Abstract
Accumulating evidence suggests that Alzheimer’s disease (AD) has a long preclinical phase, during which time its characteristic pathology accumulates and patient function declines, but symptoms are insufficient to warrant a clinical diagnosis of dementia. There have been increasing reports of noncognitive symptoms, including loss of motor function, reported to be associated with incident AD. To understand the link between motor function and preclinical AD, this article examines: our understanding of motor function and its clinical assessment in cohort studies; the relationship of motor function and loss of cognition in older persons; risk factors for cognitive and motor decline; and the relation of post-mortem indices of AD and motor function prior to death. Together, these data suggest that age-related cognitive and motor decline may share a common causation. Furthermore, individuals with a clinical diagnosis of AD may represent the ‘tip of the iceberg’, since AD pathology may also account for a substantial proportion of cognitive and motor dysfunction currently considered ‘normal aging’ in older persons without dementia. Thus, AD may have a much larger impact on the health and wellbeing of our aging population.
Keywords: Alzheimer’s disease, cognitive decline, dementia, mild cognitive impairment, motor decline, preclinical AD
Alzheimer’s disease
Clinical Alzheimer’s disease (AD) is characterized by insidious onset and slowly progressive cognitive dysfunction, particularly impaired memory [1]. The most widely used criteria for dementia are those developed by the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association [2]. These criteria require a history of intellectual decline and a standardized assessment of cognition, with unmistakable deterioration in two cognitive domains relative to the patient’s previous level of functioning. A clinical diagnosis of probable AD can usually be confirmed with post-mortem indices of AD in more than 80–90% of cases and in nearly all cases of typical AD [3]. The hallmark pathologic lesions of AD are neurofibrillary tangles and senile plaques [4]. Neurofibrillary lesions include neurofibrillary tangles, neurophil threads and neuritic plaques. All of these lesions contain hyperphosphorylated tau protein (PHF-tau). Neuritic senile plaques are extracellular deposits with a dense core of amyloid that are surrounded by an amyloid corona containing PHF-tau dystrophic neuritis and reactive microglia. Non-neuritic plaques lack the amyloid core, dystrophic neurites and microglia and are composed exclusively of amyloid. Several different classification schemes have been employed for the pathologic diagnosis of AD [5–7].
Preclinical AD
While a general consensus regarding the clinical and pathologic diagnoses of AD had emerged by the mid-1980s [2,8], accumulating evidence over the following decade suggested that clinical AD develops over the course of years, during which time many individuals may demonstrate mild cognitive impairment (MCI) insufficient for a clinical diagnosis of dementia [9,10]. In addition, post-mortem studies suggest that some older individuals with widespread evidence of AD pathology may not have dementia during their lifetime [11–16]. Recent ante-mortem brain imaging studies of amyloid extend these cross-sectional post-mortem findings, providing evidence that AD pathology accumulates well before the clinical diagnosis of dementia [9,10]. Thus, both ante-mortem and post-mortem data lend support to the notion that AD has a preclinical period, during which its characteristic neuropathology accumulates and cognitive function declines, but symptoms are insufficient to warrant a clinical diagnosis of AD.
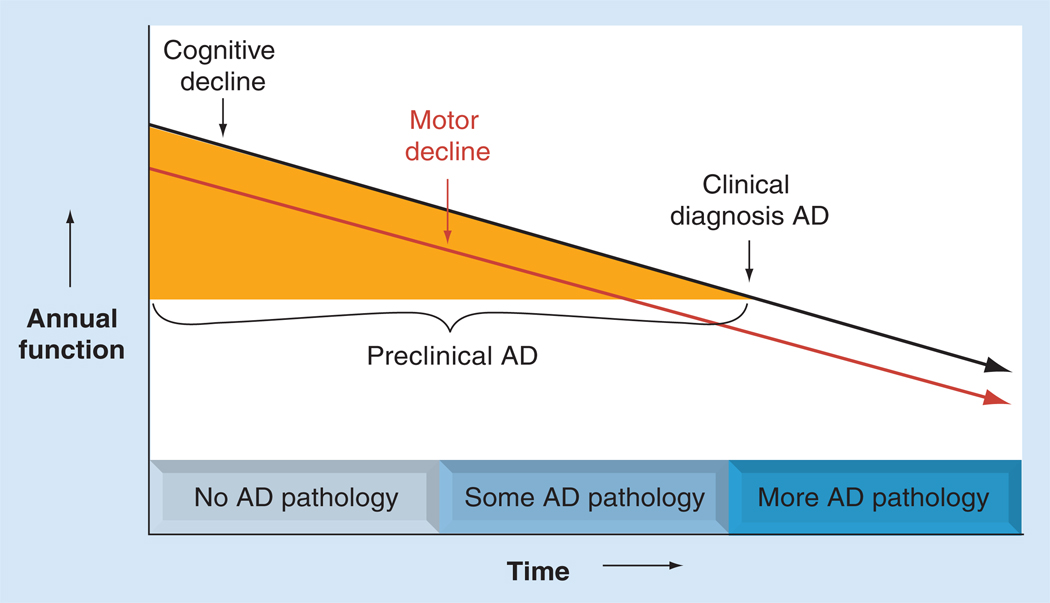
Converging evidence suggests that the pathophysiologic processes that eventually lead to AD begin years, if not decades, prior to the clinical diagnosis of AD dementia. Nonetheless, since MCI and functional impairment may represent an early stage of AD, distinct from normal aging, the long preclinical phase of AD provides a critical opportunity for potential intervention with disease-modifying therapy. However, this underscores the need to more precisely characterize the emerging clinical syndrome during the preclinical stage of AD. The recognition that several noncognitive symptoms, such as motor impairment, predict the subsequent development of AD suggests that noncognitive behaviors may serve as phenotypic markers of preclinical AD, as illustrated in Figure 1 [17–20].
Figure 1. Loss of cognitive and motor function during preclinical Alzheimer’s disease.
The figure shows the hypothesized relationship between accumulating AD pathology and declining cognitive and motor function before and after the clinical diagnosis of AD. Accumulation of AD pathology during preclinical AD may account for a substantial proportion of cognitive and motor dysfunction currently considered ‘normal aging’ in older persons without dementia.
AD: Alzheimer’s disease.
This article will focus on changes in motor function that occur prior to the clinical diagnosis of AD, during its preclinical phase. To understand the link between motor function and preclinical AD, we will review recent advances in: our understanding of motor function and its clinical assessment in cohort studies; the relationship between motor function and loss of cognition in older persons; risk factors for cognitive and motor decline; and the relationship between ante-mortem measures of motor function and post-mortem indices of AD pathology. Together these data suggest that loss of cognition and motor function in old age may share a common underlying pathophysiology, and accumulation of AD pathology contributes to age-related functional decline, including both cognitive and motor decline.
Motor function is not a unitary process
Motor function is not a unitary process, but different motor abilities derive from the coordinated activity of varied motor control systems located throughout the brain and spinal cord, and that extend via the peripheral nervous system to musculoskeletal structures [21–23]. Motor control systems that regulate the initiation, planning and execution of motor performances are located in multiple interconnected cortical and subcortical motor regions [24–29]. Descending white matter tracts provide the means for these supraspinal motor systems to influence spinal motor systems that directly control muscle, the final effector of all movement [22,23,30–34].
The best known role of muscle is its pivotal function in motor function. However, muscle has other essential roles in maintaining homeostasis, including temperature control and systemic metabolism, and it provides the body’s only reserve of amino acids. Furthermore, as muscle is situated outside of the CNS, it is not protected by the BBB. Therefore, muscle is vulnerable to a host of systemic diseases, as well as catabolic, inflammatory, immune, endocrine and metabolic processes that may not affect the motor control systems located within the CNS. Consequently, changes in muscle structure and function that can affect motor function may reflect neurologic disorders or other systemic disorders or both [23,35,36].
Recent advances in imaging and neurophysiologic testing have begun to elucidate the complex processes necessary to ensure accurate movements. Integration of a wide range of sensory and visuospatial information is essential for accurate movements (i.e., postural control, spatial navigation and joint position) [37,38], and different motor-related brain regions may control distinct aspects of movements (i.e., speed vs balance) [39–41]. Finally, the increasing complexity and novelty of motor tasks demand increasing cognitive and sensory information processing for the accuracy of successful movements.
