Ghisla S., Kuster T., Leimbacher W., Niederwieser A. Tetrahydrobiopterin biosynthesis: studies with specifically labeled (2H)NAD(P)H and 2H2O and of the enzymes involved. Eur. J. Biochem. 1985;148:413–419. doi: 10.1111/j.1432-1033.1985.tb08855.x. [DOI] [PubMed] [Google Scholar]
- 11.Crabtree M.J., Tatham A.L., Hale A.B., Alp N.J., Channon K.M. Critical role for tetrahydrobiopterin recycling by dihydrofolate reductase in regulation of endothelial nitric-oxide synthase coupling: relative importance of the de novo biopterin synthesis versus salvage pathways. J. Biol. Chem. 2009;284:28128–28136. doi: 10.1074/jbc.M109.041483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugiyama T., Levy B.D., Michel T. Tetrahydrobiopterin recycling, a key determinant of endothelial nitric-oxide synthase-dependent signaling pathways in cultured vascular endothelial cells. J. Biol. Chem. 2009;284:12691–12700. doi: 10.1074/jbc.M809295200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khoo J.P., Nicoli T., Alp N.J., Fullerton J., Flint J., Channon K.M. Congenic mapping and genotyping of the tetrahydrobiopterin-deficient hph-1 mouse. Mol. Genet. Metab. 2004;82:251–254. doi: 10.1016/j.ymgme.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 14.Bendall J.K., Alp N.J., Warrick N., Cai S., Adlam D., Rockett K., Yokoyama M., Kawashima S., Channon K.M. Stoichiometric relationships between endothelial tetrahydrobiopterin, eNOS activity and eNOS coupling in vivo: insights from transgenic mice with endothelial-targeted GTPCH and eNOS over-expression. Circ. Res. 2005;97:864–871. doi: 10.1161/01.RES.0000187447.03525.72. [DOI] [PubMed] [Google Scholar]
- 15.Gao L., Chalupsky K., Stefani E., Cai H. Mechanistic insights into folic acid-dependent vascular protection: dihydrofolate reductase (DHFR)-mediated reduction in oxidant stress in endothelial cells and angiotensin II-infused mice: a novel HPLC-based fluorescent assay for DHFR activity. J. Mol. Cell. Cardiol. 2009;47:752–760. doi: 10.1016/j.yjmcc.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heales S., Hyland K. Determination of quinonoid dihydrobiopterin by high-performance liquid chromatography and electrochemical detection. J. Chromatogr. 1989;494:77–85. doi: 10.1016/s0378-4347(00)82658-4. [DOI] [PubMed] [Google Scholar]
- 17.Werner-Felmayer G., Gross S.S. Analysis of tetrahydrobiopterin and its role in nitric oxide synthesis. In: Feelish M., Stamler J.S., editors. Methods in Nitric Oxide Research. Wiley; New York: 1996. pp. 271–294. [Google Scholar]
- 18.de Bono J.P., Warrick N., Bendall J.K., Channon K.M., Alp N.J. Radiochemical HPLC detection of arginine metabolism: measurement of nitric oxide synthesis and arginase activity in vascular tissue. Nitric Oxide. 2007;16:1–9. doi: 10.1016/j.niox.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Zhao H., Joseph J., Fales H.M., Sokoloski E.A., Levine R.L., Vasquez-Vivar J., Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl Acad. Sci. U. S. A. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fink B., Laude K., McCann L., Doughan A., Harrison D.G., Dikalov S. Detection of intracellular superoxide formation in endothelial cells and intact tissues using dihydroethidium and an HPLC-based assay. Am. J. Physiol. Cell Physiol. 2004;287:C895–C902. doi: 10.1152/ajpcell.00028.2004. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes D.C., Wosniak J., Jr., Pescatore L.A., Bertoline M.A., Liberman M., Laurindo F.R., Santos C.X. Analysis of DHE-derived oxidation products by HPLC in the assessment of superoxide production and NADPH oxidase activity in vascular systems. Am. J. Physiol. Cell Physiol. 2007;292:C413–C422. doi: 10.1152/ajpcell.00188.2006. [DOI] [PubMed] [Google Scholar]
- 22.Zaharko D.S., Dedrick R.L., Young D.M., Peale A.L. Tolerance of long-term methotrexate infusions by mice. Biochem. Pharmacol. 1976;25:1317–1321. doi: 10.1016/0006-2952(76)90096-4. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman R.J., Schimke R.T. Amplification and loss of dihydrofolate reductase genes in a Chinese hamster ovary cell line. Mol. Cell. Biol. 1981;1:1069–1076. doi: 10.1128/mcb.1.12.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serova M., Bieche I., Sablin M.P., Pronk G.J., Vidaud M., Cvitkovic E., Faivre S., Raymond E. Single agent and combination studies of pralatrexate and molecular correlates of sensitivity. Br. J. Cancer. 2011;104:272–280. doi: 10.1038/sj.bjc.6606063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chello P.L., McQueen C.A., DeAngelis L.M., Bertino J.R. Elevation of dihydrofolate reductase, thymidylate synthetase, and thymidine kinase in cultured mammalian cells after exposure to folate antagonists. Cancer Res. 1976;36:2442–2449. [PubMed] [Google Scholar]
- 26.Serrano E.E., Schimke R.T. Flow cytometric analysis of mammalian glial cultures treated with methotrexate. Glia. 1990;3:539–549. doi: 10.1002/glia.440030613. [DOI] [PubMed] [Google Scholar]
