Abstract
The free proline content in maize ear-leaves, silk and pollen were analyzed in field grown plants which had matured to the pollination stage. Using maize hybrids PR34F02, PR35P12 and PR36B08 field trials were set up at two locations in eastern Croatia in two different years. Two enzymes of proline metabolism were analyzed in the same leaf samples and specific activities of synthetase (P5CS) and proline dehydrogenase (PDH). Plant productivity was evaluated at harvest by the estimation of total and fully developed grain number per ear and per plant, the mean single grain mass, and the mass of grain per plant. The year in which the plants were grown had a very significant effect on the free proline content in the leaf and pollen, as well as on the enzyme activities assayed. The differences between the plants from the two localities were very significant in all tested parameters of plant grain productivity. There was a significant genotype effect on proline content and P5CS total activity in leaf and on all the productivity parameters. Some of the correlations established suggest that the rate of proline synthesis and degradation in maize ear-leaf at pollination might contribute to the final grain production of the maize plant. Multiple regression analyses was used to further analyze the relationship between proline and grain productivity, but it is clear that future work should include other environmental conditions, plant species and organs such as roots.
Key words: maize, maize silk, plant productivity, pollen, proline, proline dehydrogenase, Δ1-pyrroline-5-carboxylate synthetase, Zea mays L.
Introduction
Different enviromental conditions elicit diverse responses in plants at multiple levels. One of the common responses to abiotic stress conditions which is seen in many plant species is free proline accumulation in plant tissues. Amongst the so far discovered functions of proline osmoprotection of the protoplasm is the most frequently reported. However the expansion of oxidative stress research has given many new insights into the role of proline in plants. Today it is clear that proline is a multifunctional amino acid1–4 with many metabolism pathways being revealed.5–7 The proline concentrations of cells, tissues and plant organs are regulated by the interplay of biosynthesis, degradation and intra- as well as intercellular transport processes.1,2 There are two different precursors for proline biosynthesis in plants. The first pathway uses glutamate which is converted to proline by two successive reductions catalyzed by Δ1-pyrroline-5-carboxylate synthetase (P5CS) and pyrroline-5-carboxylate reductase (P5CR), respectively, both using NADPH as a cofactor.8 An alternative precursor for proline biosynthesis is ornithine. However glutamate pathway is the main pathway during osmotic stress.4 P5CS is the rate-limiting enzyme in proline biosynthesis and is subject to feedback inhibition by proline. Hong et al.9 reported that the feedback regulation of P5CS plays a role in controlling the level of proline under both normal and stress conditions. Zhang et al.10 further postulated that it is possible that the proline accumulation under stress occurs because of an increase in the amount of P5CS and a decrease of the activity of proline dehydrogenase (PDH). The two-step oxidation of proline in all eukaryotes is performed at the inner mitochondrial membrane by the consecutive action of PDH that produces Δ1-pyrroline-5-carboxylate (P5C) and P5C dehydrogenase (P5CDH) that oxidizes P5C to glutamate. This catabolic route is downregulated in plants during osmotic stress resulting in free proline accumulation.11
Agricultural output depends to a large extent on the success of plant reproduction which in turn is greatly influenced by the environmental conditions prevailing during the growing season.12 Drought and high temperature are considered to be key stress factors with a potentially high impact on crop yield.13 The yield component primarily responsible for variation in maize grain yield is the kernel number per plant.14 Grain numbers decrease because of several developmental changes, especially ovary abortion in maize or pollen sterility in small grains.12 The growth and emergence of maize silks has a considerable importance in yield determination under drought conditions.15 Schoper et al.16 reported that in three maize hybrids seed set was decreased when the turgor (Ψp) was low in the silk (stigmatic) tissue.