Consequently, motor impairment may derive from damage to the integrity of the gray matter of motor-related brain regions (i.e., neuronal loss), as well as damage to white matter tracts (i.e., connectivity), which connect distributed gray matter, motor-related brain regions or a combination of both types of damage. As a result, the type of damage (i.e., loss of neuronal elements or accumulating pathology), and its location within CNS structures, may lead to different clinical motor deficits. These dissociations underscore that motor function is not a unitary process, and that several motor measures may be necessary to adequately assess motor impairment in older persons.
Loss of motor function is common in older persons
Age-related motor decline is common and associated with a wide range of adverse health consequences [42–46]. There are currently approximately 40 million persons over the age of 65 years in the USA, and by 2030, there will be more than 70 million persons over the age of 65 years [201]. It is estimated that up to 50% of older persons may have some elements of motor impairment by the age of 80 years [47,48]. This would suggest that the public health challenge of motor impairment in old age may be an even larger challenge than dementia and cognitive impairment. Motor impairment can include reduced gait speed, loss of muscle strength and bulk, and reduced balance, as well as dexterity. Thus, the growing public health challenge of identifying motor impairment in old age is complicated by the variability of its clinical expression. Several constructs based on assessments of different motor abilities have been used to document mild motor symptoms in old age, including sarcopenia, based on muscle bulk or mass and strength [49]; physical frailty, based on grip strength, body composition, gait speed, fatigue and physical activity [50]; parkinsonian signs, based on signs of bradykinesia, tremor, rigidity and parkinsonian gait [51]; and various summary measures, based on testing for a wide range of common motor performances [52]. Regardless of the motor measures that have been employed, most studies have demonstrated that mild motor symptoms are all associated with adverse health consequences, including all-cause mortality, as well as incident disability, and other outcomes, including the development of AD. Assessments that employ several motor measures may more accurately identify individuals at risk for adverse health consequences in old age [53].
Motor function predicts AD, MCI & cognitive decline in older persons
Over the last decade there has been increasing recognition of a link between motor function and the risk of developing AD (Figure 1). This article focuses on motor function prior to a diagnosis of AD. Changes in motor function in individuals after a clinical diagnosis of AD have been examined in prior publications [54–57]. Advances in imaging techniques (i.e., functional MRI, PET and diffusion tensor imaging) and electrophysiologic methods have advanced our understanding of the complex structural and functional brain changes that can be demonstrated for both simple and more complex motor tasks in older individuals with and without AD [28,58–60]. This article focuses on motor testing employed in community-based cohort studies of older individuals.
Muscle bulk
Loss of muscle strength and bulk is common in older individuals and is recognized as a prominent feature of several age-related geriatric syndromes (e.g., frailty, sarcopenia or metabolic syndrome) [49,50,61]. Muscle mass is a major determinant of body weight or BMI in older persons. While body composition (e.g., BMI) has long been known to be associated with mortality and other common medical conditions, recent studies suggest a link between BMI and AD [62–67]. These studies suggest that changes in BMI may be an early noncognitive sign of AD. The two main components of BMI are muscle mass and body fat. Since both muscle and fat can vary without an appreciable change in BMI, more work is needed to explicate the relative contributions or synergy of low muscle mass (sarcopenia) or high body fat (adiposity) and the association of BMI with cognition in old age. Muscle mass and fat can be measured more precisely with dual-energy x-ray absorptiometry (DXA) or with imaging modalities. Since these techniques are not feasible in the community setting, it is difficult to disentangle these two aspects of BMI in community-based studies.
Morphologic studies of muscle demonstrate that the loss of muscle structure (i.e., number or size of muscle fibers), either from disease or from disuse, is associated with lower muscle strength (function) [68,69]. It is also important to remember that since muscle is the final effector of motor output, dysfunction of a wide variety of CNS and peripheral nervous system structures cause decreased strength without concomitant structural changes in muscle. Thus, assessing muscle structure is crucial since it clarifies whether loss of muscle structure contributes to the loss of muscle function [70]. Although many factors contribute to declining muscle function in old age, loss of muscle structure is an important element that can be assessed in community-dwelling older persons [71].
While some report that low BMI is associated with an increased risk of AD, others suggest the opposite [47,48]. Accumulating evidence suggests that the link between BMI and the risk of dementia is more complex and may vary over the lifespan. Differences between the associations of BMI at midlife versus late-life may account in part for conflicting reports [71]. Several studies report that baseline lower BMI, as well as a more rapid rate of declining BMI, are associated with an increased risk of AD. Declining BMI may occur several years before the clinical diagnosis of dementia and raises the question of whether mild motor symptoms may represent an early sign of AD [20]. BMI is also associated with incident MCI [64]. To circumvent diagnostic imprecision, and because change in cognitive function is the principal manifestation for clinical AD, studies have demonstrated that BMI is also related to the annual rate of cognitive decline [20].
Muscle strength
Several studies have reported that low grip strength is associated with an increased risk of incident AD [17]. A recent study used a summary measure of appendicular and axial muscle strength, which was also related to the risk of AD, MCI and cognitive decline [72]. Secondary analyses in this study suggested that their findings were primarily the result of grip strength and axial muscle strength [72]. Thus, accumulating evidence suggests that both lower levels of muscle structure (muscle bulk) and lower levels of function (muscle strength) are associated with an increased risk of MCI and AD, as well as with a more rapid rate of cognitive decline.
Motor performances
In contrast to strength testing, which depends mostly on motor units and muscle function, motor performances reflect the functional integrity of widely distributed cortical and subcortical motor-related brain regions, as well as sensory, visuospatial and cognitive functions. Motor performances, including gait and balance or finger tapping, are commonly impaired in older persons [73]. Lower levels of motor function may be more pronounced in older persons with cognitive impairment compared with those who are cognitively intact [74–77]. Both a lower level and more rapid rate of motor decline in cognitively intact individuals predict the subsequent development of MCI and AD, and loss of motor function can precede cognitive impairment by several years [74,75,77–83].
Parkinsonian signs
Several community-based studies report that mild parkinsonian signs, including motor slowing (bradykinesia), gait and posture disturbances, rigidity and resting tremor, may be present in up to 50% of community-dwelling older persons without clinical Parkinson’s disease (PD), and mild parkinsonian signs are associated with adverse health consequences, including death and disability [48]. In cross-sectional analyses, a global measure of parkinsonism and individual parkinsonian signs were related to a global measure of cognition and specific cognitive abilities [84]. A higher level of parkinsonian signs in individuals without cognitive impairments is associated with an increased risk of developing both MCI and AD [51,84,85]. In addition, individuals with MCI exhibiting a higher level of parkinsonism have a higher risk of the subsequent development of AD [77]. A higher level of parkinsonian signs prior to the diagnosis of AD is associated with a more rapid rate of cognitive decline both before and after the diagnosis of AD [84,86].
Physical frailty
Impaired motor function is a prominent characteristic of physical frailty, a heterogeneous syndrome whose features include loss of muscle strength and body composition, impaired gait and fatigue [50,87,88]. Physical frailty is associated with both the level of cognition and dementia. Longitudinal studies suggest that a higher level of physical frailty is associated with the subsequent development of both MCI and AD [89,90]. Furthermore, both a higher level of frailty at one point in time, as well as a more rapid rate of increasing frailty (i.e., developing more impairments), were related to a more rapid loss of cognitive function [89].
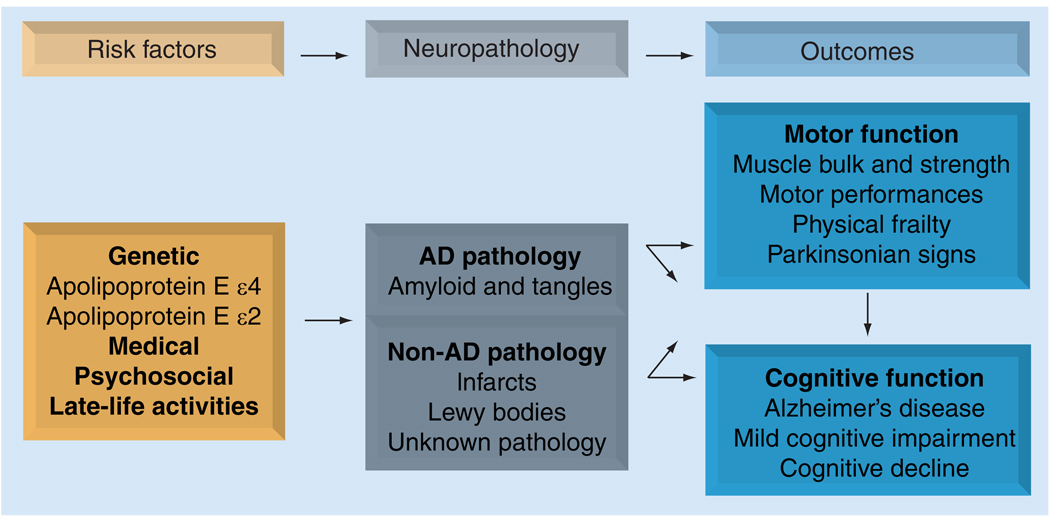
In summary, prior reports have examined a wide range of motor measures and their relationship with cognitive function in old age. Together these reports suggest that both the level and rate of motor decline are associated with adverse health outcomes, including incident AD and MCI, as well as the rate of cognitive decline (Figures 1 & 2).
