Abstract
Mitogen-activated protein kinase cascade is evolutionarily conserved signal transduction module involved in transducing extracellular signals to the nucleus for appropriate cellular adjustment. This cascade consists essentially of three components, a MAPK kinase kinase (MAPKKK), a MAPK kinase (MAPKK) and a MAPK connected to each other by the event of phosphorylation. These kinases play various roles in intra- and extra-cellular signaling in plants by transferring the information from sensors to responses. Signaling through MAP kinase cascade can lead to cellular responses including cell division, differentiation as well as responses to various stresses. MAPK signaling has also been associated with hormonal responses. In plants, MAP kinases are represented by multigene families and are involved in efficient transmission of specific stimuli and also involved in the regulation of the antioxidant defense system in response to stress signaling. In the current review we summarize and investigate the participation of MAPKs as possible mediators of various abiotic stresses in plants.
Key words: abiotic stress, cross talk, mitogen-activated protein kinases, heat map, MAPK signaling, signal transduction, stress signaling
Introduction
Signal transduction pathways in plants are very well developed while at the same time they are extremely complex to reveal all the cross talks. The simple reason behind these complexities is that the plants are sessile and experiences all cues, biotic or abiotic being stationed at one position. Signaling pathways are induced in response to environmental stresses, and recent molecular and genetic studies have revealed that these pathways involve a host of diverse responses.1,2 It has been well established that abiotic stress response is a complex trait governed by multiple genes. In the last two decades, basic biological research has taken a big leap from studying the expression of single genes or proteins to focusing on a large number of genes or gene products simultaneously, enabling genome-wide expression strategies for better understanding of these complex traits.
Out of many signaling pathways involved in abiotic stress response in plants, mitogen activated protein kinase (MAPK) cascade is one of the major pathway. This signaling module links external stimuli with several cellular responses and is evolutionary conserved among eukaryotic organisms.3,4 MAPK cascades are conserved signaling modules found in all eukaryotes, which transduce environmental and developmental cues into intracellular responses. A MAPK cascade minimally composed of MAP kinase kinase kinases (MAP3Ks/MAPKKKs/MEKKs), MAP kinase kinases (MAP2Ks/MAPKKs/MEKs/MKKs) and MAP kinases (MAPKs/MPKs).5,6 During stress, stimulated plasma membrane activates MAP3Ks or MAP kinase kinase kinase kinases (MAP4Ks).7 MAP4Ks may act as adaptors linking upstream signaling steps to the core MAPK cascades. MAP3Ks are serine/threonine kinases phosphorylating two amino acids in the S/T-X3-5-S/T motif of the MAP2K activation loop. MAP2Ks phosphorylate MAPKs on threonine and tyrosine residues at a conserved T-X-Y motif.8 MAPKs are serine/threonine kinases able to phosphorylate a wide range of substrates, including other kinases and/or transcription factors. The formation and integrity of a specific MAPK cascade can be mediated by scaffold proteins, shared docking domains and adaptor or anchoring proteins.9–11 MKPs (MAPK phosphatases) are involved in the time-dependent controller in the shut down of the pathway after signaling.12
In several species, including Arabidopsis, MAPK cascades have been shown to be involved in signaling pathways activated by abiotic stresses such as cold, salt, touch, wounding, heat, UV, osmotic shock, heavy metals, etc. We list in Table 1 all the components of MAPK cascade reported to be involved in various abiotic stress. The review will focus on the cross-talk of MAPKs and -omics strategies used to unravel the MAPK cascade.
Table 1.
List of MAPKs from different plant sources involved in abiotic stresses
| Plant | Component of MAPK cascade | Remarks | Reference |
| Alfalfa | SIMK | Activated by hyper-osmotic conditions and metal stress | Munnik, et al. 1999;22 Jonak, et al. 200474 |
| MMK2, MMK3, SAMK | Activated by heavy metal stress | Jonak, et al. 200474 | |
| P44MKK4 | Activated by cold and drought | Jonak, et al. 199637 | |
| MMK4/MKK4 | Wound stress | Bogre, et al. 199771 | |
| OMTK1 | Oxidative stress | Nakagami, et al. 200457 | |
| HAMK | Heat stress | Sangwan, et al. 200246 | |
| SAMK | Cold stress | Jonak, et al. 199637 | |
| Arabidopsis | AtMEKK1, AtMPK3 | Touch, cold and salt stress | Mizoguchi, et al. 199619 |
| AtMPK3, AtMPK6 | Hypo-osmolarity Ozone | Droillard, et al. 2002;25 Ahlfors, et al. 200463 |
|
| AtMPK1, AtMPK4,6 | Salt stress, Low temperature, dehydration, touch, wounding, hyper-osmotic stress | Ichimura, et al. 200021 | |
| MAPKK, MKK2 | Cold and salt stress | Teige, et al. 200413 | |
| Chorispora bungeana | CbMAPK3 | Cold, salt | Zhang, et al. 200631 |
| Cotton | GhMAPK | Wounding, cold, salinity | Wang, et al. 200775 |
| GhMPK7 | Salt, wounding | Shi, et al. 201029 | |
| Maize | ZmMPK3 | Activated by cold, drought, UV, salinity, heavy metal, wound | Wang, et al. 201029 |
| ZmMPK7 | H2O2, Osmotic stress | Zong, et al. 200958 | |
| ZmMAPK5 | H2O2, PEG, NaCl, CdCl2, cold, wound, UV | Ding, et al. 200927 | |
| ZmMPK5 | Low temperature stress | Berberich, et al. 199942 | |
| ZmSIMK1 | Salt dtress | Gu, et al. 201028 | |
| Pea | PsMPK2 | Wounding, ABA, H2O2 | Ortiz-Masia, et al. 200859 |
| Potato | StMPK1 | Wound, heat | Blanco, et al. 200649 |
| Rice | OsMSRMK2 | Wound, UV, metal, salt, drought, ozone, high and low temperature | Agrawal, et al. 200238 |
| MAPKK4, 6 | Cold and salt | Kumar, et al. 200892 | |
| MAPKK1 | Salt, drought | ||
