Abstract
Background: Breast cancer cells deficient for BRCA1 are hypersensitive to agents inducing DNA double-strand breaks (DSB), such as bifunctional alkylators and platinum agents. Earlier, we had developed a comparative genomic hybridisation (CGH) classifier based on BRCA1-mutated breast cancers. We hypothesised that this BRCA1-likeCGH classifier could also detect loss of function of BRCA1 due to other causes besides mutations and, consequently, might predict sensitivity to DSB-inducing agents.
Patients and methods: We evaluated this classifier in stage III breast cancer patients, who had been randomly assigned between adjuvant high-dose platinum-based (HD-PB) chemotherapy, a DSB-inducing regimen, and conventional anthracycline-based chemotherapy. Additionally, we assessed BRCA1 loss through mutation or promoter methylation and immunohistochemical basal-like status in the triple-negative subgroup (TN subgroup).
Results: We observed greater benefit from HD-PB chemotherapy versus conventional chemotherapy among patients with BRCA1-likeCGH tumours [41/230 = 18%, multivariate hazard ratio (HR) = 0.12, 95% confidence interval (CI) 0.04–0.43] compared with patients with non-BRCA1-likeCGH tumours (189/230 = 82%, HR = 0.78, 95% CI 0.50–1.20), with a significant difference (test for interaction P = 0.006). Similar results were obtained for overall survival (P interaction = 0.04) and when analyses were restricted to the TN subgroup. Sixty-three percent (20/32) of assessable BRCA1-likeCGH tumours harboured either a BRCA1 mutation (n = 8) or BRCA1 methylation (n = 12).
Conclusion: BRCA1 loss as assessed by CGH analysis can identify patients with substantially improved outcome after adjuvant DSB-inducing chemotherapy when compared with standard anthracycline-based chemotherapy in our series.
Keywords: array comparative genomic hybridisation, BRCA1, breast cancer, high-dose chemotherapy, platinum salt, predictive marker
introduction
Most evidence for benefit of adjuvant systemic treatment comes from large clinical trials carried out in the general breast cancer population [1]. However, these trials do not generally consider the molecular heterogeneity of breast cancers, which may be related to treatment benefit of individual patients. The disadvantage of these traditional trials can be best illustrated with the example of trastuzumab. Its efficacy among breast cancer patients with an amplification of the human epidermal growth factor receptor-2 (HER2-positive) would likely have been overlooked in analyses of the general population since a large percentage of breast cancers is HER2 negative and therefore does not benefit from trastuzumab. Several systemic treatments might therefore have been discarded in the past, although they may have been proven beneficial if tested in a predefined targeted population.
Among these discarded agents are bifunctional alkylators and platinum salts, which are not commonly used, with the exception of cyclophosphamide (Endoxan; Baxter International, Deefield, IL, USA), due to their relatively high toxicity and low level of efficacy in unselected breast cancer patients [2–5]. These agents act via formation of DNA cross links resulting in DNA double-strand breaks (DSBs). Preclinical and clinical evidence has emerged that a possible target of DSB-inducing agents are tumours with a non-functional BRCA1 protein, such as tumours with BRCA1 mutations [6–9]. BRCA1-mutated tumours showed hypersensitivity to these agents, which may be related to the role of BRCA1 in homologous recombination, a conservative mechanism for error-free repair of DSBs. Absence of homologous recombination, such as in BRCA1-mutated tumours, prohibits error-free repair of DSBs, which is reported to lead to cell death [10].
Furthermore, defects in homologous recombination activate alternative more error-prone mechanisms such as non-homologous end joining, presumably leading to genomic instability [11–13]. BRCA1-loss-related instability can be visualised by array comparative genomic hybridisation (aCGH) showing characteristic copy number aberrations (CNAs) in defined genomic loci in a tumour [14–17].
We have previously developed an aCGH BRCA1-like classifier aimed to differentiate between BRCA1-mutated and sporadic breast cancers with reasonable accuracy based on their characteristic CNAs [17]. This test has been shown to have a relatively high sensitivity but a somewhat lower specificity for BRCA1-mutated tumours. We hypothesised that tumours testing “false positive” with the classifier could represent tumours with functional BRCA1 loss due to other causes than mutations, such as BRCA1 promoter methylation. If true, the BRCA1-likeCGH classifier would identify a larger fraction of breast cancer patients, who might benefit from DSB-inducing agents.
The aim of this study was to determine whether the BRCA1-likeCGH classifier was capable of identifying patients benefiting from DSB-inducing agents. For this purpose, we studied a representative sample of stage III HER2-negative breast cancer patients who had been randomly assigned between two treatment arms; high-dose, platinum-based, alkylating chemotherapy (HD-PB chemotherapy), which is a DSB-inducing regimen, and a standard anthracycline-based regimen (conventional chemotherapy) in a trial with long-term follow-up [18]. We restricted our analyses to HER2-negative patients, as in the pivotal study HER2-positive patients did not benefit from HD-PB chemotherapy [18]. Since patients in our study had been randomised, we could differentiate between selective HD-PB chemotherapy benefit and general chemotherapy benefit. Accordingly, we evaluated whether the effect of HD-PB chemotherapy on survival differed by BRCA1-likeCGH classification based on multivariate proportional hazards regression with an interaction term. To explore the biology of BRCA1-likeCGH classified tumours, we studied their association with other markers for BRCA1 loss. We studied basal-like status defined by immunohistochemistry (IHC) since this had been associated with BRCA1-mutated breast cancers [19, 20]. Secondly, we assessed BRCA1 promoter methylation, which has been reported as an alternative mechanism for reduced BRCA1 expression in basal-like breast cancer [21, 22]. Lastly, BRCA1 mutation status was determined. Since we found a strong association between the BRCA1-likeCGH classified tumours and triple-negative status and these markers have all been associated with triple negativity, we investigated them in the triple-negative subgroup (TN subgroup).
patients and methods
BRCA1-likeCGH classification
A BRCA1-likeCGH classifier, which calculates the probability of belonging to the BRCA1-mutated class, had previously been constructed (see supplemental Appendix B, available at Annals of Oncology online). We determined the optimal cut-off of the BRCA1-likeCGH probability score to identify breast cancer patients likely to benefit from DSB-inducing agents (for details, see supplemental Appendix B, available at Annals of Oncology online). For this purpose, we studied metastatic breast cancer (MBC) patients who had participated in phase II studies of HD-PB chemotherapy (n = 39, MBC series described in supplemental Appendix B, available at Annals of Oncology online) [23–25]. We carried out BRCA1-likeCGH class detection on each individual aCGH tumour profile, resulting in either a BRCA1-likeCGH or a non-BRCA1-likeCGH score.