Figure 2. Model summarizing possible links between risk factors and neuropathology with motor and cognitive function prior to death.
Prior studies suggest that: motor and cognitive function in older persons are related; motor function predicts incident mild cognitive impairment and AD and is related to both the level and rate of cognitive decline; age-related motor and cognitive decline share common risk factors; motor function and cognition prior to death are related to post-mortem indices of AD and other common neuropathologies. Together, these data suggest that age-related motor and cognitive decline may share a common causation.
AD: Alzheimer’s disease.
Motor & cognitive decline share common risk factors
Developing interventions that decrease the burden of age-related motor and cognitive decline requires an understanding of their underlying neurobiology. Mechanisms of disease known to damage the brain and neuronal elements, such as oxidative stress, inflammation and impaired energy metabolism, have been the focus of intense study [91–94]. In addition to studies of these basic mechanisms, identifying risk factors that are associated with functional decline can also direct efforts to understand their underlying neurobiology and the development of novel intervention strategies. For example, prior reports demonstrating that apolipoprotein E4 is a risk factor for developing AD and cognitive decline led to a body of work that has identified a causal chain linking apolipoprotein E4, AD pathology and incident AD (Figure 2) [95,96]. This work has led to the use of apolipoprotein E4 as a biomarker for individuals at risk for AD, as well as efforts to treat AD by modifying the accumulation of amyloid.
Over the past decade, a wide range of genetic, medical and psychosocial factors have been demonstrated to predict the development of incident AD and MCI, as well as cognitive decline. In light of the associations of motor function with the loss of cognitive function in older persons (Figure 1), risk factors for cognitive decline may also be risk factors for motor decline. Here, we review several genetic, medical, psychosocial and modifiable experiential factors that are risk factors for both cognitive and motor decline.
Genetic
The increased availability of tests for genetic polymorphisms has led to studies that have demonstrated that the presence of one or more alleles of apolipoprotein E allele (APOE) ε4 is associated with a number of adverse health consequences, including mortality, AD, cognitive decline, cardiovascular disease, infection and stroke [97–101]. In a recent study of community-dwelling older persons, the presence of at least one copy of the APOE ε4 allele was associated with an increased rate of motor decline, and this association increased with age [102]. These findings suggest that apolipoprotein E allele status is a risk factor for age-related motor decline. In contrast to APOE ε4, several other genes (CLU, PICALM, CR1 and BIN1) have been linked with an increased risk of AD, but these genes have not been reported to be associated with motor decline [103,104], with the exception of a recent case of an individual with centronuclear myopathy and a novel mutation of BIN1 [105].
Odor identification
Impaired odor identification has been associated with a variety of age-related neurodegenerative conditions that impair cognitive and motor function [106–111]. Loss of odor identification was associated with incident AD and MCI, as well as a greater rate of cognitive decline and an increased risk of MCI [106,107]. Similarly, in a recent study, lower ability to identify odors was associated with increased impairment on a global measure of parkinsonism at baseline and more rapid progression of global parkinsonism and parkinsonian gait disturbance during the study period [109]. The results suggest that difficulty recognizing familiar odors is associated with increased severity and progression of parkinsonian motor impairment in old age.
Medical factors
Several common medical conditions, such as hypertension and diabetes, are associated with an increased risk of AD, MCI and cognitive decline [112–118]. The presence of hypertension has been reported to be associated with lower walking speed and more rapid decline [119]. Recent studies have demonstrated that impaired cerebral blood flow regulation is associated with both cognitive function and gait slowing in old age, but not muscle strength [120]. In cross-sectional analyses, diabetes was associated with a global measure of parkinsonism and, in particular, with a more severe parkinsonian gait disturbance [121,122]. Progression of parkinsonian gait disturbance and rigidity were more severe in older persons with diabetes [122]. Respiratory muscle strength was associated with the rate of change in mobility, even after controlling for leg strength and physical activity [123]. In a subsequent study, respiratory muscle strength, leg strength, physical activity and pulmonary function were all found to make relatively independent contributions to the development of mobility disability based on assessment of gait speed [124].
Modifiable experiential factors
Physical activity is a modifiable risk factor for overall musculoskeletal fitness (i.e., muscular strength, endurance, power and flexibility) and cardiovascular disease, as well as a variety of other chronic diseases [125]. Many, but not all, observational studies suggest that higher levels of physical exercise are beneficial for cognition and may be associated with a lower risk of AD [126,127]. These findings are supported by several recent exercise intervention studies, which have demonstrated statistically significant improvement in cognitive function [126–132].
Previous studies have also demonstrated that a higher level of physical activity at one point in time predicts a higher level of motor function years later. However, the extent to which this association is due to a higher level of motor function at baseline is unclear because it is difficult to fully assess change in motor function as an outcome and distinguish it from initial level of performance on the basis of only two observations [125,133]. A recent study using repeated observations reported that self-reported physical activity is associated with a slower rate of decline in motor function, even after controlling for baseline levels of physical activity [134]. This association was primarily owing to the effect of physical activity on motor performance, rather than muscle strength, underscoring the need for further work to clarify the beneficial mechanisms of physical activity on motor function. Accumulating literature suggests that participation in a broad spectrum of late-life activities are associated with positive health outcomes in old age and, in particular, that more frequent participation in social activity may be protective against motor decline in older persons [135].
Psychosocial
There is increasing recognition of the importance of lifestyle and psychosocial factors for healthy aging in older persons [136]. Distress, neuroticism and loneliness have been reported to be associated with incident AD, MCI and cognitive decline [137–140]. Similarly, in a recent study, self-perceived isolation, loneliness and social engagement as measured by late-life social activities were relatively independent predictors of the rate of motor decline. The association of loneliness and motor decline persisted even after controlling for a wide range of leisure activities, including physical and cognitive activities, depressive symptoms and other possible confounding covariates, as well as after controlling for baseline disability or history of stroke and PD [141]. Cross-sectional studies have reported that personality traits, such as neuroticism and extraversion, are related to levels of physical activity and walking speed [142,143]. A recent longitudinal study reported that neuroticism and extraversion were independently associated with the rate of change in motor function, such that a higher level of neuroticism and a lower level of extraversion were associated with more rapid motor decline [Buchman AS, Boyle PA, Wilson RS, Leurgans SE, Arnold SE, Bennett DA. Neuroticism, extraversion, and motor function in community-dwelling older persons. Manuscript submitted].
Motor function prior to death is related to AD pathology
Since motor and cognitive function are related and common risk factors predict declines in both, one may expect that they share a common causation [144]. AD pathology is also related to MCI and cognitive decline in old age and is common, even in older persons without cognitive complaints prior to death [145,146]. Since AD pathology is associated with loss of cognition, the accumulation of AD pathology in motor-related brain regions could also contribute to the loss of motor function. It has been suggested that β-amyloid and its precursors are abnormally and specifically present in inclusion body myositis muscle fibers and may link muscle with AD pathology, but there is limited evidence to support this notion [147,148]. AD pathology in cognitive systems may affect motor function by impairing the widespread distributed neural systems that are recognized to play an important role in the serial or parallel processes, which are essential for the planning and execution of movements [149].
Muscle bulk, as measured by BMI, has been reported to demonstrate an inverse linear relationship with the amount of AD pathology in both individuals with and without dementia during life [150]. Similarly, a higher level of a composite measure of physical frailty prior to death was related to a higher level of AD neuropathology at autopsy [151]. In the latter study, AD neuropathology was related to grip strength and BMI prior to death and there was a trend for gait speed [151]. The associations of BMI and frailty with AD pathology was similar in both individuals with and without dementia, suggesting that loss of BMI and increasing frailty may occur before both MCI and AD [150,151].