| MAPKK10-2 | cold | ||
| OsMAPK5 | Wound, drought, salt, cold | Xiong and Yang 200330 | |
| OsBWMK1 | Mechanical wounding | He, et al. 199972 | |
| DSM1(MAPKKK) | drought | Ning, et al. 201039 | |
| OsMPK3, OsMPK4, OsMKK4 | Arsenic stress | Rao, et al. 201079 | |
| Salicornia brachiata | SbMAPKK | Dehydration, cold, salt | Agarwal, et al. 201044 |
| Soybean | MAPK | Activated by light, Wound stress | Lee, et al. 200174 |
| Tobacco | SIPK | Salt and osmotic stress, Ozone | Mikołajczyk, et al. 2000;24 Samuel, et al. 200061 |
| NtWIPK1 | Wound | Seo, et al. 199570 | |
| NtMPK4 | Wound, ozone tolerance | Gomi, et al. 200565 | |
| Tomato | tMEK2 | Wound | Xing, et al. 200173 |
Cross-talk between Plant MAP Kinases in Abiotic Stress Signaling
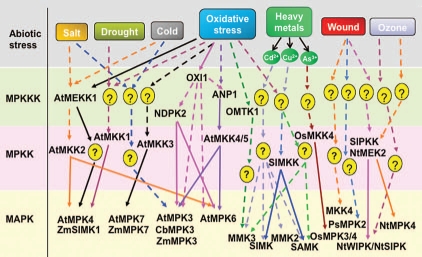
The term ‘cross-talk’ is used generally to refer to situations where different signalling pathways share one or more intermediates/components or have some common outputs. Various abiotic stresses result in both general and specific effects on plant growth and development. Based on the presence of general and specific abiotic stress tolerance mechanisms, it is logical to expect plants to have multiple stress perception and signal transduction pathways, which may cross-talk at various steps in the pathways. As discussed above, MAP kinases play a central role in transduction of different types of signals. Perhaps some of the strongest evidence for cross-talk during abiotic stress signaling in plants comes from studies of MAPK cascades. The Arabidopsis genome contains approximately 80 MAPKKKs, 10 MAPKKs and 20 MAPKs which offer scope for cross-talk between different stress signals. MAPKs are involved in developmental, hormonal, biotic and abiotic stress signaling.6 Members of MAPK cascades are activated by more than one types of stress (Fig. 1) for example, AtMPK6 is involved in O3, H2O2, Ethylene, ABA and JA signaling pathways, and also in important developmental processes such as epidermal patterning, and anther and embryo development. The functional interaction of MPK6 has been demonstrated by a wide set of MAP2Ks such as MKK2,13 MKK3,14 MKK4, MKK5,15 and MKK9.16 Thus, it suggests that MAPK cascades act as points of convergence in stress signalling.
Figure 1.
Schematic representation of cross-talk among different plant MAP kinase signaling components. The scheme of general signal transduction is shown on the left. The homologs in Arabidopsis (At), tobacco (Nt), maize (Zm), pea (Ps) and Chorispora bungeana (Cb) are shown. Solid arrows show proven pathways; dashed arrows indicate postulated pathways; question marks indicate unknown cascade components.
Involvement of MAPK Cascades in Abiotic Stresses
Salt stress.
Plant agriculture in many parts of the world, particularly irrigated land are severely afflicted with salinity stress.17,18 In Arabidopsis, previous study has demonstrated that the MEKK1 (a MAPKKK) mRNA accumulated in response to environmental stresses, including high salinity.19 Yeast two-hybrid analyses showed protein-protein interactions between MEKK1 and MKK2/MEK1 (MAPKKs), between MKK2/MEK1 and MPK4 (a MAPK), and between MPK4 and MEKK1.20 Further studies demonstrated that environmental stress signals are transmitted to at least two MAPK cascades. One is the MPK4 cascade (MEKK1-MEK1/MKK2-MPK4) and the other involves MPK6 and p44MAPK.21 Under salt or cold stress, MAPK pathway involves MEKK1 as an upstream activator of MKK2 and the downstream MAPKs MPK4 and MPK6.13 MKP1 plays a negative role in salt stress signaling through MAPKs (MPK6 and MPK4).12
A 46 kDa SIMK (salt stress-induced MAPK) in alfalfa was reported to be activated by salt.22 Yeast-2-hybrid identified an upstream activator kinase SIMKK that interacts specifically with SIMK and enhanced the salt-induced activation of SIMK in vivo, as well as in vitro.23 It was also reported that tobacco protoplasts exposed to salt and osmotic stress showed enhancement of a 48-kDa kinase, the SIPK (salicylic acid-induced protein kinase).24 Osmotic stress reportedly activated the expression of AtMPK3, AtMPK4 and AtMPK6 in Arabidopsis.25,26 Recently three salt stress-induced MAPKs, ZmMPK3, ZmMAPK5 and ZmSIMK1, have been identified in Zea mays.27–29 Overexpression of OsMAPK5 in rice transgenic plants increased tolerance while suppression led to hypersensitivity to various stresses including salt.30 Salt stress also activated expression of CbMAPK3,31 and GhMPK7.32
Drought stress.
Among the stresses, drought is a major environmental factor limiting productivity and distribution of plants.33,34 When soil moisture is continuously low, water extraction by root and water transport within the plant is reduced and a drought like situation prevails. Drought is a major constraint to increase yield in crop plants. Many stress-responsive genes have been identified and their altered gene expression plays an important role in plant drought resistance.17,35,36 In gel kinase assays followed by immunoprecipitation with specific peptide antibodies raised against different alfalfa MAP kinases showed that alfalfa p44MKK4 (MAP kinase kinase) gene expression and kinase activity got activated under drought conditions in an ABA independent manner.37 Research in Arabidopsis found that the expression of AtMEKK1 and AtMPK3 could be induced by drought.19 Drought stress resulted in the activation of OsMSRMK2 and OsMAPK5 in rice plants.30,38 Overexpression of DSM1 (a putative rice MAPKKK gene in rice) increased the tolerance to dehydration stress.39 Peng et al.40, investigated the expression patterns of MaMAPK and showed that activity of MAPK might be one of the molecular mechanisms of different drought tolerance in Malus. ZmMPK3 also play an important role in response to environmental stresses including drought stress.29
Temperature stress.