Whole-genome gene-expression changes of maize plants in response to water-deficit stress at the heading stage were investigated by Yue et al.17 The most notable genes highlighted appear to be involved in osmolyte metabolism, particularly in proline, sucrose, trehalose and raffinose metabolism in the leaves. Mattioli et al.18 proposed that proline may act both as a metabolic substrate to sustain the needs of rapidly dividing cells and, in turn, as a feedback signal molecule to fine-tune developmental processes such as flower transition. Furthermore, during flower, embryo and other developmental processes it may support the energetic needs of rapidly dividing or elongating cells.3,19 Proline was found to be the most abundant amino acid in mature and germinated Arabidopsis pollen, accounting for 60–65% of the free amino acid pool.20 Water stress at maize tassel initiation showed a greater influence on proline, ABA levels and yield than that at anthesis. However, it was found that proline and ABA levels accumulated under water stress conditions were negatively correlated with corn yield.21
As well as the importance of stress tolerance during vegetative growth, the sensitivity to stress of the reproductive phase also deserves more attention, especially in the case of short, but extreme, stress conditions particularly during fertilization and early grain filling in cereals.13 Short hot and dry spells before or during silking have an inordinately large effect on maize grain yield.22 Morison et al.23 suggested that the development and release of new varieties with characteristics that will improve water usage through combined physiological, biotechnological and agronomic research will help with the ‘Blue Revolution’ that is being called for and as such would have a benefit for crop production. Therefore, further understanding of proline accumulation and its relationship to plant productivity in different field conditions will help in creating new more efficient genotypes. Maize, as one of the most important crop species, seems to be very suitable model plant for this research.
Results
To gain a better understanding of how proline metabolism may influence the productivity of a crop plant the proline levels the ear-leaves, silk and pollen of three maize hybrids (PR34F02, PR35P12 and PR36B08) which had been grown in field trials were determined, along with a variety of determinants of crop yield. Total mean proline content in the maize ear-leaf was 0.943 µmol g−1 FW, the lowest proline content was estimated in silk tissue (0.632 µmol g−1) while pollen had by far the highest proline level (196.8 µmol g−1). However it was found that leaf proline content was very variable having variation coefficient of over 70% (Table 1). Further analysis revealed that the leaf proline content was influenced by the year in which the maize was grown, showing a much higher level in 2008 (Table 2). The influence of the locality on leaf proline level was also very significant and there was also a genotype effect, although two of the tested hybrids (PR34F02 and PR36B08) did not differ in leaf proline content. As for proline content in silk, there was no influence of the year established, whereas locality and genotype effects were very significant. All interactions of the main effects were important for leaf proline levels whereas the silk proline content was only influenced by year × genotype interaction (A × C). Pollen proline content was influenced by all the main effects and their interactions, with the exception of the interaction of locality × genotype (B × C). Maize plants grown in 2009 and at the Porec field site had increased proline in pollen. Considering the different genotypes studied, PR36B08 showed the lowest proline content in silk and pollen, with the highest values being obtained in PR34F02.
Table 1.
Mean values, standard deviation (SD) and variation coefficients (CV%) for tested parameters of proline content, enzymes activities and maize plant productivity
| Tested parameter | Symbol | Total mean | SD | CV% |
| Leaf proline content (µmolg−1 FW) | PRO-l | 0.943 | 0.669 | 70.90 |
| Silk proline content (µmolg−1 FW) | PRO-s | 0.632 | 0.158 | 24.98 |
| Pollen proline content (µmolg−1 FW) | PRO-p | 196.8 | 31.66 | 16.08 |
| P5CS-total activity (Ug−1 FW) | P5CS-t | 0.634 | 0.091 | 14.37 |
| P5CS-specific activity (Umg−1 proteins) | P5CS-s | 0.608 | 0.247 | 40.54 |
| PDH-total activity (Ug−1 FW) | PDH-t | 0.395 | 0.129 | 32.69 |
| PDH-specific activity (Umg−1 proteins) | PDH-s | 0.200 | 0.066 | 32.85 |
| Total grain number per ear | TGN/e | 514.8 | 41.27 | 8.02 |
| Developed grain number per ear | DGN/e | 491.7 | 48.86 | 9.94 |
| Mean developed grain mass (g) | MDGM | 0.345 | 0.04 | 11.59 |
| Total grain number per plant | TGN/p | 504.9 | 58.16 | 11.52 |
| Developed grain number per plant | DGN/p | 482.8 | 65.45 | 13.56 |
| Developed grain mass per plant (g) | DGM/p | 168.2 | 35.69 | 21.22 |
FW, frozen tissue weight.
Table 2.