While prior studies measured post-mortem indices of AD in cognitive regions, recent studies suggest that AD pathology also accumulates in regions known to subserve motor function, such as the primary and supplementary motor cortices, striatum and substantia nigra [152–157]. Furthermore, reports that have documented early clinical motor signs in patients demonstrated to have severe post-mortem AD pathology in the motor cortex emphasize the correlation between the location of AD pathology in the brain and ante-mortem clinical motor signs [155]. Recent data demonstrate that neurofibrillary tangles in the substantia nigra are related to parkinsonian signs in persons both with and without dementia [152,156]. Together, these data suggest that AD pathology in cortical and subcortical regions may contribute not only to loss of cognition, but also to age-related motor decline.
Other possible links between risk factors, pathology & motor function
Identification of risk factors for motor decline does not clarify the underlying mechanism that accounts for the association. Some risk factors, such as APOE, are likely to lead to motor decline, in part through an association with the accumulation of AD pathology, whereas other risk factors are likely to be associated with motor decline through other common neuropathologies or unknown mechanisms. Assuming that the association of AD pathology with motor decline represents the consequence rather than the cause of AD pathology, of the risk factors discussed above we can only be certain that APOE is directly associated with AD pathology. By contrast, psychological and experiential risk factors either modify the relation of AD pathology with motor function or have effects on motor decline separate from AD and other pathologies. Since cognitive and motor decline may share a common causation, prior work with cognitive decline suggests that other neuropathologies (i.e., infarcts and Lewy bodies), as well as factors that provide ‘neural reserve’, may modify the deleterious effects of AD pathology on motor function [9,158,159]. These observations have led us to consider a model (Figure 2) whereby the accumulation of AD pathology is primarily under the control of genomic variation. By contrast, experiential and psychological risk factors may primarily affect how the brain responds to the accumulation of pathology – that is, neural reserve. Further studies are needed to demonstrate these hypothesized relationships in motor decline.
Expert commentary
AD is best conceptualized as a continuum
Patients, families, payers, public health planners and physicians generally employ a categorical approach to disease – that is, the disease is present or absent. The demand for categorical definitions has also dominated aging research, in which the counting of cases is predicated on the assumption that disease can be clearly defined [160]. However, it has been epidemiologic research that has demonstrated that diseases such as osteoporosis, hypertension and chronic obstructive pulmonary disease behave as a continuum without natural definitions. These disorders have an insidious onset over years and possibly decades, and the demarcation between the presence and absence of the disease is a matter of consensus criteria based on imperfect data. For some conditions, a designation that is an intermediate between normality and disease is employed, such as prehypertension and osteopenia. The situation is analogous for AD and preclinical AD. The public health problem posed by AD is already large and growing in our aging population. Currently, the notion that clinically diagnosed AD disease is but the ‘tip of the iceberg’ with respect to cognitive impairment has gained acceptance. It is well-recognized that many individuals with MCIs are likely to represent mild or early AD. Since it is likely that the pathophysiologic processes that lead to AD pathology occur even before MCIs, efforts are underway to identify biomarkers that can be used to characterize ‘AD in situ’ or that can be used like laboratory tests to identify hypertension or renal insufficiency to identify individuals at risk for AD before there are any clinical deficits.
Similarly, it is probable that individuals with noncognitive manifestations of AD, such as mild motor impairments or impaired odor identification described above, also manifest early AD (Figure 1). Just as the clinical manifestations of seizures or stroke vary with the region of the brain that is affected, it is probable that noncognitive manifestations of AD vary with the location of AD pathology. Further work is necessary to characterize the clinical profile of preclinical AD, including both cognitive and noncognitive manifestations, to delineate the clinical profile for AD from other neuropathologies – that is, cerebrovascular disease or synuclienopathies. It is also necessary to characterize the contribution of AD pathology to what is often referred to as ‘normal’ age-related functional decline in individuals who do not meet clinical criteria for AD prior to death. Understanding the relationship of the pathophysiologic processes that lead to AD and age-related functional decline among older persons would increase the numbers of older persons who may benefit from interventions that are developed to treat AD and may improve or slow functional decline. Thus, recognition that AD is on a continuum will alter the paradigm of AD research and, ultimately, have a large impact on the health and wellbeing of our aging population.
Evidence of a common causation for cognitive & motor decline
The association between cognitive and motor decline, as well as the accumulating reports that they share similar risk factors for decline, suggest that there may be other common factors that lead to both cognitive and motor decline. For example, AD pathology is one example of common neuropathologies, such as Lewy bodies and infarcts, that may contribute to the loss of cognitive and motor function in older individuals (Figure 2). Alternatively, there are likely to be other processes and pathways that do not act through known neuropathologies to cause both cognitive and motor decline. For example, motor function may be a proxy for the level of physical activity and low levels of physical activity may lead to both reduced activity in central pathways, such as those involving BDNF, which may affect both cognitive and motor function.
Evidence of neural reserve
While there is accumulating evidence that AD pathology may account for cognitive and motor symptoms during preclinical AD, post-mortem studies suggest that the extent of AD histopathologic changes at autopsy do not always correlate with the degree of clinical impairment. This suggests that there are factors, such as experiential and psychosocial factors, that may increase the brain’s ability to tolerate the pathology of AD without manifesting functional impairment [9]. Further work is needed to clarify which risk factors may serve as buffers and counter the contribution of AD pathology to the loss of cognitive and motor function [161].
Five-year view
The phenotype of AD will continue to expand beyond its traditional characterization as a cognitive disorder. As more sophisticated clinical testing of a broader array of clinical behaviors, including not only cognitive function, but also motor, olfaction and psychosocial factors, are integrated and combined with post-mortem indices of AD pathology obtained from cognitive and noncognitive brain regions in research studies, the full characterization of the diverse cognitive and noncognitive symptoms manifested by individuals with the earliest stages of AD will become apparent. Efforts will continue to explicate the neurobiology of AD and to develop testing that can identify individuals with AD (defined by its pathology) without any clinical symptoms. Technological advances for the ante-mortem imaging of AD pathology and the processes that lead to its accumulation, along with efforts to identify new biomarkers, are likely to lead to further successes in identifying individuals at risk for AD with minimal clinical symptoms. These studies are likely to benefit from concomitant advances in rapidly expanding disciplines, including genomics and proteomics. Current epigenetic and proteomic studies are likely to identify a host of new potential biologic pathways and factors that may provide neural reserve and counter the effects of accumulating AD pathology.
Key issues.
Motor and cognitive function in older persons are related.
Motor function predicts incident mild cognitive impairment and Alzheimer’s disease (AD) and is related to both the level and rate of cognitive decline.
Age-related motor and cognitive decline share common risk factors.
Motor function and cognition prior to death are both related to post-mortem indices of AD pathology.
Together, these data suggest that age-related motor and cognitive decline may share a common causation that may be exemplified by AD neuropathology.
Individuals with a clinical diagnosis of AD may only represent the ‘tip of the iceberg’, since accumulation of AD pathology during preclinical AD may account for a substantial proportion of cognitive and motor dysfunction currently considered ‘normal aging’ in older persons without dementia and who may also benefit from future interventions to treat AD.