Change of temperature is one of the most common responses a plant experience during its complete life cycle. They depend on the perception of both high and low temperatures, both for their survival and for the regulation of key developmental events. Although environmental change is expected to increase average temperatures, this will also have important consequences for the way in which plants perceive low temperature. A lot of studies have been carried out in Arabidopsis which indicated the role of MAPKs in temperature stress. AtMEKK1 and AtMPK3 are transcriptionally induced19 while AtMPK4 and AtMPK6 are activated by cold stress.21 Arabidopsis MAPKK, AtMKK2, also got upregulated in response to cold stress.13 Yeast-two-hybrid as well as protein kinase assays revealed that AtMPK4 and AtMPK6 were direct and specific substrates of MKK2.41 Functional and interaction analysis in yeast suggested that MEKK1 functions upstream of MKK1, MKK2 and MPK4,20 and a role for the MAPK module consisting of MEKK1-MKK2-MPK4/6 has now been con- firmed in cold stress. The transcript level of ZmMPK3 increased markedly within 30 min and remained high during a 4 h period.29 Cold stress also induced the expression and activity of ZmMAPK5.42,43 Recently, a lot of information has been gathered where cold stress led to the activation of MAPKs in different plant genera for example, Chorispora bungeana,31 Gossypium hirsutum (GhMAPK),32 and Salicornia brachiata (SbMAPKK).44
It was not only low temperature, but also high temperature led to the activation of MAPKs. The sudden increase in ambient maximum temperature, in a matter of few days, by 5–7°C with corresponding increase in the minimum temperature, creates heat stress on plants. The normal physiology of the plant gets affected and the plant maturity is accelerated. In practical agriculture, such heat stress inflicts enormous crop losses. In the European heat-wave of 2003, crop production was reduced by around 30%.45 Due to global weather change, the frequency of heat stress is predicted to increase in different parts of the world. Sangwan et al.46, identified the first plant heat shock-activated MAPK (HAMK) from alfalfa cells. In tomato photoautotrophic cell cultures, a partially purified heat activated MAPK was shown to phosphorylate HsFA3 transcription factor.47 Recently, high and low temperature exhibited an internal rhythm in the activity of MAPK in rice.48 Another report was published in Solanum tuberosum where heat treatment to potato tubers elevated StMPK1 transcript levels.49 In rice, changes in temperature affected the transcript levels of OsMSRMK2.38 High temperature (37°C) resulted in a considerable decrease in its transcript level at 30 min, where as at 25°C an increase was observed at 30 min, which drastically decreased with time. Interestingly, at low temperature (12°C) the OsMSRMK2 transcript started to accumulate only around 60 min, reaching a maximum at 90 min, followed by a slight decline at 120 min. Thus, rapid induction of OsMSRMK2 mRNA at 37°C suggests its role in sensing high temperatures.38
Oxidative stress.
Most types of abiotic stresses such as drought, salinity, heat and cold stresses disrupt the metabolic balance of cells, resulting in oxidative stress.50 Oxidative stress is a term used to describe the effect of oxidation in which an abnormal level of reactive oxygen species (ROS), such as the free radicals (e.g., hydroxyl, nitric acid, superoxide) or the non-radicals (e.g., hydrogen peroxide, lipid peroxide) lead to damage (called oxidative damage) to specific molecules with consequential injury to cells or tissue.51 Removal or neutralization of ROS is achieved with antioxidants, endogenous (e.g., catalase, glutathione, superoxide dismutase) or exogenous (e.g., vitamins A, C, E, bioflavonoids, carotenoids). Plants overcome oxidative stress with the production of scavenger enzymes such as catalases, which decompose H2O2. For example, A. thaliana CAT1 is regulated by ABA, and Xing et al.,52 found that the MAP2K inhibitor PD98059 hindered ABA-mediated CAT1 expression. In addition, the A. thaliana mkk1 and mpk6 mutants were altered in their responses to ABA and desiccation stress. These results, together with the lack of ABA-mediated activation of MPK6 in mkk1 mutants, suggested that MKK1-MPK6 regulate H2O2 metabolism through CAT1.53 In contrast with CAT1, the closely related CAT2 expression seems to be regulated by MEKK1 and MPK4,54 which are involved in plant defense and SA accumulation. The MEKK1-MPK4 cascade playing an important role in ROS metabolism was first demonstrated by Nakagami et al.55 In addition, other MAPKKKs are activated in A. thaliana protoplasts by H2O2 that include ANP1, which may cause the downstream activation of MPK3 and MPK6.56 These findings imply that multiple MAPK modules mediate oxidative stress responses and that MAP kinase cascades are not only induced by ROS but may also regulate ROS levels by affecting catalase activity. Notably, ROS homeostasis is a convergence point that indicates plant stress status because oxidative stress is a common response to biotic and abiotic stress. A recent review compiled ROS-mediated MAPK signaling literature.54 The continued examination of available A. thaliana mutants and other in planta studies of stress-specific protein interactions will help dissect the roles of MAPK modules. An important issue that has emerged in this field is how cellular redox status determines cell growth and differentiation and, thus, development. In alfalfa, a novel MAPKKK, OMTK1 (oxidative stress-activated MAP triple- kinase 1) was identified which further activated downstream MAPK, MMK3.57 H2O2 induced activation of plant MAPK has also been reported in various genera e.g., maize27,58 and pea.59 Lately, Lumbreras et al.,60 demonstrated that MKP2 positively controls abiotic oxidative stress responses and is a key regulator of MPK3 and MPK6 networks controlling stress responses in plants.
Ozone stress.
Ozone is a strong oxidant and atmospheric pollutant and is known to activate MAPK signaling pathway. MAP kinases in plants are also activated by exposure to ozone.61 A 46 kDa MBP kinase activity immunoprecipitable with anti- SIPK is induced in tobacco leaves and cells after ozone treatment. Ozone treatment also triggers the accumulation of H2O2, superoxide anion and hydroxyl radicals that ultimately cause an oxidative burst in cells.62 Ozone also showed a dramatic increase in the transcript level of OsMSRMK2 gene (MAP kinase) in rice.38 In A. thaliana, MPK3 and MPK6 were activated by ozone exposure,63 and plants lacking these kinases became hypersensitive to ozone.64 Similarly, NtMPK4 silenced tobacco plants showed enhanced sensitivity to ozone.65 In poplar, ozone treatment activated two MAPKs and activation of these MAPKs was dependent on the production of reactive oxygen species (ROS); the influx of calcium ions via membrane channels; the activation of an upstream, membrane-localized component; and a cognate MAPK kinase.66 Recently, a MKP2 was identified as an important regulator for controlling both ozone induced MPK3, and MPK6 and MKP2 RNAi plants were shown to exhibit hypersensitivity to ozone.67
Wounding.