patient selection
We studied stage III HER2-negative breast cancer patients from a large randomised controlled trial (RCT) carried out in the Netherlands between 1993 and 1999 in the adjuvant setting (stage III series). Eligibility criteria have been published previously [18] (see supplemental Appendix A, available at Annals of Oncology online). Patients were randomly assigned between conventional chemotherapy (5*FEC: 5-fluorouracil 500 mg/m2, epirubicin 90 mg/m2, cyclophosphamide 500 mg/m2) and HD-PB chemotherapy (4*FEC, followed by 1*CTC: cyclophosphamide 6000 mg/m2, thiotepa 480 mg/m2 and carboplatin 1600 mg/m2) [18].
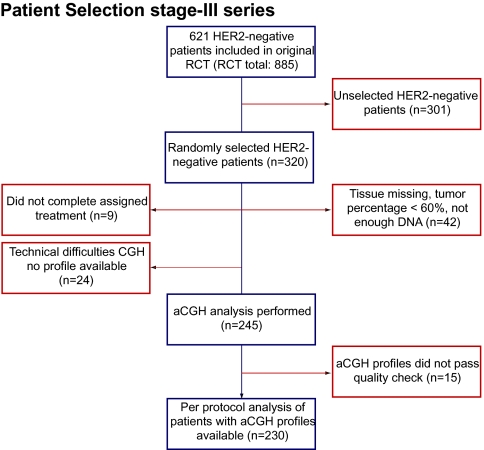
Due to practical (financial) constraints, we did not evaluate all 621 HER2-negative breast cancer patients, but randomly selected 320 HER2-negative patients (320/621 HER2-negative cases, 51%). Patient samples were included in analyses if formalin-fixed paraffin-embedded (FFPE) primary tumour tissue consisting of more than 60% of tumour cells was available and if they had been treated per-protocol. Figure 1 summarises the flow of patients through the study. All trials described in this article were approved by the Institutional Review Board of the Netherlands Cancer Institute. This study was designed according to the REporting recommendations for tumor MArker prognostic studies (REMARK) guidelines [27] following the predictive marker trial design of ‘Indirect assessment: Marker by treatment interaction design, test of interaction’ as described by Sargent et al. [28].
Figure 1.
Flow diagram of patients in the study. Flow of patients through the study including number of patients in each stage. Reasons for dropout are listed. aCGH, array comparative genomic hybridisation.
comparative genomic hybridisation
Genomic DNA was extracted from FFPE primary tumours as previously described [29]. For 11 patients, only lymph node tissue containing primary tumour tissue, removed at first diagnosis, was available. Of 11 samples, DNA concentrations were too low for direct aCGH analysis and these samples were amplified with the BioScore™ Screening and Amplification Kit (42440, Enzo Life Sciences BVBA, Zandhoven, Belgium). Tumour DNA and reference DNA were labelled and hybridised as published previously (see supplemental Appendix A, available at Annals of Oncology online) [30]. To determine the quality of each CGH profile and to be able to compare experiments, we used a profile quality and hybridisation quality score (see supplemental Appendix A, available at Annals of Oncology online).
mutation and methylation analyses
We screened for 38 known BRCA1 mutations using allelic discrimination and multiplex PCR accounting for 853 of 1166 BRCA1 families (∼73%) in the Netherlands (Supplemental Table S1, available at Annals of Oncology online) (M. K. Schmidt et al., unpublished data). Each putative mutation identified was validated using capillary sequencing.
Hypermethylation of the BRCA1 promoter was assessed using a custom methylation specific MLPA set according to the manufacturer’s protocol (ME005-custom; MRC-Holland, Amsterdam, the Netherlands). Probe sequences of the MLPA set are available on request (info@mlpa.com). DNA fragments were analysed on a 3730 DNA Analyzer (Applied Biosystems, Foster City, CA, USA). For normalisation and analysis, the Coffalyzer program was used (MRC-Holland, Amsterdam, The Netherlands); peak heights below 250 were excluded from further analyses. When both BRCA1 probes showed methylation (threshold of 0.2; MRC-Holland), we classified the result as BRCA1 promoter methylation.
histopathology
Haematoxylin and eosin-stained slides were scored for tumour percentages. Estrogen receptor (ER), progesterone receptor (PR), P53 and HER2 status were determined by IHC as described previously [18, 32]. We used Pronase pretreatment for the epidermal growth factor receptor (EGFR Ab-10 clone 111.6; 1 : 200; Neomarkers / Lab Vision Corporation, Fremont, CA, USA; EGFR clone 31G7, 1 : 400; Zymed / Invitrogen, Carlsbad, CA, USA) and the standard procedure for cytokeratin 5/6 staining (CK5/6, clone D5/16 B4, M7237, 1 : 200; Dako, Glostrup, Denmark). CK5/6 and EGFR were considered positive if any staining of tumour cells was observed. Tumours were classified as basal-like according to the Nielsen basal-like breast cancer IHC definition [33].
statistical analysis
Differences between groups of interest were tested using Fisher’s exact tests. Survival curves were generated using the Kaplan–Meier method and compared using log-rank tests. Hazard ratios (HR) were calculated using Cox proportional hazards regression.
Recurrence-free survival (RFS) was calculated from randomisation to appearance of local or regional recurrence, metastases or to death from any cause [18]. All other events were censored. Overall survival (OS) was time from randomisation to death from any cause or end of follow-up. Median RFS and OS were 7.6 and 8.2 years, respectively, for all 230 patients. Patients alive at last follow-up were censored at that time. All treatment comparisons were based on patients who completed their assigned treatment (per-protocol analysis) to secure the correct correlation between molecular subtype and treatment received. We assessed whether the effect of HD-PB chemotherapy versus conventional chemotherapy on survival, expressed as the HR, differed by BRCA1-likeCGH status based on multivariate proportional hazards regression with an interaction term, adjusting for potential confounders. All calculations were carried out using the statistical package SPSS 15.0.1 (for Windows) (SPSS Inc., Chicago, IL, USA).
results
Of the 320 randomly selected patients, 90 could not be analysed with aCGH due to unavailability or low quality of tumour tissue (i.e. tumour percentage, DNA yield, quality of DNA reflected by the aCGH quality score). In Figure 1, reasons for dropout are listed. Our selection held more ER- and PR-negative patients than the HER2-negative patients not selected for these analyses. Otherwise, characteristics and treatments of these 230 cases did not differ from those HER2-negative cases of the RCT not in current analyses (Supplemental Table S2, available at Annals of Oncology online).