Acknowledgments
This research was supported by National Institute on Aging Grants P30AG10161, R01AG15819 R01AG17917, R01AG24480, P01AG09466, R01AG30142 and K23AG23040, the Illinois Department of Public Health and the Robert C Borwell endowment fund.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Bennett DA. Part II. Clinical diagnosis and course of Alzheimer’s disease. Dis. Mon. 2000;46(10):666–687. doi: 10.1016/s0011-5029(00)90029-4. [DOI] [PubMed] [Google Scholar]
- 2.Mckhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 3.Bennett DA, Schneider JA, Aggarwal NT, et al. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27(3):169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- 4.Arnold SE. Part III. Neuropathology of Alzheimer’s disease. Dis. Mon. 2000;46(10):687–705. doi: 10.1016/s0011-5029(00)90030-0. [DOI] [PubMed] [Google Scholar]
- 5.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Neurology. 1991;41(4):479–479. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 7.Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol. Aging. 1997;18(4 Suppl.):S1–S2. [PubMed] [Google Scholar]
- 8.Khachaturian ZS. Diagnosis of Alzheimer’s disease. Arch. Neurol. 1985;42(11):1097–1105. doi: 10.1001/archneur.1985.04060100083029. [DOI] [PubMed] [Google Scholar]
- 9.Rentz DM, Locascio JJ, Becker JA, et al. Cognition, reserve, and amyloid deposition in normal aging. Ann. Neurol. 2010;67(3):353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperling RA, Laviolette PS, O’Keefe K, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63(2):178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomlinson BE, Blessed G, Roth M. Observations on the brains of non-demented old people. J. Neurol. Sci. 1968;7(2):331–356. doi: 10.1016/0022-510x(68)90154-8. [DOI] [PubMed] [Google Scholar]
- 12.Katzman R, Terry R, Deteresa R, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann. Neurol. 1988;23(2):138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- 13.Crystal HA, Dickson DW, Sliwinski MJ, et al. Pathological markers associated with normal aging and dementia in the elderly. Ann. Neurol. 1993;34(4):566–573. doi: 10.1002/ana.410340410. [DOI] [PubMed] [Google Scholar]
- 14.Morris JC, Storandt M, Mckeel DW, Jr, et al. Cerebral amyloid deposition and diffuse plaques in ‘normal’ aging: evidence for presymptomatic and very mild Alzheimer’s disease. Neurology. 1996;46(3):707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 15.Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, Mcintyre LM. Neuropathological and neuropsychological changes in ‘normal’ aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J. Neuropathol. Exp. Neurol. 1998;57(12):1168–1174. doi: 10.1097/00005072-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J. Neuropathol. Exp. Neurol. 1999;58(4):376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Grip strength and the risk of incident Alzheimer’s disease. Neuroepidemiology. 2007;29(1–2):66–73. doi: 10.1159/000109498. [DOI] [PubMed] [Google Scholar]
- 18.Wilson RS, Arnold SE, Schneider JA, Boyle PA, Buchman AS, Bennett DA. Olfactory impairment in presymptomatic Alzheimer’s disease. Ann. NY Acad. Sci. 2009;1170:730–735. doi: 10.1111/j.1749-6632.2009.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson RS, Barnes LL, Mendes de Leon CF, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59(3):364–370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- 20.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65(6):892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 21.Hikosaka O, Nakamura K, Sakai K, Nakahara H. Central mechanisms of motor skill learning. Curr. Opin. Neurobiol. 2002;12(2):217–222. doi: 10.1016/s0959-4388(02)00307-0. [DOI] [PubMed] [Google Scholar]
- 22.Burke RE. Some unresolved issues in motor unit research. Adv. Exp. Med. Biol. 2002;508:171–178. doi: 10.1007/978-1-4615-0713-0_20. [DOI] [PubMed] [Google Scholar]
- 23.Burke RE, editor. Motor Units: Anatomy, Physiology and Functional Organization. DC, USA: American Physiological Society; 1981. [Google Scholar]
- 24.Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci. 1995;18:314–320. [PubMed] [Google Scholar]
- 25.Fogassi L, Luppino G. Motor functions of the parietal lobe. Curr. Opin. Neurobiology. 2005;15(6):626–631. doi: 10.1016/j.conb.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308(5722):662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- 27.Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31(6):889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- 28.Halsband U, Lange RK. Motor learning in man: a review of functional and clinical studies. J. Physiol. Paris. 2006;99(4–6):414–424. doi: 10.1016/j.jphysparis.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Lehericy S, Bardinet E, Tremblay L, et al. Motor control in basal ganglia circuits using fMRI and brain atlas approaches. Cereb. Cortex. 2006;16(2):149–161. doi: 10.1093/cercor/bhi089. [DOI] [PubMed] [Google Scholar]
- 30.Poppele R, Bosco G. Sophisticated spinal contributions to motor control. Trends Neurosci. 2003;26:269–276. doi: 10.1016/S0166-2236(03)00073-0. [DOI] [PubMed] [Google Scholar]
- 31.Bizzi E, D’Avella A, Saltiel P, Tresch M. Modular organization of spinal motor systems. Neuroscientist. 2002;8(5):437–442. doi: 10.1177/107385802236969. [DOI] [PubMed] [Google Scholar]
- 32.Gosgnach S, Lanuza GM, Butt SJB, et al. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440(7081):215–219. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- 33.Lanuza GM, Gosgnach S, Pierani A, Jessell TM, Goulding M. Genetic identification of spinal interneurons that coordinate left–right locomotor activity necessary for walking movements. Neuron. 2004;42(3):375–373. doi: 10.1016/s0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]
- 34.Gordon IT, Whelan PJ. Deciphering the organization and modulation of spinal locomotor central pattern generators. J. Exp. Biol. 2006;209(11):2007–2014. doi: 10.1242/jeb.02213. [DOI] [PubMed] [Google Scholar]
- 35.Buford TW, Anton SD, Judge AR, et al. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res. Rev. 2010;9(4):369–383. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buford TW, Cooke MB, Manini TM, Leeuwenburgh C, Willoughby DS. Effects of age and sedentary lifestyle on skeletal muscle NF-κB signaling in men. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65A(5):532–537. doi: 10.1093/gerona/glp196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaehle T, Jordan K, Wüstenberg T, Baudewig J, Dechent P, Mast FW. The neural basis of the egocentric and allocentric spatial frame of reference. Brain Res. 2007;1137:92–103. doi: 10.1016/j.brainres.2006.12.044. [DOI] [PubMed] [Google Scholar]
- 38.Bakker M, De Lange FP, Helmich RC, Scheeringa R, Bloem BR, Toni I. Cerebral correlates of motor imagery of normal and precision gait. Neuroimage. 2008;41(3):998–1010. doi: 10.1016/j.neuroimage.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 39.Rosano C, Aizenstein HJ, Studenski S, Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2007;62(9):1048–1055. doi: 10.1093/gerona/62.9.1048. [DOI] [PubMed] [Google Scholar]
- 40.Rosano C, Brach J, Longstreth WT, Jr, Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology. 2006;26(1):52–60. doi: 10.1159/000089240. [DOI] [PubMed] [Google Scholar]
- 41.Rosano C, Brach J, Studenski S, Longstreth WT, Jr, Newman AB. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology. 2007;29(3–4):193–200. doi: 10.1159/000111582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buchman AS, Wilson RS, Bienias JL, Bennett DA. Change in frailty and risk of death in older persons. Exp. Aging Res. 2009;35(1):61–82. doi: 10.1080/03610730802545051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delmonico MJ, Harris TB, Lee J-S, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J. Am. Geriatr. Soc. 2007;55(5):769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 44.Louis ED, Schupf N, Marder K, Tang MX. Functional correlates of mild parkinsonian signs in the community-dwelling elderly: poor balance and inability to ambulate independently. Mov. Disord. 2006;21(3):411–416. doi: 10.1002/mds.20735. [DOI] [PubMed] [Google Scholar]
- 45.Onder G, Penninx BW, Ferrucci L, Fried LP, Guralnik JM, Pahor M. Measures of physical performance and risk for progressive and catastrophic disability: results from the Women’s Health and Aging Study. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60(1):74–79. doi: 10.1093/gerona/60.1.74. [DOI] [PubMed] [Google Scholar]
- 46.Louis ED, Tang MX, Schupf N, Mayeux R. Functional correlates and prevalence of mild parkinsonian signs in a community population of older people. Arch. Neurol. 2005;62(2):297–302. doi: 10.1001/archneur.62.2.297. [DOI] [PubMed] [Google Scholar]
- 47.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J. Gerontol. A Biol. Sci. Med. Sci. 2004;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 48.Louis ED, Bennett DA. Mild parkinsonian signs: an overview of an emerging concept. Mov. Disord. 2007;22(12):1681–1688. doi: 10.1002/mds.21433. [DOI] [PubMed] [Google Scholar]
- 49.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998;147(8):755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 50.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 51.Louis ED, Schupf N, Manly J, Marder K, Tang MX, Mayeux R. Association between mild parkinsonian signs and mild cognitive impairment in a community. Neurology. 2005;64(7):1157–1161. doi: 10.1212/01.WNL.0000156157.97411.5E. [DOI] [PubMed] [Google Scholar]
- 52.Buchman AS, Wilson RS, Boyle PA, Bienias JL, Bennett DA. Change in motor function and risk of mortality in older persons. J. Am. Geriatr. Soc. 2007;55(1):11–19. doi: 10.1111/j.1532-5415.2006.01032.x. [DOI] [PubMed] [Google Scholar]