Many physical injuries caused by anthropogenic activity, herbivore or insect attack results in wounding. When wounded, plants express several sets of defense-related genes that are involved in healing damaged tissues and protecting against pathogen infection and insect attack.68,69 These genes are activated through signaling pathways that include various protein kinases. Many plant species demonstrate an increase in MAPK levels after being wounded. First report of the activation of a MAP kinase in response to wounding was published in tobacco70 and named as WIPK (wound induced MAP kinase). Bogre et al.,71 demonstrated that wounding alfalfa leaves specifically induced the activation of MMK4 (MAPK). AtMPK4 and AtMPK6 are also shown to get rapidly activated by wounding.21 NtMPK4, a tobacco homolog of AtMPK4, revealed wound induced activation along with two other wound-responsive tobacco MAPKs, WIPK and SIPK.65 Molecular characterization of StMPK1 (potato MAPK) revealed its transcriptional upregulation upon wounding.49 In last 10 years, several wound-activated MAPKs have been identified in various plant species for example, rice,30,38,72 tomato,73 soybean,74 cotton,32,75 pea59 and maize.27,29
Heavy metal stress.
Higher dose of these heavy metals adversely affects plant growth and development even though heavy metal ions are essential in many physiological and developmental processes. The presence of enhanced level of heavy metal ions triggers a wide range of cellular responses. In plants, higher amount of copper, cadmium and mercury ions resulted in the activation of a novel MAPK gene OsMSRMK2 from japonica-type rice (cv. Nipponbare).38 Yeh et al.,76 confirmed the activation of a MAPK gene and MBP kinases in rice in response to cadmium stress. Exposure of Medicago seedlings to excess copper or cadmium ions resulted in a complex activation pattern of four distinct MAPKs: SIMK, MMK2, MMK3 and SAMK (stress activated MAPK).77 In protoplasts, the Medicago MAPKK, SIMKK, only conveyed activation of SIMK and SAMK, but not of MMK2 and MMK3. Furthermore, SIMKK only mediated activation by copper but not by cadmium ions. Gupta et al.,78 reported the activation of MAPK activity in response to As(III) treatment indicating a role of this important cascade in transducing As(III) mediated signals. Recently, involvement of OsMPK3, OsMPK4 and OsMKK4 has been shown in As(III) mediated in rice seedlings.79 Heavy metals activation of MAPKs was also demonstrated in maize.27,29 These data show that MAPK cascades are involved in signaling activated by different heavy metals.
Conclusion
The information generated by studying abiotic responses in plants is basically implied to improve the abiotic tolerance of plants by different means of genetic manipulation. The findings reported using model research plants like Arabidopsis have been used to improve several plants, including crop species.80–82 The complete genome sequence of rice, and Arabidopsis and emerging sequence information for several other plant genomes, such as Populus, Medicago, lotus, tomato, maize and chickpea, have given rise to the use of tools which can aid in the determination of the function of many genes simultaneously. All these studies have made it convenient for the researchers around the globe to answer important biological question which can be used for improving the crop plants. The omics approaches like transcriptomics, proteomics, metabolomics, bioinformatics and high-throughput DNA sequencing have enabled active analysis of regulating networks that control abiotic stress responses. Recently, Popescu et al.,83 identified several MKK/MPK/substrate signaling pathways by employing Arabidopsis protein microarrays. Ding et al.,84 generated a Rice Kinase-Protein interaction map and reported a protein interaction map of 116 representative rice kinases and 254 of their interacting partners. Similarly, a directed protein-protein interaction screen between all the Arabidopsis MAPKs and their upstream activators MAPKKs was carried out to gain insight into their potential relationships.85 Recently, Jung et al.,86 described the application of phylogenomics to elucidate the functions of individual members of the large rice kinase gene-family. The authors developed rice kinase database for 1,508 rice kinases87 and also identified the functions of MAPKs, MAP2Ks and six MAP3K genes playing important roles in a broad range of stress responses. Here we used the publicly available microarray GEO database to generate a heat map of rice MAPK, MAP2K and MAP3K in different abiotic stress and hormone treatments (Fig. 2). The information of rice MAP3K was gathered from Rao et al.,88 where 75 members are reported through an in-silico analysis of the rice genome. Based on the expression information of Figure 2A, we deduced a possible chain of MAPK components working in abiotic stress and hormone signaling in rice in Figure 2B. Though this is just a speculation, stringent biochemical, molecular and genetic studies are required to appropriate validation. Integrating the orthologous gene information from other recently sequenced crop plants with rice database will enable the prediction of gene function in these species. Recently, it has been shown that MPK2 from Reaumuria soongorica (a stress tolerant woody shrub) is involved in the regulation of the antioxidant defense system in the response to stress signaling, which suggests that MAPKs also function as possible mediators of abiotic stresses.89 Recently, the MAPK machinery in plants has also been reviewed by Taj et al.90 Recently, DNA sequence of the AtMPK3 promoter for responses to drought, high salinity, low temperature, and wounding has been identified, which advances our understanding of the molecular mechanisms controlling AtMPK3 expression in response to abiotic stress.91
Figure 2.
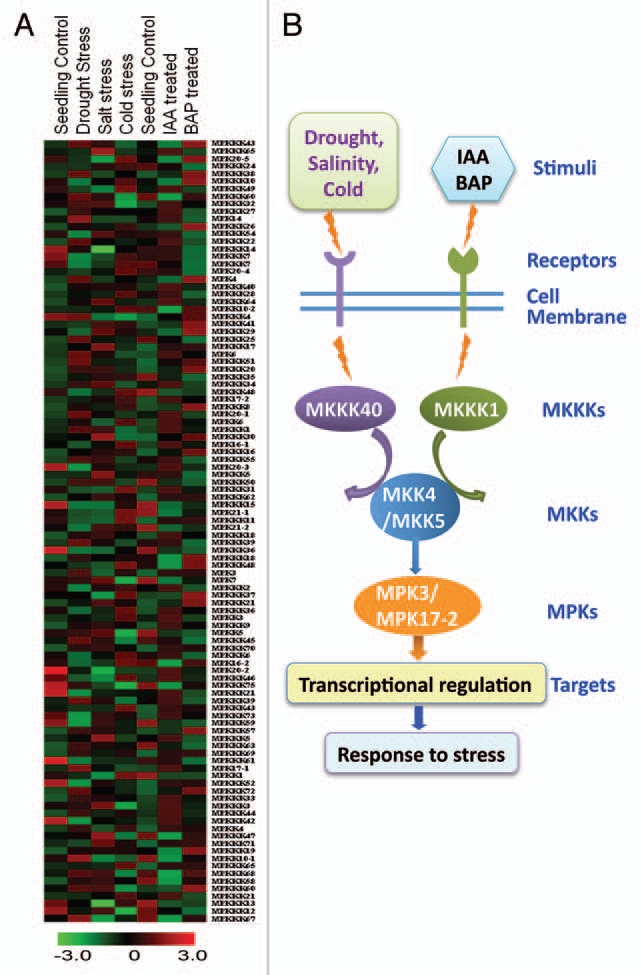
Differential expression of rice MAP kinase genes in response to various abiotic stress and hormonal treatment. (A) Heat map showing the expression patterns of mitogen activated protein kinase (MAPK), MAPK kinase (MAP2K) and MAP2 kinase (MAP3K) genes in rice under abiotic stress and hormonal treatment. The microarray data publicly available at GEO database under the series accession numbers GSE6901 (abiotic stress) and GSE5167 (auxin and cytokinin treatment) were used for expression analysis. The color scale is give at the bottom. (B) Deduced MAPK cascade from heat map under abiotic stress and hormone treatment.