Forty-one of the 230 tumours (18%) were scored as BRCA1 likeCGH. Patient characteristics did not differ by treatment arm within the BRCA1- or non-BRCA1-likeCGH subgroups (Table 1). When compared with patients with non-BRCA1-likeCGH tumours, patients with BRCA1-likeCGH tumours were generally younger and more often treated with breast-conserving surgery; their tumours were more often poorly differentiated, triple-negative, basal-like and P53-positive (Table 1).
Table 1.
Patient characteristics distributed by treatment arm and BRCA1 classification of the stage III series
| Variable | Patients with non-BRCA1-likeCGH tumours |
Patients with BRCA1-likeCGH tumours |
Pb | |||||||||||||
| Conventional chemotherapy |
HD-PB chemotherapy |
Total |
Pa | Conventional chemotherapy |
HD-PB chemotherapy |
Total |
Pa | |||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |||||
| Total | 95 | 50.3 | 94 | 49.7 | 189 | 100.0 | 23 | 56.1 | 18 | 43.9 | 41 | 100.0 | n.s | |||
| Age in categories, years | ||||||||||||||||
| ≤40 | 21 | 22.1 | 22 | 23.4 | 43 | 22.8 | n.s | 11 | 47.8 | 9 | 50.0 | 20 | 48.8 | n.s | 0.002 | |
| >40 | 74 | 77.9 | 72 | 76.6 | 146 | 77.2 | 12 | 52.2 | 9 | 50 | 21 | 51.2 | ||||
| Type of surgery | ||||||||||||||||
| Breast-conserving therapy | 16 | 16.8 | 18 | 19.1 | 34 | 18.0 | n.s | 8 | 34.8 | 6 | 33.3 | 14 | 34.1 | n.s | 0.03 | |
| Mastectomy | 79 | 83.2 | 76 | 80.9 | 155 | 82.0 | 15 | 65.2 | 12 | 66.7 | 27 | 65.9 | ||||
| Pathological tumour classification | ||||||||||||||||
| pT1 or pT2 | 80 | 84.2 | 78 | 83.0 | 158 | 83.6 | n.s | 19 | 82.6 | 17 | 94.4 | 36 | 87.8 | n.s | n.s | |
| pT3 | 15 | 15.8 | 14 | 14.9 | 29 | 15.3 | 4 | 17.4 | 1 | 5.6 | 5 | 12.2 | ||||
| Unknown | 0 | 0.0 | 2 | 2.1 | 2 | 1.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||||
| Number of positive lymph nodes | ||||||||||||||||
| 4–9 | 66 | 69.5 | 59 | 62.8 | 125 | 66.1 | n.s | 15 | 65.2 | 11 | 61.1 | 26 | 63.4 | n.s | n.s | |
| ≥10 | 29 | 30.5 | 35 | 37.2 | 64 | 33.9 | 8 | 34.8 | 7 | 38.9 | 15 | 36.6 | ||||
| Histological grade | ||||||||||||||||
| I + II | 63 | 66.3 | 63 | 67.0 | 126 | 66.7 | n.s | 4 | 17.4 | 1 | 5.6 | 5 | 12.2 | n.s | <0.001 | |
| III | 30 | 31.6 | 27 | 28.7 | 57 | 30.2 | 19 | 82.6 | 14 | 77.8 | 33 | 80.5 | ||||
| Not determined | 2 | 2.1 | 4 | 4.3 | 6 | 3.2 | 0 | 0.0 | 3 | 16.7 | 3 | 7.3 | ||||
| Triple-negative status | ||||||||||||||||
| ER or PR positive (≥10%) | 82 | 86.3 | 81 | 86.2 | 163 | 86.2 | n.s | 4 | 17.4 | 1 | 5.6 | 5 | 12.2 | n.s | <0.001 | |
| Triple negative | 13 | 13.7 | 13 | 13.8 | 26 | 13.8 | 18 | 78.3 | 16 | 88.9 | 34 | 82.9 | ||||
| Unknown | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 4.3 | 1 | 5.6 | 2 | 4.9 | ||||
| Nielsen basal-like breast cancer definition | ||||||||||||||||
| Negative | 89 | 93.7 | 85 | 90.4 | 174 | 92.1 | n.s | 7 | 30.4 | 2 | 11.1 | 9 | 22.0 | n.s | <0.001 | |
| Basal-like | 6 | 6.3 | 9 | 9.6 | 15 | 7.9 | 15 | 65.2 | 15 | 83.3 | 30 | 73.2 | ||||
| Unknown | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 4.3 | 1 | 5.6 | 2 | 4.9 | ||||
| P53 status | ||||||||||||||||
| Negative (<10%) | 51 | 53.7 | 65 | 69.1 | 116 | 61.4 | 0.05 | 8 | 34.8 | 7 | 38.9 | 15 | 36.6 | n.s | 0.02 | |
| Positive (≥10%) | 40 | 42.1 | 26 | 27.7 | 66 | 34.9 | 12 | 52.2 | 8 | 44.4 | 20 | 48.8 | ||||
| Unknown | 4 | 4.2 | 3 | 3.2 | 7 | 3.7 | 3 | 13.0 | 3 | 16.7 | 6 | 14.6 | ||||
Patients with unknown values were omitted and P values were calculated using the Fisher’s exact test.
Association within subgroup.
Association between subgroups.