- 53.Buchman AS, Leurgans SE, Boyle PA, Schneider JA, Arnold SE, Bennett DA. Combinations of motor measures more strongly predict adverse health outcomes in old age: the Rush Memory and Aging Project, a community-based cohort study. BMC Med. 2011 doi: 10.1186/1741-7015-9-42. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chainay H, Louarn C, Humphreys GW. Ideational action impairments in Alzheimer’s disease. Brain Cogn. 2006;62(3):198–205. doi: 10.1016/j.bandc.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 55.Derouesné C, Lagha-Pierucci S, Thibault S, Baudouin-Madec V, Lacomblez L. Apraxic disturbances in patients with mild to moderate Alzheimer’s disease. Neuropsychologia. 2000;38(13):1760–1769. doi: 10.1016/s0028-3932(00)00081-6. [DOI] [PubMed] [Google Scholar]
- 56.Della Sala S, Spinnler H, Venneri A. Walking difficulties in patients with Alzheimer’s disease might originate from gait apraxia. J. Neurol. Neurosurg. Psychiatry. 2004;75(2):196–201. [PMC free article] [PubMed] [Google Scholar]
- 57.Hebert LE, Bienias JL, Mccann JJ, Scherr PA, Wilson RS, Evans DA. Upper and lower extremity motor performance and functional impairment in Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 2010;25(5):425–431. doi: 10.1177/1533317510367636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Agosta F, Rocca MA, Pagani E, et al. Sensorimotor network rewiring in mild cognitive impairment and Alzheimer’s disease. Hum. Brain Mapp. 2010;31(4):515–525. doi: 10.1002/hbm.20883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Babiloni C, Frisoni GB, Vecchio F, et al. Stability of clinical condition in mild cognitive impairment is related to cortical sources of α rhythms: an electroencephalographic study. Hum. Brain Mapp. 2010 doi: 10.1002/hbm.21157. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jahng G-H, Xu S, Weiner M, Meyerhoff D, Park S, Schuff N. DTI studies in patients with Alzheimer’s disease, mild cognitive impairment, or normal cognition with evaluation of the intrinsic background gradients. Neuroradiology. 2011 doi: 10.1007/s00234-011-0845-3. 1-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stump CS, Henriksen EJ, Wei Y, Sowers JR. The metabolic syndrome: role of skeletal muscle metabolism. Ann. Med. 2006;38(6):389–402. doi: 10.1080/07853890600888413. [DOI] [PubMed] [Google Scholar]
- 62.Atti AR, Palmer K, Volpato S, Winblad B, De Ronchi D, Fratiglioni L. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen Project. J. Am. Geriatr. Soc. 2008;56(1):111–116. doi: 10.1111/j.1532-5415.2007.01458.x. [DOI] [PubMed] [Google Scholar]
- 63.Dahl A, Hassing LB, Fransson E, et al. Being overweight in midlife is associated with lower cognitive ability and steeper cognitive decline in late life. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65A(1):57–62. doi: 10.1093/gerona/glp035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ogunniyi A, Gao S, Unverzagt FW, et al. Weight loss and incident dementia in elderly Yoruba Nigerians: a 10-year follow-up study. Int. Psychogeriatr. 2010;23(3):387–394. doi: 10.1017/S1041610210001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71(14):1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 66.Stewart R, Masaki K, Xue Q-L, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu–Asia Aging study. Arch. Neurol. 2005;62(1):55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 67.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I. An 18-year follow-up of overweight and risk of Alzheimer disease. Arch. Intern. Med. 2003;163(13):1524–1528. doi: 10.1001/archinte.163.13.1524. [DOI] [PubMed] [Google Scholar]
- 68.Verdijk LB, Snijders T, Beelen M, et al. Characteristics of muscle fiber type are predictive of skeletal muscle mass and strength in elderly men. J. Am. Geriatr. Soc. 2010;58(11):2069–2075. doi: 10.1111/j.1532-5415.2010.03150.x. [DOI] [PubMed] [Google Scholar]
- 69.Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med. 2004;34(12):809–824. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- 70.Hairi NN, Cumming RG, Naganathan V, et al. Loss of muscle strength, mass (sarcopenia), and quality (specific force) and its relationship with functional limitation and physical disability: the concord Health and Ageing in Men Project. J. Am. Geriatr. Soc. 2010;58(11):2055–2062. doi: 10.1111/j.1532-5415.2010.03145.x. [DOI] [PubMed] [Google Scholar]
- 71. Gustafson D. Adiposity indices and dementia. Lancet Neurol. 2006;5(8):713–720. doi: 10.1016/S1474-4422(06)70526-9.. • Suggests that that the link between BMI and risk of dementia is more complex and may vary over the lifespan.
- 72. Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch. Neurol. 2009;66(11):1339–1344. doi: 10.1001/archneurol.2009.240.. • Loss of strength is common in old age and this study demonstrated that appendicular and axial muscle strength in older persons is associated with the risk of developing mild cognitive impairment (MCI), Alzheimer’s disease (AD) and cognitive decline.
- 73.Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve. 2002;25(1):17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- 74.Camicioli R, Howieson D, Oken B, Sexton G, Kaye J. Motor slowing precedes cognitive impairment in the oldest old. Neurology. 1998;50(5):1496–1498. doi: 10.1212/wnl.50.5.1496. [DOI] [PubMed] [Google Scholar]
- 75. Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch. Neurol. 2010;67(8):980–989. doi: 10.1001/archneurol.2010.159.. •• Motor decline, as indexed by gait speed, accelerates up to 12 years before MCI. Thus, the rate of change in motor decline may identify individuals at risk for developing dementia during its preclinical stage.
- 76.Boyle PA, Wilson RS, Buchman AS, et al. Lower extremity motor function and disability in mild cognitive impairment. Exp. Aging Res. 2007;33(3):355–371. doi: 10.1080/03610730701319210. [DOI] [PubMed] [Google Scholar]
- 77.Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Arch. Neurol. 2006;63(12):1763–1769. doi: 10.1001/archneur.63.12.1763. [DOI] [PubMed] [Google Scholar]
- 78.Watson NL, Rosano C, Boudreau RM, et al. Executive function, memory, and gait Speed decline in well-functioning older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65A(10):1093–1100. doi: 10.1093/gerona/glq111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang L, Larson EB, Bowen JD, Van Belle G. Performance-based physical function and future dementia in older people. Arch. Intern. Med. 2006;166(10):1115–1120. doi: 10.1001/archinte.166.10.1115. [DOI] [PubMed] [Google Scholar]
- 80.Inzitari M, Newman AB, Yaffe K, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: the Health Aging and Body Composition Study. Neuroepidemiology. 2007;29:156–162. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Waite LM, Grayson DA, Piguet O, Creasey H, Bennett HP, Broe GA. Gait slowing as a predictor of incident dementia: 6-year longitudinal data from the Sydney Older Persons Study. J. Neurol. Sci. 2005:229–230. 89–93. doi: 10.1016/j.jns.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 82.Ble A, Volpato S, Zuliani G, et al. Executive function correlates with walking speed in older persons: the InCHIANTI study. J. Am. Geriatr. Soc. 2005;53(3):410–415. doi: 10.1111/j.1532-5415.2005.53157.x. [DOI] [PubMed] [Google Scholar]
- 83.Soumare A, Tavernier B, Alperovitch A, Tzourio C, Elbaz A. A cross-sectional and longitudinal study of the relationship between walking speed and cognitive function in community-dwelling elderly people. J. Gerontol. A Biol. Sci. Med. Sci. 2009:1058–1065. doi: 10.1093/gerona/glp077. [DOI] [PubMed] [Google Scholar]
- 84.Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Parkinsonianlike signs and risk of incident Alzheimer disease in older persons. Arch. Neurol. 2003;60(4):539–544. doi: 10.1001/archneur.60.4.539. [DOI] [PubMed] [Google Scholar]
- 85.Richards M, Stern Y, Mayeux R. Subtle extrapyramidal signs can predict the development of dementia in elderly individuals. Neurology. 1993;43(11):2184–2188. doi: 10.1212/wnl.43.11.2184. [DOI] [PubMed] [Google Scholar]
- 86.Portet F, Scarmeas N, Cosentino S, Helzner EP, Stern Y. Extrapyramidal signs before and after diagnosis of incident Alzheimer disease in a prospective population study. Arch. Neurol. 2009;66(9):1120–1126. doi: 10.1001/archneurol.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Jr, Walston JD. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J. Am. Geriatr. Soc. 2004;52(4):625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 88.Bandeen-Roche K, Xue Q-L, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J. Gerontol. A Biol. Sci. Med. Sci. 2006;61(3):262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- 89.Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident Alzheimer’s disease and cognitive decline in the elderly. Psychosom. Med. 2007;69(5):483–489. doi: 10.1097/psy.0b013e318068de1d. [DOI] [PubMed] [Google Scholar]