Acknowledgements
Postdoctoral fellowship to M.J. from NIPGR, New Delhi Inida and Junior research fellowship to B.R. from Department of Biotechnology, Government of Inda is gratefully acknowledged. Work on plant abiotic stress tolerance in A.K.S. and N.T's laboratories are partially supported by Department of Biotechnology (DBT), and Department of Science and Technology (DST), Government of India. We also thank grants-in aid from COST Action FA0605.
Abbreviations
- MAPK
mitogen activated protein kinase
- MAP2K/MAPKK/MKK
MAPK kinase
- MAP3K/MAPKKK/MEKK
MAP2K kinase
Note
Please visit http://www.landesbioscience.com/journals/psb/article/14701 to download a color versions of the images in this article.
References
- 1.Chinnusamy V, Schumaker K, Zhu JK. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J Exp Bot. 2004;55:225–236. doi: 10.1093/jxb/erh005. [DOI] [PubMed] [Google Scholar]
- 2.Sreenivasulu N, Sopory SK, Kavi Kishor PB. Deciphering the regulatory mechanisms of abiotic stress tolerance in plants by genomic approaches. Gene. 2007;388:1–13. doi: 10.1016/j.gene.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Tena G, Asai T, Chiu WL, Sheen J. Plant mitogen-activated protein kinase signaling cascades. Curr Opin Plant Biol. 2001;4:392–400. doi: 10.1016/s1369-5266(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 4.Jonak C, Okrész L, Bögre L, Hirt H. Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol. 2002;5:415–424. doi: 10.1016/s1369-5266(02)00285-6. [DOI] [PubMed] [Google Scholar]
- 5.Mishra NS, Tuteja R, Tuteja N. Signaling through MAP kinase networks in plants. Arch Biochem Biophys. 2006;452:55–68. doi: 10.1016/j.abb.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez MC, Petersen M, Mundy J. Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol. 2010;61:621–649. doi: 10.1146/annurev-arplant-042809-112252. [DOI] [PubMed] [Google Scholar]
- 7.Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol. 2001;11:220–230. doi: 10.1016/s0962-8924(01)01980-8. [DOI] [PubMed] [Google Scholar]
- 8.Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 9.Whitmarsh AJ, Davis RJ. Structural organization of MAP-kinase signaling modules by scaffold proteins in yeast and mammals. Trends Biochem Sci. 1998;23:481–485. doi: 10.1016/s0968-0004(98)01309-7. [DOI] [PubMed] [Google Scholar]
- 10.Bardwell AJ, Flatauer LJ, Matsukuma K, Thorner J, Bardwell L. A conserved docking site in MEKs mediates high-affinity binding to MAP kinases and cooperates with a scaffold protein to enhance signal transmission. J Biol Chem. 2001;276:10374–1086. doi: 10.1074/jbc.M010271200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takekawa M, Tatebayashi K, Saito H. Conserved docking site is essential for activation of mammalian MAP kinase kinases by specific MAP kinase kinase kinases. Mol Cell. 2005;18:295–306. doi: 10.1016/j.molcel.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Ulm R, Ichimura K, Mizoguchi T, Peck SC, Zhu T, Wang X, et al. Distinct regulation of salinity and genotoxic stress responses by Arabidopsis MAP kinase phosphatase 1. EMBO J. 2002;21:6483–6493. doi: 10.1093/emboj/cdf646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teige M, Scheikl E, Eulgem T, Dóczi R, Ichimura K, Shinozaki K, et al. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol Cell. 2004;15:141–152. doi: 10.1016/j.molcel.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi F, Yoshida R, Ichimura K, Mizoguchi T, Seo S, Yonezawa M, et al. The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell. 2007;19:805–818. doi: 10.1105/tpc.106.046581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, et al. MAP kinase signaling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 16.Yoo SD, Cho YH, Tena G, Xiong Y, Sheen J. Dual control of nuclear EIN3 by bifurcate MAPK cascades in C2H4 signaling. Nature. 2008;451:789–795. doi: 10.1038/nature06543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuteja N. Mechanisms of high salinity tolerance in plants. Methods Enzymol. 2007;428:419–438. doi: 10.1016/S0076-6879(07)28024-3. [DOI] [PubMed] [Google Scholar]
- 19.Mizoguchi T, Irie K, Hirayama T, Hayashida N, Yamaguchi-Shinozaki K, Matsumoto K, et al. A gene encoding a mitogen-activated protein kinase kinase kinase is induced simultaneously with genes for a mitogen-activated protein kinase and an S6 ribosomal protein kinase by touch, cold and water stress in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1996;93:765–769. doi: 10.1073/pnas.93.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ichimura K, Mizoguchi T, Irie K, Morris P, Giraudat J, Matsumoto K, et al. Isolation of ATMEKK1 (a MAP Kinase kinase kinase)-interacting proteins and analysis of a MAP kinase cascade in Arabidopsis. Biochem Biophys Res Commun. 1998;253:532–543. doi: 10.1006/bbrc.1998.9796. [DOI] [PubMed] [Google Scholar]
- 21.Ichimura K, Mizoguchi T, Yoshida R, Yuasa T, Shinozaki K. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 2000;24:655–665. doi: 10.1046/j.1365-313x.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- 22.Munnik T, Ligterink W, Meskiene II, Calderini O, Beyerly J, Musgrave A, Hirt H. Distinct osmo-sensing protein kinase pathways are involved in signaling moderate and severe hyper-osmotic stress. Plant J. 1999;20:381–388. doi: 10.1046/j.1365-313x.1999.00610.x. [DOI] [PubMed] [Google Scholar]