CGH, comparative genomic hybridisation; ER, estrogen receptor; n.s., non-significant; PR, progesterone receptor.
outcome according to treatment in stage III series by BRCA1-likeCGH classification
The beneficial effect of HD-PB chemotherapy compared with conventional chemotherapy differed between patients with BRCA1-likeCGH tumours and those with non-BRCA1-likeCGH tumours (adjusted test for interaction P = 0.006). Among patients with BRCA1-likeCGH tumours, the risk of recurrence was eightfold decreased after HD-PB chemotherapy compared with conventional chemotherapy (adjusted HR 0.12, 95% CI 0.04–0.43; Table 2 and Figure 2B), while in patients with non-BRCA1-likeCGH tumours, no significant treatment difference was observed (adjusted HR 0.78, 95% CI 0.50–1.20; Table 2 and Figure 2A). Similar results were observed for OS (Figure 2C and D, adjusted test for interaction P = 0.04, data not shown). All analyses were adjusted for pathological tumour size, number of positive lymph nodes, Bloom–Richardson grade, triple-negative status and treatment as these were significantly associated with RFS (supplemental Table S3, available at Annals of Oncology online).
Table 2.
Multivariate Cox proportional hazard analysis of the risk of recurrence (recurrence-free survival) in the stage III series
| Variable | All patients stage III series |
|||
| Number of events/number of patients | Hazard ratio | 95% CI | P | |
| Lymph nodes | ||||
| 4–9 | 61/151 | 1.00 | ||
| ≥10 | 43/79 | 1.71 | 1.13–2.59 | 0.01 |
| p T-stage | ||||
| 1 or 2 | 82/194 | 1.00 | ||
| 3 | 22/34 | 1.95 | 1.19–3.22 | 0.009 |
| Histological grade | ||||
| I + II | 55/131 | 1.00 | ||
| III | 47/90 | 1.54 | 0.98–2.40 | n.s. |
| Hormone receptor status | ||||
| ER and PR negative (<10%) | 32/60 | 1.00 | ||
| ER or PR positive (≥10%) | 71/168 | 0.74 | 0.43–1.25 | n.s. |
| aCGH classifier | ||||
| Non-BRCA1-likeCGH tumour | 83/189 | 1.00 | ||
| BRCA1-likeCGH tumour | 21/41 | 2.07 | 1.02–4.17 | 0.04 |
| BRCA1-likeCGH tumour | ||||
| Conventional chemotherapy | 17/23 | 1.00 | ||
| High-dose chemotherapy | 4/18 | 0.12* | 0.04–0.43 | 0.001 |
| Non-BRCA1-likeCGH tumour | ||||
| Conventional chemotherapy | 47/95 | 1.00 | ||
| High-dose chemotherapy | 36/94 | 0.78* | 0.50–1.20 | n.s. |
Homogeneity of both hazard ratios was rejected based on an interaction term with *P =0.006; Number of events is not equal for all variables since some patients have missing data; maximum missing variables (i.e. events) of all patients stage III series is 2/104 events.
aCGH, array comparative genomic hybridisation; CI, confidence interval; CGH, comparative genomic hybridisation; ER, estrogen receptor; n.s., non-significant; p, pathological; PR, progesterone receptor.
Figure 2.
Association of BRCA1-likeCGH classification with outcome after high-dose platinum-based (HD-PB) chemotherapy and conventional chemotherapy in all patients of the stage III series and the triple-negative subgroup. Kaplan–Meier survival curves according to the BRCA1 classification of patients who had been randomly assigned between HD chemotherapy and conventional chemotherapy. (A) Recurrence-free survival of non-BRCA1-likeCGH HER2-negative patients. (B) Recurrence-free survival of BRCA1-likeCGH HER2-negative patients. (C) Overall survival of non-BRCA1-likeCGH HER2-negative patients. (D) Overall survival of BRCA1-likeCGH HER2-negative patients. (E) Recurrence-free survival of non-BRCA1-likeCGH ‘triple-negative’ patients. (F) Recurrence-free survival of BRCA1-likeCGH triple-negative patients. CGH, comparative genomic hybridisation.
association of the BRCA1-likeCGH classifier within the triple-negative subgroup with BRCA1 mutation status, BRCA1 promoter methylation status and basal-like Nielsen phenotype
In the TN subgroup (n = 60), eight of the 13 BRCA1-mutated tumours had a BRCA1-likeCGH profile (Table 3). All 12 tumours with methylation of the BRCA1 promoter displayed a BRCA1-likeCGH profile (Table 3). All BRCA1-mutated tumours had an unmethylated BRCA1 promoter. TN BRCA1-likeCGH tumours displayed in 88% (30/34), a basal-like phenotype. Conversely, 33% (15/45) of the basal-like tumours scored as non-BRCA1-likeCGH (Table 3). To explore the predictive potential of above markers and to put the BRCA1-likeCGH classifier in perspective, we assessed whether the effect of HD-PB chemotherapy on RFS differed by each separate marker with an interaction term.
Table 3.
Distribution of patients with a BRCA1 mutation, a BRCA1 methylation and basal-like status between BRCA1-likeCGH and non-BRCA1-likeCGH patients
| Variable | Patients with non-BRCA1-likeCGH tumours |
Patients with BRCA1-likeCGH tumours |
P | ||
| N | % | N | % | ||
| BRCA1 mutation statusa | |||||
| No mutation detected | 19 | 73.1 | 26 | 76.5 | n.s. |
| Mutation present | 5b | 19.2 | 8 | 23.5 | |
| Undetermined | 2 | 7.7 | 0 | 0.0 | |
| BRCA1-promoter methylation statusa | |||||
| Unmethylated | 25 | 96.2 | 20 | 58.8 | 0.001 |
| Methylated | 0 | 0.0 | 12 | 35.3 | |
| Undetermined | 1 | 3.8 | 2 | 5.9 | |
| Nielsen basal-like breast cancer definitiona | |||||
| Negative | 11 | 42.3 | 4 | 11.8 | 0.01 |
| Basal-like | 15 | 57.7 | 30 | 88.2 | |
Analyses carried out in the triple-negative subset of the stage III series (n = 60). In seven BRCA1-likeCGH tumours, only ∼62% of the types of BRCA1 mutations prevalent in the Netherlands were determined due to technical difficulties instead of the intended ∼73%. Similarly, of one non-BRCA1-likeCGH tumours ∼40% of the type of BRCA1 mutations could be tested.
One patient scored just below the predetermined threshold of 0.63 the BRCA1-likeCGH classifier (score: 0.61). BRCA1 mutations were not necessarily germ line mutations since we tested DNA derived from the tumours. In all undetermined cases, all DNA had been used for array comparative genomic hybridisation analysis and no additional analyses could be carried out. Patients with unknown values were omitted and P values were calculated using the Fisher’s exact test.