- 90.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Physical frailty is associated with incident mild cognitive impairment in community-based older persons. J. Am. Geriatr. Soc. 2010;58(2):248–255. doi: 10.1111/j.1532-5415.2009.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ungvari Z, Kaley G, De Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J. Gerontol. A Biol. Sci. Med. Sci. 2010;65A(10):1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J. Leukoc. Biol. 2008;84(4):932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011;10(2):187–198. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sultana R, Perluigi M, Butterfield D. Oxidatively modified proteins in Alzheimer’s disease (AD), mild cognitive impairment and animal models of AD: role of Aβ in pathogenesis. Acta Neuropathologica. 2009;118(1):131–150. doi: 10.1007/s00401-009-0517-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch. Neurol. 2004;61(3):378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 96.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Berry-Kravis E, Arnold SE. Amyloid mediates the association of apolipoprotein E ε4 allele to cognitive function in older people. J. Neurol. Neurosurg. Psychiatry. 2005;76(9):1194–1199. doi: 10.1136/jnnp.2004.054445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mahley RW, Weisgraber KH, Huang Y. Inaugural article: apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc. Natl Acad. Sci. 2006;103(15):5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Houlden H, Greenwood R. Apolipoprotein E4 and traumatic brain injury. J. Neurol. Neurosurg. Psychiatry. 2006;77(10):1106–1107. doi: 10.1136/jnnp.2006.095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wilson RS, Schneider JA, Barnes LL, et al. The apolipoprotein E ε4 allele and decline in different cognitive systems during a 6-year period. Arch. Neurol. 2002;59(7):1154–1160. doi: 10.1001/archneur.59.7.1154. [DOI] [PubMed] [Google Scholar]
- 100.Sudlow C, Martinez Gonzalez NA, Kim J, Clark C. Does Apolipoprotein E genotype influence the risk of ischemic stroke, intracerebral hemorrhage, or subarachnoid hemorrhage?: systematic review and meta-analyses of 31 studies among 5961 cases and 17,965 controls. Stroke. 2006;37(2):364–370. doi: 10.1161/01.STR.0000199065.12908.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Burt TD, Agan BK, Marconi VC, et al. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE ε4/ε4 genotype accelerates HIV disease progression. Proc. Natl Acad. Sci. 2008;105(25):8718–8723. doi: 10.1073/pnas.0803526105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Buchman AS, Boyle PA, Wilson RS, Beck T, Kelly JF, Bennett DA. Apolipoprotein E ε4 allele is associated with more rapid motor decline in older persons. Alzheimer Dis. Assoc. Disord. 2009;23:63–69. doi: 10.1097/wad.0b013e31818877b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jun G, Naj AC, Beecham GW, et al. Meta-analysis confirms CR1, CLU, and PICALM as Alzheimer disease risk loci and reveals interactions with APOE genotypes. Arch. Neurol. 2010;67(12):1473–1484. doi: 10.1001/archneurol.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seshadri S, Fitzpatrick AL, Ikram MA, et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303(18):1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Claeys KG, Maisonobe T, Böhm J, et al. Phenotype of a patient with recessive centronuclear myopathy and a novel BIN1 mutation. Neurology. 2010;74(6):519–521. doi: 10.1212/WNL.0b013e3181cef7f9. [DOI] [PubMed] [Google Scholar]
- 106.Wilson RS, Arnold SE, Tang Y, Bennett DA. Odor identification and decline in different cognitive domains in old age. Neuroepidemiology. 2006;26(2):61–67. doi: 10.1159/000090250. [DOI] [PubMed] [Google Scholar]
- 107.Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch. Gen. Psychiatry. 2007;64(7):802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- 108.Wilson RS, Yu L, Schneider JA, Arnold SE, Buchman AS, Bennett DA. Lewy bodies and olfactory dysfunction in old age. Chem. Senses. 2011 doi: 10.1093/chemse/bjq139. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wilson RS, Arnold SE, Buchman AS, Tang Y, Bennett DA. Odor identification and progression of parkinsonian signs in older persons. Exp. Aging Res. 2008;34(3):173–187. doi: 10.1080/03610730802070001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schubert CR, Carmichael LL, Murphy C, Klein BE, Klein R, Cruickshanks KJ. Olfaction and the 5-year incidence of cognitive impairment in an epidemiological study of older adults. J. Am. Geriatr. Soc. 2008;56(8):1517–1521. doi: 10.1111/j.1532-5415.2008.01826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Olofsson JK, Ronnlund M, Nordin S, Nyberg L, Nilsson LG, Larsson M. Odor identification deficit as a predictor of five-year global cognitive change: interactive effects with age and ApoE-ε4. Behav. Genet. 2009;39(5):496–503. doi: 10.1007/s10519-009-9289-5. [DOI] [PubMed] [Google Scholar]
- 112.Schrijvers EMC, Witteman JCM, Sijbrands EJG, Hofman A, Koudstaal PJ, Breteler MMB. Insulin metabolism and the risk of Alzheimer disease. Neurology. 2010;75(22):1982–1987. doi: 10.1212/WNL.0b013e3181ffe4f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rocca WA, Petersen RC, Knopman DS, et al. Trends in the incidence and prevalence of Alzheimer’s disease, dementia, and cognitive impairment in the United States. Alzheimers Demen. 2011;7(1):80–93. doi: 10.1016/j.jalz.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hoffman LB, Schmeidler J, Lesser GT, et al. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology. 2009;72(20):1720–1726. doi: 10.1212/01.wnl.0000345881.82856.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Beeri MS, Ravona-Springer R, Silverman JM, Haroutunian V. The effects of cardiovascular risk factors on cognitive compromise. Dialogues Clin. Neurosci. 2009;11(2):201–212. doi: 10.31887/DCNS.2009.11.2/msbeeri. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arvanitakis Z, Bennett DA, Wilson RS, Barnes LL. Diabetes and cognitive systems in older black and white persons. Alzheimer Dis. Assoc. Disord. 2009;24(1):37–42. doi: 10.1097/WAD.0b013e3181a6bed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch. Neurol. 2004;61(5):661–666. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 118.Arvanitakis Z, Wilson RS, Li Y, Aggarwal NT, Bennett DA. Diabetes and function in different cognitive systems in older individuals without dementia. Diabetes Care. 2006;29(3):560–565. doi: 10.2337/diacare.29.03.06.dc05-1901. [DOI] [PubMed] [Google Scholar]
- 119.Dumurgier J, Elbaz A, Dufouil C, Tavernier B, Tzourio C. Hypertension and lower walking speed in the elderly: the Three-City study. J. Hypertension. 2010;28(7):1506–1514. doi: 10.1097/HJH.0b013e328338bbec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sorond FA, Galica A, Serrador JM, et al. Cerebrovascular hemodynamics, gait, and falls in an elderly population. Neurology. 2010;74(20):1627–1633. doi: 10.1212/WNL.0b013e3181df0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arvanitakis Z, Wilson RS, Bienias JL, Bennett DA. Diabetes and parkinsonian signs in older persons. Alzheimer Dis. Assoc. Disord. 2007;21(2):144–149. doi: 10.1097/WAD.0b013e31805ba768. [DOI] [PubMed] [Google Scholar]
- 122.Arvanitakis Z, Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and progression of rigidity and gait disturbance in older persons. Neurology. 2004;63(6):996–1001. doi: 10.1212/01.wnl.0000138432.16676.4b. [DOI] [PubMed] [Google Scholar]
- 123.Buchman AS, Boyle PA, Wilson RS, Leurgans S, Shah RC, Bennett DA. Respiratory muscle strength predicts decline in mobility in older persons. Neuroepidemiology. 2008;31(3):174–180. doi: 10.1159/000154930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Buchman AS, Boyle PA, Leurgans SE, Evans DA, Bennett DA. Pulmonary function, muscle strength and mobility disability in community-dwelling elders. Proc. Am. Thorac. Soc. 2009;6:581–587. doi: 10.1513/pats.200905-030RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174(6):801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Larson EB. Physical activity for older adults at risk for Alzheimer disease. JAMA. 2008;300(9):1077–1079. doi: 10.1001/jama.300.9.1077. [DOI] [PubMed] [Google Scholar]
- 127.Barak Y, Aizenberg D. Is dementia preventable? Focus on Alzheimer’s disease. Expert Rev. Neurother. 2010;10(11):1689–1698. doi: 10.1586/ern.10.159. [DOI] [PubMed] [Google Scholar]
- 128.Scarmeas N, Luchsinger JA, Schupf N, et al. Physical activity, diet, and risk of Alzheimer disease. JAMA. 2009;302(6):627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–1037. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 130.Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch. Intern. Med. 2010;170(2):170–178. doi: 10.1001/archinternmed.2009.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dechamps A, Diolez P, Thiaudiere E, et al. Effects of exercise programs to prevent decline in health-related quality of life in highly deconditioned institutionalized elderly persons: a randomized controlled trial. Arch. Int. Med. 2010;170(2):162–169. doi: 10.1001/archinternmed.2009.489. [DOI] [PubMed] [Google Scholar]
- 132.Etgen T, Sander D, Huntgeburth U, Poppert H, Forstl H, Bickel H. Physical activity and incident cognitive impairment in elderly persons: the INVADE Study. Arch. Int. Med. 2010;170(2):186–193. doi: 10.1001/archinternmed.2009.498. [DOI] [PubMed] [Google Scholar]
- 133.Evans DA, Bienias JL. Alcohol consumption and cognition. N. Engl. J. Med. 2005;352(3):289–290. doi: 10.1056/NEJMe048315. [DOI] [PubMed] [Google Scholar]
- 134.Buchman AS, Boyle PA, Wilson RS, Bienias JL, Bennett DA. Physical activity and motor decline in older persons. Muscle Nerve. 2007;35:354–362. doi: 10.1002/mus.20702. [DOI] [PubMed] [Google Scholar]
- 135. Buchman AS, Boyle PA, Wilson RS, Fleischman DA, Leurgans S, Bennett DA. Association between late-life social activity and motor decline in older adults. Arch. Int. Med. 2009;169(12):1139–1146. doi: 10.1001/archinternmed.2009.135.. • Psychosocial factors are known to be related to the rate of cognitive decline. This study found that higher levels of social engagement in older individuals were associated with a slower rate of motor decline. Thus, motor and cognitive decline may share similar risk factors.