- 23.Kiegerl S, Cardinale F, Siligan C, Gross A, Baudouin E, Liwosz A, et al. SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress-induced MAPK, SIMK. Plant Cell. 2000;12:2247–2258. doi: 10.1105/tpc.12.11.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mikołajczyk M, Awotunde OS, Muszynska G, Klessig DF, Dobrowolska G. Osmotic stress induces rapid activation of a salicylic acid-induced protein kinase and a homolog of protein kinase ASK1 in tobacco cells. Plant Cell. 2000;12:165–178. [PMC free article] [PubMed] [Google Scholar]
- 25.Droillard M, Boudsocq M, Barbier-Brygoo H, Laurière C. Different protein kinase families are activated by osmotic stresses in Arabidopsis thaliana cell suspensions. Involvement of the MAP kinases AtMPK3 and AtMPK6. FEBS Lett. 2002;527:43–50. doi: 10.1016/s0014-5793(02)03162-9. [DOI] [PubMed] [Google Scholar]
- 26.Droillard MJ, Boudsocq M, Barbier-Brygoo H, Lauriere C. Involvement of MPK4 in osmotic stress response pathways in cell suspensions and plantlets of A. thaliana: activation by hypoosmolarity and negative role in hyperosmolarity tolerance. FEBS Lett. 2004;574:42–48. doi: 10.1016/j.febslet.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Ding HD, Zhang XH, Xu SC, Sun LL, Jiang MY, Zhang AY, Jin YG. Induction of protection against paraquat-induced oxidative damage by abscisic acid in maize leaves is mediated through mitogen-activated protein kinase. J Integr Plant Biol. 2009;51:961–972. doi: 10.1111/j.1744-7909.2009.00868.x. [DOI] [PubMed] [Google Scholar]
- 28.Gu L, Liu Y, Zong X, Liu L, Li DP, Li DQ. Overexpression of maize mitogen-activated protein kinase gene, ZmSIMK1 in Arabidopsis increases tolerance to salt stress. Mol Biol Rep. 2010;37:4067–4073. doi: 10.1007/s11033-010-0066-6. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Ding H, Zhang A, Ma F, Cao J, Jiang M. A novel mitogen-activated protein kinase gene in maize (Zea mays), ZmMPK3, is involved in response to diverse environmental cues. J Integr Plant Biol. 2010;52:442–452. doi: 10.1111/j.1744-7909.2010.00906.x. [DOI] [PubMed] [Google Scholar]
- 30.Xiong L, Yang Y. Disease resistance and abiotic stress tolerance in rice are inversely modulated by an abscisic acid-inducible mitogen-activated protein kinase. Plant Cell. 2003;15:745–759. doi: 10.1105/tpc.008714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang T, Liu Y, Xue L, Xu S, Chen T, Yang T, et al. Molecular cloning and characterization of a novel MAP kinase gene in Chorispora bungeana. Plant Physiol Biochem. 2006;44:78–84. doi: 10.1016/j.plaphy.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Shi J, An HL, Zhang L, Gao Z, Guo XQ. GhMPK7, a novel multiple stress-responsive cotton group C MAPK gene, has a role in broad spectrum disease resistance and plant development. Plant Mol Biol. 2010;74:1–17. doi: 10.1007/s11103-010-9661-0. [DOI] [PubMed] [Google Scholar]
- 33.Boyer JS. Plant productivity and environment. Science. 1982;218:443–448. doi: 10.1126/science.218.4571.443. [DOI] [PubMed] [Google Scholar]
- 34.Mahajan S, Tuteja N. Cold, salinity and drought stresses: An overview. Arch Biochem Biophys. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 35.Li WX, Oono Y, Zhu J, He XJ, Wu JM, Iida K, et al. The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell. 2008;20:2238–2251. doi: 10.1105/tpc.108.059444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee HK, Cho SK, Son O, Xu Z, Hwang I, Kim WT. Drought stress-induced Rma1H1, a RING membrane-anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell. 2009;21:622–641. doi: 10.1105/tpc.108.061994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jonak C, Kiegerl S, Ligterink W, Barker PJ, Huskisson NS, Hirt H. Stress signaling in plants: a mitogen-activated protein kinase pathway is activated by cold and drought. Proc Natl Acad Sci USA. 1996;93:11274–11279. doi: 10.1073/pnas.93.20.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agrawal GK, Rakwal R, Iwahashi H. Isolation of novel rice (Oryza sativa L.) multiple stress responsive MAP kinase gene, OsMSRMK2, whose mRNA accumulates rapidly in response to environmental cues. Biochem Biophys Res Commun. 2002;294:1009–1016. doi: 10.1016/S0006-291X(02)00571-5. [DOI] [PubMed] [Google Scholar]
- 39.Ning J, Li X, Hicks LM, Xiong L. A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol. 2010;152:876–890. doi: 10.1104/pp.109.149856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peng LX, Gu LK, Zheng CC, Li DQ, Shu HR. Expression of MaMAPK gene in seedlings of Malus L. under water stress. Acta Biochim Biophys Sin (Shanghai) 2006;38:281–286. [PubMed] [Google Scholar]
- 41.Mizoguchi T, Ichimura K, Yoshida R, Shinozaki K. MAP kinase cascades in Arabidopsis: their roles in stress and hormone responses. In: Hirt H, editor. Results and Problems in Cell Di Verentiation: MAP Kinases in Plant Signal Transduction. Heidelberg: Springer; 2000. pp. 29–38. [DOI] [PubMed] [Google Scholar]
- 42.Berberich T, Sano H, Kusano T. Involvement of a MAP kinase, ZmMPK5, in senescence and recovery from low-temperature stress in maize. Mol Gen Genet. 1999;262:534–542. doi: 10.1007/s004380051115. [DOI] [PubMed] [Google Scholar]
- 43.Ding H, Zhang A, Wang J, Lu R, Zhang H, Zhang J, Jiang M. Identity of an ABA-activated 46 kDa mitogen-activated protein kinase from Zea mays leaves: partial purification, identification and characterization. Planta. 2009;230:239–251. doi: 10.1007/s00425-009-0938-y. [DOI] [PubMed] [Google Scholar]
- 44.Agarwal PK, Gupta K, Jha B. Molecular characterization of the Salicornia brachiata SbMAPKK gene and its expression by abiotic stress. Mol Biol Rep. 2010;37:981–986. doi: 10.1007/s11033-009-9774-1. [DOI] [PubMed] [Google Scholar]
- 45.Ciais P, Reichstein M, Viovy N, Granier A, Ogée J, Allard V, et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature. 2005;437:529–533. doi: 10.1038/nature03972. [DOI] [PubMed] [Google Scholar]