Free proline content in maize leaf (PRO-l), silk (PRO-s) and pollen (PRO-p), P5CS and PDH total and specific enzyme activities in maize leaf, as affected by year, locality, genotype and their interactions
| Free proline content | P5CS activity in leaf | PDH activity in leaf | |||||
| PRO-l | PRO-s | PRO-p | P5CS-t | P5CS-s | PDH-t | PDH-s | |
| µmolg−1 tissue | Ug−1 tissue | Umg−1 proteins | Ug−1 tissue | Umg−1 proteins | |||
| Year effect (A) | |||||||
| 2008 | 1.488a ± 0.104 | 0.658a ± 0.035 | 186b ± 5 | 0.668a ± 0.030 | 0.748a ± 0.059 | 0.283b ± 0.018 | 0.244a ± 0.015 |
| 2009 | 0.398b ± 0.021 | 0.606a ± 0.041 | 208a ± 9 | 0.599b ± 0.018 | 0.468b ± 0.024 | 0.507a ± 0.021 | 0.156b ± 0.010 |
| p value | <0.0001 | ns | 0.0038 | 0.0331 | 0.0005 | <0.0001 | 0.0001 |
| Locality effect (B) | |||||||
| Bicko Selo | 0.815b ± 0.088 | 0.540b ± 0.031 | 184b ± 6 | 0.688a ± 0.029 | 0.758a ± 0.059 | 0.419a ± 0.026 | 0.238a ± 0.016 |
| Poreč | 1.071a ± 0.167 | 0.724a ± 0.034 | 209a ± 9 | 0.580b ± 0.015 | 0.458b ± 0.019 | 0.371b ± 0.034 | 0.162b ± 0.011 |
| p value | 0.0017 | 0.0002 | 0.0023 | 0.0052 | 0.0003 | 0.0127 | 0.0003 |
| Genotype effect (C) | |||||||
| PR34F02 | 0.913b ± 0.150 | 0.727a ± 0.039 | 217a ± 12 | 0.585b ± 0.026 | 0.551a ± 0.043 | 0.382a ± 0.038 | 0.200a ± 0.018 |
| PR36B08 | 0.803b ± 0.120 | 0.485b ± 0.030 | 177c ± 6 | 0.624a,b ± 0.031 | 0.641a ± 0.073 | 0.400a ± 0.037 | 0.208a ± 0.018 |
| PR35P12 | 1.113a ± 0.213 | 0.684a ± 0.045 | 196b ± 6 | 0.692a ± 0.032 | 0.632a ± 0.077 | 0.405a ± 0.039 | 0.193a ± 0.022 |
| p value | 0.0049 | 0.0003 | 0.0014 | 0.0341 | ns | ns | ns |
| Significance of interactions effects (p value) | |||||||
| A × B | 0.0004 | ns | 0.0058 | ns | 0.0057 | 0.0010 | 0.0319 |
| A × C | 0.0293 | 0.0490 | 0.0096 | ns | ns | ns | ns |
| B × C | 0.0047 | ns | ns | ns | ns | ns | ns |
| A × B × C | 0.0014 | ns | 0.0098 | ns | ns | ns | ns |
3-factorial ANOVA: data are treatment means ± SE;
treatment means with the same later do not differ after LSD test, p = 0.05; ns = p value above 0.05.
Total and specific activities of the enzyme P5CS were influenced by the year and locality, showing higher values in 2008 and at Bicko Selo. The specific activity of P5CS in leaf was not significantly different in tested maize hybrids, but total enzyme activity showed some degree of genotype influence. The interactive effects of the main factors (year, locality, genotype) were mostly insignificant for P5CS activity with the exception of year × locality (A × B) that affected P5CS specific activity. With PDH activity there was a very strong influence of the year. The effects of locality as well as the interaction of the year × locality (A × B) were also significant for both indicators of PDH activity, that is total and specific activities.
The different parameters used to gauge the estimated grain yield were all influenced by the main factors (Table 3: year, locality, genotype) and plant productivity was found to be higher in 2008 and at Porec. PR34F02 had the highest TGN/e, DGN/e, TGN/p and DGN/p, but this hybrid finally showed significantly lower MDGM in comparison with other hybrids. PR36B08 had the highest DGM/p (175 g). The interactions of the main factors had very different degrees of significance in their influence on the plant productivity parameters. Only the year × locality effect was statistically significant for all the tested maize yield components.
Table 3.