- 27.Thony B., Auerbach G., Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 2000;347:1–16. [PMC free article] [PubMed] [Google Scholar]
- 28.Huang A., Zhang Y.Y., Chen K., Hatakeyama K., Keaney J.F., Jr. Cytokine-stimulated GTP cyclohydrolase I expression in endothelial cells requires coordinated activation of nuclear factor-kappaB and Stat1/Stat3. Circ. Res. 2005;96:164–171. doi: 10.1161/01.RES.0000153669.24827.DF. [DOI] [PubMed] [Google Scholar]
- 29.Katusic Z.S., Stelter A., Milstien S. Cytokines stimulate GTP cyclohydrolase I gene expression in cultured human umbilical vein endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1998;18:27–32. doi: 10.1161/01.atv.18.1.27. [DOI] [PubMed] [Google Scholar]
- 30.Linscheid P., Schaffner A., Blau N., Schoedon G. Regulation of 6-pyruvoyltetrahydropterin synthase activity and messenger RNA abundance in human vascular endothelial cells. Circulation. 1998;98:1703–1706. doi: 10.1161/01.cir.98.17.1703. [DOI] [PubMed] [Google Scholar]
- 31.Tatham A.L., Crabtree M.J., Warrick N., Cai S., Alp N.J., Channon K.M. GTP cyclohydrolase I expression, protein, and activity determine intracellular tetrahydrobiopterin levels, independent of GTP cyclohydrolase feedback regulatory protein expression. J. Biol. Chem. 2009;284:13660–13668. doi: 10.1074/jbc.M807959200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nichol C.A., Lee C.L., Edelstein M.P., Chao J.Y., Duch D.S. Biosynthesis of tetrahydrobiopterin by de novo and salvage pathways in adrenal medulla extracts, mammalian cell cultures, and rat brain in vivo. Proc. Natl Acad. Sci. U. S. A. 1983;80:1546–1550. doi: 10.1073/pnas.80.6.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren J., Duan J., Thomas D.P., Yang X., Sreejayan N., Sowers J.R., Leri A., Kajstura J., Gao F., Anversa P. IGF-I alleviates diabetes-induced RhoA activation, eNOS uncoupling, and myocardial dysfunction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R793–R802. doi: 10.1152/ajpregu.00713.2007. [DOI] [PubMed] [Google Scholar]
- 34.Ionova I.A., Vasquez-Vivar J., Whitsett J., Herrnreiter A., Medhora M., Cooley B.C., Pieper G.M. Deficient BH4 production via de novo and salvage pathways regulates NO responses to cytokines in adult cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 2008;295:H2178–H2187. doi: 10.1152/ajpheart.00748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vasquez-Vivar J., Duquaine D., Whitsett J., Kalyanaraman B., Rajagopalan S. Altered tetrahydrobiopterin metabolism in atherosclerosis: implications for use of oxidized tetrahydrobiopterin analogues and thiol antioxidants. Arterioscler. Thromb. Vasc. Biol. 2002;22:1655–1661. doi: 10.1161/01.atv.0000029122.79665.d9. [DOI] [PubMed] [Google Scholar]
- 36.Antoniades C., Shirodaria C., Warrick N., Cai S., de Bono J., Lee J., Leeson P., Neubauer S., Ratnatunga C., Pillai R., Refsum H., Channon K.M. 5-Methyltetrahydrofolate rapidly improves endothelial function and decreases superoxide production in human vessels: effects on vascular tetrahydrobiopterin availability and endothelial nitric oxide synthase coupling. Circulation. 2006;114:1193–1201. doi: 10.1161/CIRCULATIONAHA.106.612325. [DOI] [PubMed] [Google Scholar]
- 37.Ohara Y., Peterson T.E., Harrison D.G. Hypercholesterolemia increases endothelial superoxide anion production. J. Clin. Invest. 1993;91:2546–2551. doi: 10.1172/JCI116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Panza J.A., García C.E., Kilcoyne C.M., Quyyumi A.A., Cannon R.O., III Impaired endothelium-dependent vasodilation in patients with essential hypertension: evidence that nitric oxide abnormality is not localized to a single signal transduction pathway. Circulation. 1995;91:1732–1738. doi: 10.1161/01.cir.91.6.1732. [DOI] [PubMed] [Google Scholar]
- 39.White C.R., Brock T.A., Chang L.-Y., Crapo J., Briscoe P., Ku D., Bradley W.A., Gianturco S.H., Gore J., Freeman B.A., Tarpey M.M. Superoxide and peroxynitrite in atherosclerosis. Proc. Natl Acad. Sci. U. S. A. 1994;91:1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brand M.P., Hyland K., Engle T., Smith I., Heales S.J. Neurochemical effects following peripheral administration of tetrahydropterin derivatives to the hph-1 mouse. J. Neurochem. 1996;66:1150–1156. doi: 10.1046/j.1471-4159.1996.66031150.x. [DOI] [PubMed] [Google Scholar]
- 41.Kaufman S., Kapatos G., Rizzo W.B., Schulman J.D., Tamarkin L., Van Loon G.R. Tetrahydropterin therapy for hyperphenylalaninemia caused by defective synthesis of tetrahydrobiopterin. Ann. Neurol. 1983;14:308–315. doi: 10.1002/ana.410140309. [DOI] [PubMed] [Google Scholar]
- 42.Sawabe K., Wakasugi K.O., Hasegawa H. Tetrahydrobiopterin uptake in supplemental administration: elevation of tissue tetrahydrobiopterin in mice following uptake of the exogenously oxidized product 7,8-dihydrobiopterin and subsequent reduction by an anti-folate-sensitive process. J. Pharmacol. Sci. 2004;96:124–133. doi: 10.1254/jphs.fp0040280. [DOI] [PubMed] [Google Scholar]