- 46.Sangwan V, Orvar BL, Beyerly J, Hirt H, Dhindsa RS. Opposite changes in membrane fluidity mimic cold and heat stress activation of distinct plant MAP kinase pathways. Plant J. 2002;31:629–638. doi: 10.1046/j.1365-313x.2002.01384.x. [DOI] [PubMed] [Google Scholar]
- 47.Link V, Sinha AK, Vashista P, Hofmann MG, Proels RK, Ehness R, Roitsch T. A heat-activated MAP kinase in tomato: a possible regulator of the heat stress response. FEBS Lett. 2002;531:179–183. doi: 10.1016/s0014-5793(02)03498-1. [DOI] [PubMed] [Google Scholar]
- 48.Rao KP, Vani G, Kumar K, Sinha AK. Rhythmic expression of mitogen activated protein kinase activity in rice. Mol Cells. 2009;28:417–422. doi: 10.1007/s10059-009-0137-5. [DOI] [PubMed] [Google Scholar]
- 49.Blanco FA, Zanetti ME, Casalongué CA, Daleo GR. Molecular characterization of a potato MAP kinase transcriptionally regulated by multiple environmental stresses. Plant Physiol Biochem. 2006;44:315–322. doi: 10.1016/j.plaphy.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- 51.Gill SS, Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem. 2010 doi: 10.1016/j.plaphy.2010.08.016. In press. [DOI] [PubMed] [Google Scholar]
- 52.Xing Y, Jia W, Zhang J. AtMEK1 mediates stress-induced gene expression of CAT1 catalase by triggering H2O2 production in Arabidopsis. J Exp Bot. 2007;58:2969–2981. doi: 10.1093/jxb/erm144. [DOI] [PubMed] [Google Scholar]
- 53.Xing Y, Jia W, Zhang J. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. Plant J. 2008;54:440–451. doi: 10.1111/j.1365-313X.2008.03433.x. [DOI] [PubMed] [Google Scholar]
- 54.Pitzschke A, Hirt H. Disentangling the complexity of mitogen-activated protein kinases and reactive oxygen species signaling. Plant Physiol. 2009;149:606–615. doi: 10.1104/pp.108.131557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakagami H, Soukupova H, Schikora A, Zarsky V, Hirt H. A mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis. J Biol Chem. 2006;281:38697–38704. doi: 10.1074/jbc.M605293200. [DOI] [PubMed] [Google Scholar]
- 56.Kovtun Y, Chiu WL, Tena G, Sheen J. Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA. 2000;97:2940–2945. doi: 10.1073/pnas.97.6.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakagami H, Kiegerl S, Hirt H. OMTK1, a novel MAPKKK, channels oxidative stress signaling through direct MAPK interaction. J Biol Chem. 2004;279:26959–26966. doi: 10.1074/jbc.M312662200. [DOI] [PubMed] [Google Scholar]
- 58.Zong XJ, Li DP, Gu LK, Li DQ, Liu LX, Hu XL. Abscisic acid and hydrogen peroxide induce a novel maize group C MAP kinase gene, ZmMPK7, which is responsible for the removal of reactive oxygen species. Planta. 2009;229:485–495. doi: 10.1007/s00425-008-0848-4. [DOI] [PubMed] [Google Scholar]
- 59.Ortiz-Masia D, Perez-Amador MA, Carbonell P, Aniento F, Carbonell J, Marcote MJ. Characterization of PsMPK2, the first C1 subgroup MAP kinase from pea (Pisum sativum L.) Planta. 2008;227:1333–1342. doi: 10.1007/s00425-008-0705-5. [DOI] [PubMed] [Google Scholar]
- 60.Lumbreras V, Vilela B, Irar S, Solé M, Capellades M, Valls M, et al. MAPK phosphatase MKP2 mediates disease responses in Arabidopsis and functionally interacts with MPK3 and MPK6. Plant J. 2010;63:1017–1030. doi: 10.1111/j.1365-313X.2010.04297.x. [DOI] [PubMed] [Google Scholar]
- 61.Samuel MA, Miles GP, Ellis BE. Ozone treatment rapidly activates MAP kinase signaling in plants. Plant J. 2000;22:367–376. doi: 10.1046/j.1365-313x.2000.00741.x. [DOI] [PubMed] [Google Scholar]
- 62.Samuel MA, Ellis BE. Double jeopardy: both overexpression and suppression of a redox-activated plant mitogen-activated protein kinase render tobacco plants ozone sensitive. Plant Cell. 2002;14:2059–2069. doi: 10.1105/tpc.002337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahlfors R, Macioszek V, Rudd J, Brosché M, Schlichting R, Scheel D, Kangasjärvi J. Stress hormone-independent activation and nuclear translocation of mitogen-activated protein kinases in Arabidopsis thaliana during ozone exposure. Plant J. 2004;40:512–522. doi: 10.1111/j.1365-313X.2004.02229.x. [DOI] [PubMed] [Google Scholar]
- 64.Miles GP, Samuel MA, Zhang Y, Ellis BE. RNA interference-based (RNAi) suppression of AtMPK6, an Arabidopsis mitogen-activated protein kinase, results in hypersensitivity to ozone and misregulation of AtMPK3. Environ Pollut. 2005;138:230–237. doi: 10.1016/j.envpol.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 65.Gomi K, Ogawa D, Katou S, Kamada H, Nakajima N, Saji H, et al. A mitogen-activated protein kinase NtMPK4 activated by SIPKK is required for jasmonic acid signaling and involved in ozone tolerance via stomatal movement in tobacco. Plant Cell Physiol. 2005;46:1902–1914. doi: 10.1093/pcp/pci211. [DOI] [PubMed] [Google Scholar]
- 66.Hamel LP, Miles GP, Samuel MA, Ellis BE, Séguin A, Beaudoin N. Activation of stress-responsive mitogen-activated protein kinase pathways in hybrid poplar (Populus trichocarpa × Populus deltoides) Tree Physiol. 2005;25:277–288. doi: 10.1093/treephys/25.3.277. [DOI] [PubMed] [Google Scholar]
- 67.Lee JS, Ellis BE. Arabidopsis MAPK phosphatase 2 (MKP2) positively regulates oxidative stress tolerance and inactivates the MPK3 and MPK6 MAPKs. J Biol Chem. 2007;282:25020–25029. doi: 10.1074/jbc.M701888200. [DOI] [PubMed] [Google Scholar]
- 68.Lawton MA, Lamb CJ. Transcriptional activation of plant defense genes by fungal elicitor, wounding and infection. Mol Cell Biol. 1987;7:335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brederode FT, Linthorst HJ, Bol JF. Differential induction of acquired resistance and PR gene expression in tobacco by virus infection, ethephon treatment, UV light and wounding. Plant Mol Biol. 1991;17:1117–1125. doi: 10.1007/BF00028729. [DOI] [PubMed] [Google Scholar]