CGH, comparative genomic hybridisation; n.s., non-significant.
outcome according to treatment in the triple-negative subgroup by different markers
Influence of the BRCA1-likeCGH classifier on differential treatment effect in the TN subgroup was similar to that observed in the total group of 230 patients (P interaction = 0.05). Subsequently, no substantial modification was seen of the HRs for RFS in BRCA1-likeCGH (adjusted HR: 0.17, 95% CI 0.05–0.60; Figure 2F and Table 4) and non-BRCA1-likeCGH patients (adjusted HR: 0.88, 95% CI 0.30–2.57; Figure 2E and Table 4). BRCA1 methylation interacted significantly with the effect of HD-PB chemotherapy on RFS in the TN subgroup (interaction P = 0.02; Table 4). HD-PB chemotherapy effects differed less strongly by basal-like status or BRCA1 mutation status and homogeneity was not rejected (P interaction: P = 0.83, P = 0.76, respectively, Table 4).
Table 4.
Multivariate Cox proportional hazard analysis of the risk of recurrence (recurrence-free survival) for multiple markers in the triple-negative subgroup
| Variablea | Number of events/number of patients | Hazard ratio | 95% CI | P |
| Nielsen basal-like tumour | ||||
| Conventional chemotherapy | 15/21 | 1.00 | ||
| High-dose chemotherapy | 8/24 | 0.36* | 0.14–0.94 | 0.04 |
| Non-basal-like tumour | ||||
| Conventional chemotherapy | 7/10 | 1.00 | ||
| High-dose chemotherapy | 2/5 | 0.45* | 0.09–2.30 | n.s. |
| BRCA1-likeCGH tumour | ||||
| Conventional chemotherapy | 13/18 | 1.00 | ||
| High-dose chemotherapy | 4/16 | 0.17** | 0.05–0.60 | 0.006 |
| Non-BRCA1-likeCGH tumour | ||||
| Conventional chemotherapy | 9/13 | 1.00 | ||
| High-dose chemotherapy | 6/13 | 0.88** | 0.30–2.57 | n.s. |
| BRCA1-mutated tumour | ||||
| Conventional chemotherapy | 3/6 | 1.00 | ||
| High-dose chemotherapy | 3/7 | 0.48*** | 0.08–2.98 | n.s. |
| No mutation found in tumour | ||||
| Conventional chemotherapy | 19/25 | 1.00 | ||
| High-dose chemotherapy | 6/20 | 0.35*** | 0.13–0.91 | 0.03 |
| BRCA1-methylated tumour | ||||
| Conventional chemotherapy | 6/7 | 1.00 | ||
| High-dose chemotherapy | 0/5 | 0.00**** | 0–0.17b | <0.001b |
| Unmethylated tumour | ||||
| Conventional chemotherapy | 15/23 | 1.00 | ||
| High-dose chemotherapy | 9/22 | 0.55**** | 0.23–1.31 | n.s. |
All analyses shown were adjusted for marker of interest, lymph node status, pathological T-stage and histological grade as in Table 2. Homogeneity of both hazard ratios was tested with an interaction term resulting in: *P = 0.83, **P = 0.05, ***P = 0.76, ****P = 0.02.
The upper confidence bound is based on a model restricted to patients with methylated tumours because it could not be calculated in the model including methylated and unmethylated tumour patients. Number of events is not equal for all variables since some patients have missing data; maximum missing variables (i.e. events) is 2/32.
CGH, comparative genomic hybridisation; CI, confidence interval.
toxicity of HD-PB chemotherapy and marker status
There was no correlation between BRCA1 status as assessed by mutation, methylation or aCGH analysis and early or late (non-)haematological toxicity of HD-PB chemotherapy.
discussion
In this study, we observed that a BRCA1-likeCGH classifier, derived from BRCA1-mutated tumours, was capable of selecting HER2-negative patients who had a significantly better outcome after HD-PB chemotherapy compared with conventional chemotherapy while there was no such evidence for unselected non-BRCA1-likeCGH patients (significant P interactions, RFS and OS). We found a similar high proportion of triple-negative cases within BRCA1-likeCGH tumours (34/39, 87%) as in BRCA1-mutated tumours [34, 35] and therefore examined the classifier’s association with BRCA1 mutation, BRCA1 methylation and basal-like status in the TN subgroup. We found that 63% (20/32) BRCA1-likeCGH tumours harboured either a BRCA1 mutation (n = 8) or BRCA1 methylation (n = 12), and these features were mutually exclusive. Furthermore, BRCA1-methylation status showed potential for the identification of patients with selective benefit of HD-PB chemotherapy; however, due to the small numbers, these data should be interpreted with caution and no conclusions can be drawn at this stage.
The BRCA1-likeCGH classifier displayed two characteristics required for efficacy in clinical practice. It selected a substantial number of patients (41/230). Secondly, in this series, it predicted a large differential treatment effect; selected patients showed an improved outcome after HD-PB chemotherapy when compared with standard anthracycline-based adjuvant chemotherapy and, just as importantly, unselected patients did not seem to have any advantage over standard chemotherapy as demonstrated by their HRs being close to one. Furthermore, it showed a large overlap with the other markers, 8/13 BRCA1-mutated tumours scored as BRCA1 likeCGH. Why all BRCA1-mutated tumours did not score as BRCA1 likeCGH is a matter of speculation. In two cases, the tumour cell content was estimated to be below 60% at blinded repeat examination, which may have caused excess ‘dilution’ of the tumour DNA by normal DNA. In a third case, a BRCA1-likeCGH score of 0.61 was found, while 0.63 was the predetermined threshold for a BRCA1-likeCGH status. In tumours with a low tumour percentage or tumours scoring near the threshold, confirmation of the test results by BRCA1 sequencing may be advisable. In addition, many BRCA1-likeCGH tumours had a basal-like phenotype based on the Nielsen definition [33] in our series (∼75%). However, basal-like phenotype and BRCA1-likeCGH do not seem to be identical markers since a substantial amount, one-third (15/45), of the basal-like tumours scored as non-BRCA1-likeCGH. Of the BRCA1-methylated tumours, 12/12 scored as BRCA1 likeCGH; given the small numbers, it could well be that the accuracy of the BRCA1-likeCGH classifier for identifying BRCA1-methylated cases is overestimated. However, it should be noted that in our study one-third of the BRCA1-likeCGH tumours showed BRCA1-promoter methylation, supporting our hypothesis that the classifier also identifies patients with BRCA1 loss conferred by causes other than mutations. This hypothesis was further strengthened by a recent publication with a similar approach, in which BRCA1/2-mutated ovarian cancers were used to develop a gene expression profile of BRCAness [36]. In this study, 20/70 sporadic ovarian cancer patients scored as having BRCAness and had a significantly longer disease-free survival after platinum agents [36].