The components of maize plant productivity: influence of year, locality, genotype and their interactions
| * | TGN/e | DGN/e | MDGM | TGN/p | DGN/p | DGM/p |
| Year effect (A) | ||||||
| 2008 | 532a ± 8 | 508a ± 9 | 0.360a ± 0.006 | 522a ± 8 | 498a ± 8 | 178.7a ± 2.5 |
| 2009 | 498b ± 9 | 476b ± 11 | 0.330a ± 0.009 | 488b ± 17 | 467b ± 18 | 157.5a ± 9.9 |
| p value | 0.0004 | 0.0005 | <0.0001 | 0.0073 | 0.0041 | <0.0001 |
| Locality effect (B) | ||||||
| Bicko Selo | 486b ± 8 | 457b ± 9 | 0.337b ± 0.010 | 463b ± 12 | 436b ± 13 | 102.9b ± 8.0 |
| Poreč | 544a ± 6 | 527a± 6 | 0.353a ± 0.005 | 547a ± 8 | 530a ± 9 | 150.0a ± 4.3 |
| p value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Genotype effect (C) | ||||||
| PR34F02 | 538a ± 12 | 522a ± 13 | 0.327b ± 0.011 | 518a ± 18 | 502a ± 19 | 166.4b ± 10.1 |
| PR36B08 | 506b ± 10 | 484b ± 12 | 0.355a ± 0.008 | 512a ± 16 | 491a ± 17 | 175.0a ± 8.3 |
| PR35P12 | 499b ± 10 | 469c ± 11 | 0.354a ± 0.010 | 484b ± 15 | 455b ± 16 | 162.9b ± 9.2 |
| p value | 0.0012 | 0.0003 | <0.0001 | 0.0371 | 0.0037 | 0.0202 |
| Significance of interactions effects (p value) | ||||||
| A × B | 0.0365 | 0.0029 | <0.0001 | 0.0013 | 0.0002 | <0.0001 |
| A × C | ns | ns | 0.0014 | ns | 0.0361 | 0.0016 |
| B × C | ns | ns | ns | ns | ns | ns |
| A × B x C | 0.0387 | 0.0431 | 0.0079 | ns | ns | ns |
3-factorial ANOVA: data are treatment means ± SE;
treatment means labeled with the same later do not differ after LSD test, p = 0.05; ns = p value above 0.05.
TGN/e, total grain number per ear; DGN/e, developed grain number per ear; MDGM, mean developed grain mass; TGN/p, total grain number per plant; DGN/p, developed grain number per plant; DGM/p, developed grain mass per plant.
To assess whether there was a correlation between proline metabolism and plant productivity multiple regression analysis was used. This showed a significant interplay of proline content, tested enzymes activity and yield components. As an example, in the analysis where DGM/p was taken as the dependent variable and PRO-l, PRO-s, P5CS-t, P5CS-s, PDH-t, PDH-s and MDGM are independent variables, regression is significant at p = 0.05 (F(7,12) = 17.2244; R2 = 0.9679), with a particular impact of MDGM on DGM/p (t = 0.9679; p = 0.01):
DGM/p = −90.3232 − 3.1006 × PRO-l + 21.9984 × PRO-s − 110.1938 × P5CS-t − 80.6877 × P5CS-s − 28.2535 × PDH-t + 245.9423 × PDH-s + 951.4003 × MDGM.
Using linear single correlation analyses PRO-l was strongly and negatively correlated to PDH-t (r = −0.937; p ≤ 0.01). PRO-s had a positive relation to PRO-p (r = 0.783; p ≤ 0.01) and very significant positive correlation was established between P5CS-s and PDH-s (r = 0.942; p ≤ 0.01). Based on a whole data set, the correlations among TGN/e and PRO-l, PRO-s and PDH-t (Fig. 1) were also very significant (p ≤ 0.01). Measured parameters of the productivity of maize plants showed a lot of significant correlations, with the strongest link established between TGN/p and DGN/p (r = 0.995; p ≤ 0.01).
Figure 1.