- 136.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurol. 2004;3(6):343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 137.Wilson RS, Arnold SE, Schneider JA, Li Y, Bennett DA. Chronic distress, age-related neuropathology, and late-life dementia. Psychosom. Med. 2007;69(1):47–53. doi: 10.1097/01.psy.0000250264.25017.21. [DOI] [PubMed] [Google Scholar]
- 138.Wilson RS, Evans DA, Bienias JL, Mendes de Leon CF, Schneider JA, Bennett DA. Proneness to psychological distress and risk of Alzheimer’s disease. Neurology. 2003;61(11):1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- 139.Wilson RS, Krueger KR, Arnold SE, et al. Loneliness and risk of Alzheimer disease. Arch. Gen. Psychiatry. 2007;64(2):234–240. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- 140.Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- 141.Buchman A, Boyle P, Wilson R, et al. Loneliness and the rate of motor decline in old age: the Rush Memory and Aging Project, a community-based cohort study. BMC Geriatrics. 2010;10(1):77. doi: 10.1186/1471-2318-10-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rhodes RE, Smith NEI. Personality correlates of physical activity: a review and meta-analysis. Br. J. Sports Med. 2006;40(12):958–965. doi: 10.1136/bjsm.2006.028860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tolea MI, Costa PT, Terracciano A, et al. Sex-specific correlates of walking speed in a wide age-ranged population. J. Gerontol. B Psycholog. Sci. Soc. Sci. 2010;65B(2):174–184. doi: 10.1093/geronb/gbp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Buchman AS, Boyle PA, Leurgans SE, Barnes LL, Bennett DA. Cognitive function is associated with the development of mobility impairments in community-dwelling elders. Am. J. Geriatr. Psych. 2011 doi: 10.1097/JGP.0b013e3181ef7a2e. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol. 2009;66(2):200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66(12):1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6.. •• This autopsy study excluded both individuals with MCI and with dementia, yet still found that a substantial proportion (>35%) of cognitively normal adult subjects demonstrated evidence for AD pathology that met pathologic criteria for AD as well as subtle changes in episodic memory. Thus, accumulation of AD pathology in old age may account for cognitive dysfunction currently considered ‘normal aging’.
- 147.Greenberg SA. How citation distortions create unfounded authority: analysis of a citation network. BMJ. 2009;339:b2680. doi: 10.1136/bmj.b2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Salajegheh M, Pinkus JL, Nazareno R, Amato AA, Parker KC, Greenberg SA. Nature of ‘Tau’ immunoreactivity in normal myonuclei and inclusion body myositis. Muscle Nerve. 2009;40(4):520–528. doi: 10.1002/mus.21471. [DOI] [PubMed] [Google Scholar]
- 149. Cisek P, Kalaska JF. Neural mechanisms for interacting with a world full of action choices. Ann. Rev. Neurosci. 2010;33(1):269–298. doi: 10.1146/annurev.neuro.051508.135409.. • Physical frailty, which components includes grip strength, gait speed and BMI, was associated with the level of AD pathology in older persons with and without dementia. This suggests that AD pathology may contribute to loss of motor function in old age.
- 150.Buchman AS, Schneider JA, Wilson RS, Bienias JL, Bennett DA. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology. 2006;67:1949–1954. doi: 10.1212/01.wnl.0000247046.90574.0f. [DOI] [PubMed] [Google Scholar]
- 151.Buchman AS, Schneider JA, Leurgans S, Bennett DA. Physical frailty in older persons is associated with Alzheimer disease pathology. Neurology. 2008;71(7):499–504. doi: 10.1212/01.wnl.0000324864.81179.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Schneider JA, Li JL, Li Y, Wilson RS, Kordower JH, Bennett DA. Substantia nigra tangles are related to gait impairment in older persons. Ann. Neurol. 2006;59(1):166–173. doi: 10.1002/ana.20723. [DOI] [PubMed] [Google Scholar]
- 153.Gearing M, Levey AI, Mirra SS. Diffuse plaques in the striatum in Alzheimer disease (AD): relationship to the striatal mosaic and selected neuropeptide markers. J. Neuropathol. Exp. Neurol. 1997;56(12):1363–1370. doi: 10.1097/00005072-199712000-00011. [DOI] [PubMed] [Google Scholar]
- 154.Wolf DS, Gearing M, Snowdon DA, Mori H, Markesbery WR, Mirra SS. Progression of regional neuropathology in Alzheimer disease and normal elderly: findings from the Nun study. Alzheimer Dis. Assoc. Disord. 1999;13(4):226–231. doi: 10.1097/00002093-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 155.Horoupian DS, Wasserstein PH. Alzheimer’s disease pathology in motor cortex in dementia with Lewy bodies clinically mimicking corticobasal degeneration. Acta Neuropathol. (Berl.) 1999;98(3):317–322. doi: 10.1007/s004010051087. [DOI] [PubMed] [Google Scholar]
- 156.Burns JM, Galvin JE, Roe CM, Morris JC, McKeel DW. The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology. 2005;64(8):1397–1403. doi: 10.1212/01.WNL.0000158423.05224.7F. [DOI] [PubMed] [Google Scholar]
- 157.Suva D, Favre I, Kraftsik R, Esteban M, Lobrinus A, Miklossy J. Primary motor cortex involvement in Alzheimer disease. J. Neuropathol. Exp. Neurol. 1999;58(11):1125–1134. doi: 10.1097/00005072-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 158.Bennett DA, Wilson RS, Schneider JA, et al. Education modifies the relation of AD pathology to level of cognitive function in older persons. Neurology. 2003;60(12):1909–1915. doi: 10.1212/01.wnl.0000069923.64550.9f. [DOI] [PubMed] [Google Scholar]
- 159.Boyle PA, Wilson RS, Schneider JA, Bienias JL, Bennett DA. Processing resources reduce the effect of Alzheimer pathology on other cognitive systems. Neurology. 2008;70(17):1534–1542. doi: 10.1212/01.wnl.0000304345.14212.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rose G. The Strategy of Preventive Medicine. Oxford, UK: Oxford University Press; 1992. [Google Scholar]
- 161.Nithianantharajah J, Hannan AJ. The neurobiology of brain and cognitive reserve: mental and physical activity as modulators of brain disorders. Prog. Neurobiol. 2009;89(4):369–382. doi: 10.1016/j.pneurobio.2009.10.001. [DOI] [PubMed] [Google Scholar]
Website
- 201.US Census, Bureau News Report. DC, USA: Department of Commerce; 2006. Dramatic Changes in US Aging. www.census.gov/newsroom/releases/archives/aging_population/cb06-36.html. [Google Scholar]