Our study is in line with previous findings in which BRCA1 methylation was associated with good response to a platinum agent in 28 triple-negative breast cancer (TNBC) patients in the neoadjuvant setting [37]. In that study using tumour response according to Miller–Payne criteria as a surrogate end point for outcome, 2/2 TNBC patients with a BRCA1 mutation achieved a pathological complete remission (pCR) on conventionally dosed cisplatin [37]. Similarly, Byrski et al. [9] studied a cohort of 102 BRCA1 mutation carriers from 16 hospitals who had received various chemotherapy regimens in the neoadjuvant setting. Ten of the 12 patients (83%) achieved pCR on cisplatin monotherapy, while only 11 of the 51 (22%) patients who had received an anthracycline-based regimen achieved pCR [9]. Byrski et al. [9] cautioned, however, that their study was an observational study and patients in the cisplatin group had smaller tumours, were more often node negative and none of them had received prior chemotherapy, making direct comparison among treatment groups difficult. We did not observe a greater beneficial effect of platinum-based HD-PB chemotherapy over conventional chemotherapy in BRCA1-mutated compared with non-BRCA1-mutated breast cancers in the context of a RCT, which might at least partly be caused by the small numbers. Furthermore, we studied survival data with a median follow-up time of 7 years as the end point, instead of response to neoadjuvant chemotherapy. Additionally, it has been suggested that mutation site in the BRCA1 gene could influence sensitivity to these agents [38]. Similarly, secondary mutations restoring the BRCA1 reading frame in BRCA1-mutated cancers could lead to resistance, as has been described for ovarian cancers [39]. The low incidence of BRCA1-mutated breast cancer will make it challenging to resolve these remaining questions.
We did not find a significantly different benefit of HD-PB chemotherapy over conventional chemotherapy between basal-IHC and non-basal-IHC patients within the TN subgroup. In contrast, Diallo-Danebrock et al. [40] found an improved outcome after high-dose chemotherapy compared with dose-dense chemotherapy in high-risk breast cancer patients with a basal-IHC phenotype. However, this was not studied in the TN subgroup of patients and high-dose chemotherapy used in this study did not include a platinum salt. It is important to dissect TNBC in at least two subgroups, as TNBC has been shown to derive substantial benefit from addition of taxanes [41, 42], while in preclinical studies, relative resistance against taxanes has been demonstrated for breast cancer cells lacking functional BRCA1 [6, 7]. We hypothesise therefore that BRCA1-likeCGH TNBC patients should receive DSB-inducing regimens, while non-BRCA1-likeCGH TNBC patients should receive taxane-based regimens. A neoadjuvant study has been initiated to test this hypothesis (NCT01057069).
The resolution of the CGH platform used in our study was lower compared with newer commercially available platforms. Nevertheless, it is unlikely that findings based on low resolution disappear on high resolution. Moreover, as we tested an existing classifier developed several years ago, we were confined to using the same platform. A limitation of our study was that it consisted of an unplanned subgroup analysis in a RCT. However, the use of a RCT allowed us to determine whether the association between markers and improved survival was related to either selective sensitivity to high-dose platinum-based chemotherapy or to general chemotherapy sensitivity/resistance.
Despite increased toxicity of HD-PB chemotherapy in the whole group, we did not observe a difference between patients with or without a BRCA1-likeCGH, BRCA1-methylated or BRCA1-mutated tumour. This corroborates with the synthetic lethality concept in which cells with a functional BRCA1 protein maintain their homologous recombination function and are capable of repairing the DSBs induced by the HD-PB chemotherapy, including normal tissues of BRCA1 mutation carriers that did not lose the wild-type allele. In an era where we have largely abandoned HD-PB chemotherapy as a toxic regimen with no survival benefit, it is tempting to disregard the potential of the predictive markers investigated in our study, especially given the controversy surrounding this subject [43–45]. However, RFS differences observed between HD-PB chemotherapy and conventional, anthracycline-based chemotherapy in the BRCA1-likeCGH are remarkable. Presumably, this difference observed is an overestimation of the actual effect and should be confirmed in other studies. Because of constraints of the trial, unfortunately, we could not determine whether the platinum-based DSB-inducing regimen would have resulted in a similar improved outcome had it been conventionally dosed. We can only speculate that given the molecular background of the aCGH classifier (derived from BRCA1-mutated tumours) the type of agents is mandatory, all causing DSBs in the DNA, and explains the beneficial effect of HD-PB chemotherapy. This is particularly interesting given the fact that, recently a far less toxic, new DSB-inducing agent has been introduced in the form of poly(ADP) (PARP)-ribose inhibitors, which has been shown to target BRCA1-mutated breast cancer[8, 46]. Therefore, it would be interesting to consider the subgroup identified by the aCGH classifier for studies with PARP inhibitors only or in combination with alkylating or platinum agents. To assess the usefulness of these markers for prediction of PARP inhibitor benefit, we have initiated a small pilot study in patients treated with an olaparib-containing regimen in the metastatic setting.
In conclusion, our data suggest that the BRCA1-likeCGH classifier might be predictive for selective HD-PB chemotherapy benefit, a DSB-inducing regimen. However, what the role of BRCA1 methylation, basal-like and BRCA1-mutation status is remains unclear due to small numbers. This is the first study in breast cancer patients in which all these markers were evaluated in the context of a RCT with long-term outcome. However, these findings do not justify the introduction of HD-PB chemotherapy as a standard treatment option for breast cancer patients with a BRCA1-likeCGH tumour. The use of the aCGH classifier as a predictive marker for HD-PB chemotherapy, but especially for other DSB-inducing regimens (such as other alkylators, preferably in combination with PARP inhibitors) and the additive value of additional biomarkers, such as BRCA1 methylation, separately and in combination warrants further investigation and validation, preferably in prospective RCTs.
funding
Dutch Cancer Society (NKI 2006-3706) and A Sister’s Hope/Pink Ribbon.
disclosure
SCL, MAV and PMN are named inventors on a provisional patent application for the aCGH BRCA1-likeCGH classifier used in this study; above authors have declared no further conflicts. All other authors of this paper have declared no conflicts of interest.