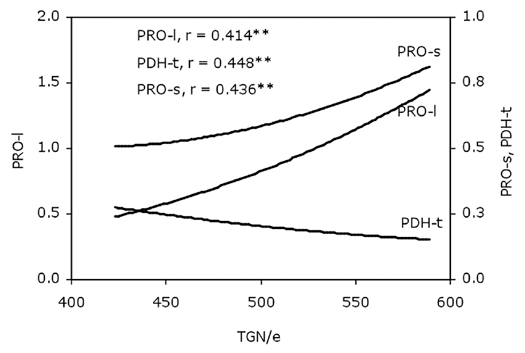
The correlations among total grain number per ear (TGN/e) and leaf proline content (PRO-l, µmol g−1 tissue), silk proline content (PRO-s, µmol g−1 tissue) and proline dehydrogenase total activity (PDH-t, Ug−1 tissue) in maize plants (n = 48; **p ≤ 0.01).
Discussion
Factors affecting grain set under drought are of special interest in commercial breeding endeavors.24 Because maize is an outcrossing species, pollen must move from the anthers at the top of the plant to the exposed silk of the same and surrounding plants. This growth stage in Croatia takes place at the beginning of July in mostly dry and warm weather conditions. Here, three maize hybrids were grown at two locations in eastern Croatia in two successive years (2008 and 2009). As shown in Table 4, the mean air temperature over the vegetation period of April to September was higher than the multiannual mean at both experimental localities, with 2009 being the warmest. 2009 was much dryer as well at both localities with 35% and 27% (Bicko Selo and Porec, respectively) less precipitation in comparison with multiannual mean. The effect of year was significant for leaf proline content (Table 2) but unexpectedly it was significantly lower in 2009, whereas pollen proline content was higher in that year. Concurrently, the year effect was seen with both enzyme activities measured. P5CS-t activity was lower whilst PDH-t activity was much higher in 2009 when it was dryer and warmer. A possible explanation could be that inhibition of P5CS took place in such conditions. The very significant negative correlation between PRO-l and PDH-t (r = −0,937; p ≤ 0.01) indicates the possible utilization of proline for some purpose other than osmoprotection in 2009. As shown in Figure 1, high TGN/e was accompanied with high PRO-l and low PDH-t. If proline content in the leaves was depleted for some reason, plant productivity declined. A possible reason for low proline accumulation in the leaves could be enhanced protein synthesis or proline translocation to other parts of the plant, such as generative organs, or the roots which have to grow into deeper soil levels under drought conditions. That suggestion is in concordance with the significantly higher proline content detected in pollen in 2009 and also with the results of Valentovič et al.25 who found that proline levels increased significantly under stress conditions in all studied tissues of 13-days old maize seedling. It was much higher in roots and mesocotyl tissues than in leaves of a drought-sensitive cultivar. Similarly, Raymond and Smirnoff26 stated that proline accumulation in maize root tips was the result of decreased oxidation and incorporation into protein at low water potential.
Table 4.
Climate characteristics-mean air temperatures and precipitation amounts at localities Bicko Selo and Poreč in experimental years 2008, 2009 in comparison with multiannual mean
| Locality | Mean air temperature (°C) | Precipitation (L m−2) | ||||
| 2008 | 2009 | 1981–2009 | 2008 | 2009 | 1981–2009 | |
| Bicko Selo | 18.3 | 19.2 | 17.9 | 430 | 279 | 431 |
| Poreč | 17.8 | 18.7 | 17.7 | 394 | 323 | 441 |
Data obtained from the nearest weather stations; maize vegetation period April–September.