- 70.Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y. Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science. 1995;270:1988–1992. doi: 10.1126/science.270.5244.1988. [DOI] [PubMed] [Google Scholar]
- 71.Bogre L, Ligterink W, Meskiene I, Barker PJ, Heberle-Bors E, Huskisson NS, et al. Wounding induces the rapid and transient activation of a specific MAP kinase Pathway. Plant Cell. 1997;9:75–83. doi: 10.1105/tpc.9.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.He C, Fong SH, Yang D, Wang GL. BWMK1, a novel MAP kinase induced by fungal infection and mechanical wounding in rice. Mol Plant Microbe Interact. 1999;12:1064–1073. doi: 10.1094/MPMI.1999.12.12.1064. [DOI] [PubMed] [Google Scholar]
- 73.Xing T, Malik K, Martin T, Miki BL. Activation of tomato PR and wound-related genes by a mutagenized tomato MAP kinase kinase through divergent pathways. Plant Mol Biol. 2001;46:109–120. doi: 10.1023/a:1010633215445. [DOI] [PubMed] [Google Scholar]
- 74.Lee S, Hirt H, Lee Y. Phosphatidic acid activates a wound-activated MAPK in Glycine max. Plant J. 2001;26:479–486. doi: 10.1046/j.1365-313x.2001.01037.x. [DOI] [PubMed] [Google Scholar]
- 75.Wang M, Zhang Y, Wang J, Wu X, Guo X. A novel MAP kinase gene in cotton (Gossypium hirsutum L.), GhMAPK, is involved in response to diverse environmental stresses. J Biochem Mol Biol. 2007;40:325–332. doi: 10.5483/bmbrep.2007.40.3.325. [DOI] [PubMed] [Google Scholar]
- 76.Yeh CM, Hsiao LJ, Huang HJ. Cadmium activates a mitogen-activated protein kinase gene and MBP kinases in rice. Plant Cell Physiol. 2004;45:1306–1312. doi: 10.1093/pcp/pch135. [DOI] [PubMed] [Google Scholar]
- 77.Jonak C, Nakagami H, Hirt H. Heavy metal stress. Activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol. 2004;136:3276–3283. doi: 10.1104/pp.104.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gupta M, Sharma P, Sarin NB, Sinha AK. Differential response of arsenic stress in two varieties of Brassica juncea L. Chemosphere. 2009;74:1201–1208. doi: 10.1016/j.chemosphere.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 79.Rao KP, Vani G, Kumar K, Wankhede DP, Mishra M, Gupta M, Sinha AK. Arsenic stress activates MAP kinase in rice roots and leaves. Arch Biochem Biophys. 2010;506:73–82. doi: 10.1016/j.abb.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 80.Flowers TJ. Improving crop salt tolerance. J Exp Bot. 2004;55:307–319. doi: 10.1093/jxb/erh003. [DOI] [PubMed] [Google Scholar]
- 81.Bartels D, Sunkar R. Drought and salt tolerance in plants. Crit Rev Plant Sci. 2005;24:23–58. [Google Scholar]
- 82.Umezawa T, Fujita M, Fujita Y, Yamaguchi-Shinozaki K, Shinozaki K. Engineering drought tolerance in plants: discovering and tailoring genes to unlock the future. Curr Opin Biotechnol. 2006;17:113–122. doi: 10.1016/j.copbio.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 83.Popescu SC, Popescu GV, Bachan S, Zhang Z, Gerstein M, Snyder M, Dinesh-Kumar SP. MAPK target networks in Arabidopsis thaliana revealed using functional protein microarrays. Genes Dev. 2009;23:80–92. doi: 10.1101/gad.1740009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ding X, Richter T, Chen M, Fujii H, Seo YS, Xie M, et al. A rice kinase-protein interaction map. Plant Physiol. 2009;149:1478–1492. doi: 10.1104/pp.108.128298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee JS, Huh KW, Bhargava A, Ellis BE. Comprehensive analysis of protein-protein interactions between Arabidopsis MAPKs and MAPK kinases helps define potential MAPK signaling modules. Plant Signal Behav. 2008;3:1037–1041. doi: 10.4161/psb.3.12.6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jung KH, Cao P, Seo YS, Dardick C, Ronald PC. The Rice Kinase Phylo-genomics Database: a guide for systematic analysis of the rice kinase super-family. Trends Plant Sci. 2010;15:595–599. doi: 10.1016/j.tplants.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 87.Dardick C, Chen J, Richter T, Ouyang S, Ronald P. The rice kinase database. A phylogenomic database for the rice kinome. Plant Physiol. 2007;143:579–586. doi: 10.1104/pp.106.087270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rao KP, Richa T, Kumar K, Raghuram B, Sinha AK. In silico analysis reveals 75 members of mitogen-activated protein kinase kinase kinase gene family in rice. DNA Res. 2010;17:139–153. doi: 10.1093/dnares/dsq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Y, Li X, Tan H, Liu M, Zhao X, Wang J. Molecular characterization of RsMPK2, a C1 subgroup mitogen-activated protein kinase in the desert plant Reaumuria soongorica. Plant Physiol Biochem. 2010;48:836–844. doi: 10.1016/j.plaphy.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 90.Taj G, Agarwal P, Grant M, Kumar A. MAPK machinery in plants: Recognition and response to different stresses through multiple signal transduction pathways. Plant Signal Behav. 2010;5:1370–1378. doi: 10.4161/psb.5.11.13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gao F, Su Q, Fan Y, Wang L. Expression pattern and core region analysis of AtMPK3 promoter in response to environmental stresses. Sci China Life Sci. 2010;53:1315–1321. doi: 10.1007/s11427-010-4079-0. [DOI] [PubMed] [Google Scholar]
- 92.Kumar K, Rao KP, Sharma P, Sinha AK. Differential regulation of rice mitogen activated protein kinase kinase (MKK) by abiotic stress. Plant Physiol Biochem. 2008;46:891–897. doi: 10.1016/j.plaphy.2008.05.014. [DOI] [PubMed] [Google Scholar]