Supplementary Material
Acknowledgments
We thank the Netherlands Cancer Institute - Antoni van Leeuwenhoek Hospital (NKI-AvL) central microarray facility, Richard van Hien, Esther Scheerman, Lennart Mulder, Renske Fles, Ingrid van der Heijden, Sybren Meijer and Bastiaan Evers for technical assistance; Marleen Kok, Tesa Severson, Jos Jonkers, Christiaan Klijn and Piet Borst for critical appraisal of the manuscript. The authors would like to commemorate Hans Peterse, an excellent surgical pathologist and a highly esteemed colleague, who passed away unexpectedly during the course of this project.
Presentation of work: Presented in part at the AACR 101st Annual Meeting 2010, oral presentation in the minisymposia, abstract no. 4820.
Contributors: SCL and SR were responsible for the study design. MAV coordinated the study. MKS and EHvB developed the research methods used. LFAW, PMN and EHvB constructed the BRCA1-likeCGH classifier. MAV, EHL, FEF, JGS, MHo, EGEdV, HvT took part in data collection. JW and MJvdV carried out all histopathological analyses. MAV, EHL and MdB carried out all experiments, except the mutation analyses, which were carried out by SC. MAV, MHa and HvT carried out the data analysis. MAV, EHL, SCL, PMN, LFAW and MHa took part in data interpretation. MAV and SCL drafted the manuscript with substantial contributions from all other authors. All authors gave their approval for submission. No medical writers were involved in writing this manuscript.
References
- 1.Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Yap HY, Salem P, Hortobagyi GN, et al. Phase II study of cis-dichlorodiammineplatinum(II) in advanced breast cancer. Cancer Treat Rep. 1978;62(3):405–408. [PubMed] [Google Scholar]
- 3.Eisen T, Smith IE, Johnston S, et al. Randomized phase II trial of infusional fluorouracil, epirubicin, and cyclophosphamide versus infusional fluorouracil, epirubicin, and cisplatin in patients with advanced breast cancer. J Clin Oncol. 1998;16(4):1350–1357. doi: 10.1200/JCO.1998.16.4.1350. [DOI] [PubMed] [Google Scholar]
- 4.Crown JP. The platinum agents: a role in breast cancer treatment? Semin Oncol. 2001;28(1 Suppl 3):28–37. doi: 10.1016/s0093-7754(01)90190-3. [DOI] [PubMed] [Google Scholar]
- 5.Nieto Y, Shpall EJ. High-dose chemotherapy for high-risk primary and metastatic breast cancer: is another look warranted? Curr Opin Oncol. 2009;21(2):150–157. doi: 10.1097/CCO.0b013e328324f48b. [DOI] [PubMed] [Google Scholar]
- 6.Quinn JE, Kennedy RD, Mullan PB, et al. BRCA1 functions as a differential modulator of chemotherapy-induced apoptosis. Cancer Res. 2003;63(19):6221–6228. [PubMed] [Google Scholar]
- 7.Rottenberg S, Nygren AO, Pajic M, et al. Selective induction of chemotherapy resistance of mammary tumors in a conditional mouse model for hereditary breast cancer. Proc Natl Acad Sci USA. 2007;104(29):12117–12122. doi: 10.1073/pnas.0702955104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 9.Byrski T, Gronwald J, Huzarski T, et al. Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;28(3):375–379. doi: 10.1200/JCO.2008.20.7019. [DOI] [PubMed] [Google Scholar]
- 10.Jaspers JE, Rottenberg S, Jonkers J. Therapeutic options for triple-negative breast cancers with defective homologous recombination. Biochim Biophys Acta. 2009;1796(2):266–280. doi: 10.1016/j.bbcan.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Karran P. DNA double strand break repair in mammalian cells. Curr Opin Genet Dev. 2000;10(2):144–150. doi: 10.1016/s0959-437x(00)00069-1. [DOI] [PubMed] [Google Scholar]
- 12.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27(3):247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 13.van Gent DC, Hoeijmakers JH, Kanaar R. Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet. 2001;2(3):196–206. doi: 10.1038/35056049. [DOI] [PubMed] [Google Scholar]
- 14.Wessels LF, van Welsem T, Hart AA, et al. Molecular classification of breast carcinomas by comparative genomic hybridization: a specific somatic genetic profile for BRCA1 tumors. Cancer Res. 2002;62(23):7110–7117. [PubMed] [Google Scholar]
- 15.Jonsson G, Naylor TL, Vallon-Christersson J, et al. Distinct genomic profiles in hereditary breast tumors identified by array-based comparative genomic hybridization. Cancer Res. 2005;65(17):7612–7621. doi: 10.1158/0008-5472.CAN-05-0570. [DOI] [PubMed] [Google Scholar]
- 16.van Beers EH, van Welsem T, Wessels LF, et al. Comparative genomic hybridization profiles in human BRCA1 and BRCA2 breast tumors highlight differential sets of genomic aberrations. Cancer Res. 2005;65(3):822–827. [PubMed] [Google Scholar]
- 17.Joosse SA, van Beers EH, Tielen IH, et al. Prediction of BRCA1-association in hereditary non-BRCA1/2 breast carcinomas with array-CGH. Breast Cancer Res Treat. 2009;116(3):479–489. doi: 10.1007/s10549-008-0117-z. [DOI] [PubMed] [Google Scholar]
- 18.Rodenhuis S, Bontenbal M, Beex LV, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for high-risk breast cancer. N Engl J Med. 2003;349(1):7–16. doi: 10.1056/NEJMoa022794. [DOI] [PubMed] [Google Scholar]
- 19.Foulkes WD, Stefansson IM, Chappuis PO, et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst. 2003;95(19):1482–1485. doi: 10.1093/jnci/djg050. [DOI] [PubMed] [Google Scholar]
- 20.Lakhani SR, Reis-Filho JS, Fulford L, et al. Prediction of BRCA1 status in patients with breast cancer using estrogen receptor and basal phenotype. Clin Cancer Res. 2005;11(14):5175–5180. doi: 10.1158/1078-0432.CCR-04-2424. [DOI] [PubMed] [Google Scholar]