Schafleitner et al.27 reported that the expression of both P5CS and PDH did not correlate with the proline levels found in leaf tissue indicating that mechanisms other than transcription participate in the regulation of proline accumulation in potato leaves. Chandra and Dubey28 reported that although the levels of proline increased with the magnitude of water stress the P5CS activity did not show a corresponding increase in all Cenchrus species which were examined. Here, the specific activities of P5CS and PDH showed a significant positive correlation (r = 0.942; p ≤ 0.01). This correlation along with an influence of the year in which they were grown was seen as both P5CS-s and PDH-s were lower in 2009, which was the dryer year. However there was also a higher total protein content found in leaf samples taken in 2009 (data not shown). It can be assumed that drought stimulated protein synthesis in the leaves, such as heat shock proteins, molecular chaperones, antioxidative enzymes or protein factors that might be involved in the regulation of signal transduction and expression of genes related to stress response, as reported by Marino et al.29
There was a large influence of both the locality in which they were grown and genotype on leaf, silk and pollen proline levels in the plants analysed. Plants grown at Porec had higher proline content in all tissues which were examined and both P5CS and PDH activities were lower in comparison with plants grown at Bicko Selo (Table 2). The estimated grain yield components were all influenced by year, locality and genotype (Table 3). Plant productivity was found to be higher in 2008 and at Porec, where higher proline levels in leaf, silk and pollen were observed. Taking into account that hybrid PR36B08 showed the lowest proline levels (Table 2) but the highest DGM/p (Table 3), a significant relationship between proline content and metabolism on maize plant productivity are feasible, although this will be influenced by the agroecological conditions of the year and locality, as well as genotype of the plants grown. It is worth noting that the hybrid PR34F02 obviously had better pollination because it showed higher TGN/e, DGN/e, TGN/p and DGN/p than PR36B08, however the latters significantly smaller MDGN resulted in a lower DGM/p (Table 3). PR34F02 also had higher proline levels in leaf, silk and pollen, accompanied with less expressed enzymatic activity for P5CS and PDH (Table 2). This indicates that genotype effects on both proline metabolism and on grain set and development can not be neglected in maize plants.
Luna et al.30 reported that maize pollen dehiscence normally occurs in mid-morning when the temperature is typically increasing, relative humidity decreasing and radiation load increasing so that pollen loses viability quickly. Therefore, the prevention of water loss from both the pollen and stigma is very important for successful fertilization and seed set. In this context proline might be acting as an osmoprotectant at pollination. As mentioned above, hybrid PR34F02 had the highest silk and pollen proline level amongst the maize genotypes tested, and its grain productivity in terms of TGN/e and TGN/p was the highest. The main effect of heat stress during or after floral initiation is observed on kernel number.13 Grain numbers decrease because of several developmental changes, especially ovary abortion in maize or pollen sterility in small grains,12 as well as delayed silk emergence.15 Here, the abortion of grain during post-anthesis development was only 3% in PR34F02, 4% in PR36B08 and 6% in PR35P12, respectively.
In summary, here it was found that the measure of productivity TGN/e was positively correlated to proline content in the maize silk (PRO-s; Fig. 1). Furthermore, multiple regression analyses showed a significant interplay between proline content (PRO-l, PRO-s), activities of enzymes involved in proline metabolism (P5CS-t, P5CS-s, PDH-t, PDH-s) and grain productivity of the plants (MDGM and DGM/p). However, it is quite obvious that proline content in maize tissues was influenced by various internal and external factors and single measurements can not reveal enough about the dynamics of proline metabolism and the roles of proline in plant growth, development, stress tolerance and productivity in field conditions. Hence, further evaluation in other environmental conditions, plant species and organs such as the root, would contribute to our knowledge on this exceptional amino acid and how it influences crop yields.
Materials and Methods
Plant material and experiment description.
Three maize hybrids (PR34F02, PR36B08, PR35P12) were grown in two different years (2008 and 2009) at two localities in eastern Croatia (Bicko Selo and Porec) in field trials. These were planted with a block design with four replicates. Each plot consisted of 4 rows which were 15 m in length. The seed was planted in April with leaf, silk and pollen sampling being done during the morning hours in early July once the plants were at the silking-tasseling stage of development. Grain yield parameters were determined before harvest in late September. The mean air temperature and precitipation were recorded at the two localities and compared to the mean values from the years 1981 to 2009. Both 2008 and 2009 were characterized by different temperature and moisture parameters during maize vegetation from April to September, depending on locality. As shown in Table 4, the area around Bicko Selo had in both years a higher mean temperature than usual in the April–September period, whereas Porec had a higher mean air temperature for the whole maize vegetation only in 2009. Considering total precipitation sum from planting to harvest, the area around Porec is usually more humid than Bicko Selo. Total precipitation recorded in 2008 for the area of Bicko Selo did not differ from the multiannual mean (years 1981–2009), but in 2009 there was only 279 Lm−2 recorded (35.3% less than average). The area around Porec received less rain throughout maize vegetation period in both years, especially in 2009 (26.7% less than multiannual mean).
Proline content analyses.
The middle section of the ear-leaf and silk samples were taken from 5 uniformly developed plants selected from the middle two rows of each plot, whereas pollen was collected from different plants in the middle rows, depending on the intensity of pollen shedding. Proline was determined spectrophotometrically using the ninhydrin method described by Bates et al.31
Enzyme activities analyses.
Frozen leaf tissue samples (0.5 g) were ground to a fine powder with liquid nitrogen and homogenized in the appropriate extraction buffer. The ratio of buffer volume:g tissue was 2:1. The extraction buffer used for P5CS assays was 50 mM Tris-HCl (pH 7.5) containing 10 mM MgCl2, 10 mM β-mercaptoethanol, 4 mM DTT, 2 mM PMSF, 1 mM EDTA and 2% PVPP. The extracts were centrifuged at 4°C for 15 min at 20,000 g and the resulting supernatant was used as the enzyme source. The buffer used for extraction of PDH was 100 mM sodium phosphate (pH 8.0) containing 0.1 mM cysteine and 0.1 mM EDTA. After centrifugation at 15,000 g for 10 min at 4°C, the supernatant was used for enzyme assay. Total enzyme activity was expressed per g of frozen tissue (FW) and specific activity was calculated taking into account total protein content in the same tissue sample, determined spectrophotometricaly with Bovine serum albumin as a standard.32
P5CS activity assay.
The activity of P5CS was assayed following the method described by Hayzer and Leisinger.33 The assay mixture contained 50 mM Tris-HCl buffer (pH 7.0), 20 mM MgCl2, 50 mM L-glutamate, 100 mM hydroxylamine-HCl and 10 mM ATP. The reaction was initiated by addition of enzyme extract. After incubation at 15 min for 37°C the reaction was stopped by adding 1 mL of stop buffer (2.5 g FeCl3 and 6 g trichloroacetic acid in a final volume of 100 mL of 2.5 M HCl). The precipitated proteins were removed by centrifugation (4°C for 15 min at 10,000 g) and the absorbance recorded (UV-VIS spectrophotometer Carry 50, Varian Medical Systems, Inc.,) at 535 nm against a blank identical to the above but lacking ATP. The amount of γ-glutamyl hydroxamate complex produced was estimated from the molar extinction coefficient of 250 M−1cm−1 reported for the Fe3+ hydroxamate complex of the compound.33 One unit of enzyme activity was defined as the amount of enzyme required to release 1 µmol of γ-glutamyl hydroxamate per minute.
PDH activity assay.
PDH activity was measured as described by Rena and Splittstoesser.34 Briefly, enzyme extract was incubated in the reaction buffer contained 100 mM Na2CO3-NaHCO3 (pH 10.3), 20 mM L-proline, 10 mM NAD+ at 28°C, and then PDH dependent NAD+ reduction was monitored at 340 nm. One unit of PDH activity is defined as the amount of enzyme catalyzing the formation 1 µmol of NADH per min.
Plant productivity determination.
At the full maturity stage of the maize plants one of two middle rows was selected for plant and ear number per plot determination. All ears were harvested and a mean ear weight was estimated. Five average ears were used for the determination of total and fully developed grain number per ear. The developed grain from each ear was dried to constant mass at 70°C and mean developed grain mass was calculated. Total and developed grain number per plant was estimated using data on ear number per plant, whereas developed grain mass per plant was calculated using developed grain number per plant and single developed grain mass.
Statistical analysis.
The results were statistically analyzed using ANOVA (GLM procedure, SAS statistical software version 8, SAS Institute, Cary, NC) with the F-test for the evaluation of year, locality, genotype and their interactions effects, the LSD test (p = 0.05) for comparison of treatment means, as well as the t-test for the evaluation of the established correlations (*p ≤ 0.05; **p ≤ 0.01) and multiple regression analyses.
Acknowledgements
This work was an integral part of the research project no.: 079-0790494-0559 (“Physiological mechanisms of plant tolerance to abiotic stress”) supported by The Ministry of Science, Education and Sports, Croatia.