- 21.Esteller M, Silva JM, Dominguez G, et al. Promoter hypermethylation and BRCA1 inactivation in sporadic breast and ovarian tumors. J Natl Cancer Inst. 2000;92(7):564–569. doi: 10.1093/jnci/92.7.564. [DOI] [PubMed] [Google Scholar]
- 22.Turner NC, Reis-Filho JS, Russell AM, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007;26(14):2126–2132. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 23.Rodenhuis S, Westermann A, Holtkamp MJ, et al. Feasibility of multiple courses of high-dose cyclophosphamide, thiotepa, and carboplatin for breast cancer or germ cell cancer. J Clin Oncol. 1996;14(5):1473–1483. doi: 10.1200/JCO.1996.14.5.1473. [DOI] [PubMed] [Google Scholar]
- 24.Schrama JG, Baars JW, Holtkamp MJ, et al. Phase II study of a multi-course high-dose chemotherapy regimen incorporating cyclophosphamide, thiotepa, and carboplatin in stage IV breast cancer. Bone Marrow Transplant. 2001;28(2):173–180. doi: 10.1038/sj.bmt.1703105. [DOI] [PubMed] [Google Scholar]
- 25.de Gast GC, Vyth-Dreese FA, Nooijen W, et al. Reinfusion of autologous lymphocytes with granulocyte-macrophage colony-stimulating factor induces rapid recovery of CD4+ and CD8+ T cells after high-dose chemotherapy for metastatic breast cancer. J Clin Oncol. 2002;20(1):58–64. doi: 10.1200/JCO.2002.20.1.58. [DOI] [PubMed] [Google Scholar]
- 26.Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci USA. 2002;99(10):6567–6572. doi: 10.1073/pnas.082099299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97(16):1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 28.Sargent DJ, Conley BA, Allegra C, Collette L. Clinical trial designs for predictive marker validation in cancer treatment trials. J Clin Oncol. 2005;23(9):2020–2027. doi: 10.1200/JCO.2005.01.112. [DOI] [PubMed] [Google Scholar]
- 29.van Beers EH, Joosse SA, Ligtenberg MJ, et al. A multiplex PCR predictor for ACGH success of FFPE samples. Br J Cancer. 2006;94(2):333–337. doi: 10.1038/sj.bjc.6602889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joosse SA, van Beers EH, Nederlof PM. Automated array-CGH optimized for archival formalin-fixed, paraffin-embedded tumor material. BMC Cancer. 2007;7:43. doi: 10.1186/1471-2407-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrij-Bosch A, Peelen T, van Vliet M, et al. BRCA1 genomic deletions are major founder mutations in Dutch breast cancer patients. Nat Genet. 1997;17(3):341–345. doi: 10.1038/ng1197-341. [DOI] [PubMed] [Google Scholar]
- 32.Van De Vijver MJ, Peterse JL, Mooi WJ, et al. Neu-protein overexpression in breast cancer. Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. N Engl J Med. 1988;319(19):1239–1245. doi: 10.1056/NEJM198811103191902. [DOI] [PubMed] [Google Scholar]
- 33.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10(16):5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 34.Lakhani SR, Van De Vijver MJ, Jacquemier J, et al. The pathology of familial breast cancer: predictive value of immunohistochemical markers estrogen receptor, progesterone receptor, HER-2, and P53 in patients with mutations in BRCA1 and BRCA2. J Clin Oncol. 2002;20(9):2310–2318. doi: 10.1200/JCO.2002.09.023. [DOI] [PubMed] [Google Scholar]
- 35.Palacios J, Honrado E, Osorio A, et al. Immunohistochemical characteristics defined by tissue microarray of hereditary breast cancer not attributable to BRCA1 or BRCA2 mutations: differences from breast carcinomas arising in BRCA1 and BRCA2 mutation carriers. Clin Cancer Res. 2003;9(10 Pt 1):3606–3614. [PubMed] [Google Scholar]
- 36.Konstantinopoulos PA, Spentzos D, Karlan BY, et al. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J Clin Oncol. 2010;28(22):3555–3561. doi: 10.1200/JCO.2009.27.5719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant cisplatin in triple-negative breast cancer. J Clin Oncol. 2010;28(7):1145–1153. doi: 10.1200/JCO.2009.22.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ben David Y, Chetrit A, Hirsh-Yechezkel G, et al. Effect of BRCA mutations on the length of survival in epithelial ovarian tumors. J Clin Oncol. 2002;20(2):463–466. doi: 10.1200/JCO.2002.20.2.463. [DOI] [PubMed] [Google Scholar]
- 39.Swisher EM, Sakai W, Karlan BY, et al. Secondary BRCA1 mutations in BRCA1-mutated ovarian carcinomas with platinum resistance. Cancer Res. 2008;68(8):2581–2586. doi: 10.1158/0008-5472.CAN-08-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diallo-Danebrock R, Ting E, Gluz O, et al. Protein expression profiling in high-risk breast cancer patients treated with high-dose or conventional dose-dense chemotherapy. Clin Cancer Res. 2007;13(2 Pt 1):488–497. doi: 10.1158/1078-0432.CCR-06-1842. [DOI] [PubMed] [Google Scholar]
- 41.Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357(15):1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 42.Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27(8):1168–1176. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crown J. Smart bombs versus blunderbusses: high-dose chemotherapy for breast cancer. Lancet. 2004;364(9442):1299–1300. doi: 10.1016/S0140-6736(04)17207-3. [DOI] [PubMed] [Google Scholar]
- 44.Hortobagyi GN. What is the role of high-dose chemotherapy in the era of targeted therapies? J Clin Oncol. 2004;22(12):2263–2266. doi: 10.1200/JCO.2004.02.927. [DOI] [PubMed] [Google Scholar]
- 45.Rodenhuis S. Is high-dose chemotherapy dead? Eur J Cancer. 2005;41(1):9–11. doi: 10.1016/j.ejca.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376(9737):245–251. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.