Abbreviations
- PRO-l
proline content in leaf
- PRO-s
proline content in silk
- PRO-p
proline content in pollen
- ;P5CS-t-total
activity of Δ1-pyrroline-5-carboxylate synthetase
- P5CS-s
specific activity of Δ1-pyrroline-5-carboxylate synthetase
- PDH-t
total activity of proline dehydrogenase
- PDH-s
specific activity of proline dehydrogenase
- TGN/e
total grain number per ear
- DGN/e
developed grain number per ear
- MDGM
mean developed grain mass
- TGN/p
total grain number per plant
- DGN/p
developed grain number per plant
- DGM/p
developed grains mass per plant
References
- 1.Lehmann S, Funck D, Szabados L, Rentsch D. Proline metabolism and transport in plant development. Amino Acids. 2010;39:949–962. doi: 10.1007/s00726-010-0525-3. [DOI] [PubMed] [Google Scholar]
- 2.Szabados L, Savouré A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Trovato M, Mattioli R, Costantino P. Multiple roles of proline in plant stress tolerance and development. Rend Lincei-Sci Fis. 2008;19:325–346. [Google Scholar]
- 4.Verbruggen N, Hermans C. Proline accumulation in plants: a review. Amino Acids. 2008;35:753–759. doi: 10.1007/s00726-008-0061-6. [DOI] [PubMed] [Google Scholar]
- 5.Brugière N, Dubois F, Limami AM, Lelandais M, Roux Y, Sangwan RS, et al. Glutamine synthetase in the phloem plays a major role in controlling proline production. Plant Cell. 1999;11:1995–2011. doi: 10.1105/tpc.11.10.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hare PD, Cress WA, van Stadten J. Proline synthesis and degradation: a model system for elucidating stress-related signal transduction. J Exp Bot. 1999;50:413–434. [Google Scholar]
- 7.Kishor KPB, Sangam S, Amrutha RN, Sri Laxmi P, Naidu KR, Rao KRSS, et al. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr Sci India. 2005;88:424–438. [Google Scholar]
- 8.Deuschle K, Funck D, Forlani G, Stransky H, Biehl A, Leister D, et al. The role of Δ1-pyrroline-5-carboxylate dehydrogenase in proline degradation. Plant Cell. 2004;16:3413–3425. doi: 10.1105/tpc.104.023622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong ZL, Lakkineni K, Zhang ZM, Verma DPS. Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 2000;122:1129–1136. doi: 10.1104/pp.122.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang CS, Lu Q, Verma DPS. Removal of feedback inhibition of Δ1-pyrroline-5-carboxylate synthetase, a bifunctional enzyme catalyzing the first two steps of proline biosynthesis in plants. J Biol Chem. 1995;270:20491–20496. doi: 10.1074/jbc.270.35.20491. [DOI] [PubMed] [Google Scholar]
- 11.Miller G, Honig A, Stein H, Suzuki N, Mittler R, Zilberstein A. Unraveling Δ1-pyrroline-5-carboxylate-proline cycle in plants by uncoupled expression of proline oxidation enzymes. J Biol Chem. 2009;284:26482–26492. doi: 10.1074/jbc.M109.009340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyer JS, Westgate ME. Grain yields with limited water. J Exp Bot. 2004;55:2385–2394. doi: 10.1093/jxb/erh219. [DOI] [PubMed] [Google Scholar]
- 13.Barnabás B, Jäger K, Fehér A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008;31:11–38. doi: 10.1111/j.1365-3040.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- 14.Fonseca AE, Lizaso JI, Westgate ME, Grass L, Dornbos DL. Simulating Potential Kernel Production in Maize Hybrid Seed Fields. Crop Sci. 2004;44:1696–1709. doi: 10.2135/cropsci2004.1696. [DOI] [Google Scholar]
- 15.Fuad-Hassan A, Tardieu F, Turc O. Drought-induced changes in anthesis-silking interval are related to silk expansion: a spatio-temporal growth analysis in maize plants subjected to soil water deficit. Plant Cell Environ. 2008;31:1349–1360. doi: 10.1111/j.1365-3040.2008.01839.x. [DOI] [PubMed] [Google Scholar]
- 16.Schoper JB, Lambert RJ, Vasilas BL, Westgate ME. Plant factors controlling seed set in maize. Plant Physiol. 1987;83:121–125. doi: 10.1104/pp.83.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yue GD, Zhuang YL, Li ZX, Sun L, Zhang JR. Differential gene expression analysis of maize leaf at heading stage in response to water-deficit stress. Bioscience Rep. 2008;28:125–134. doi: 10.1042/BSR20070023. [DOI] [PubMed] [Google Scholar]
- 18.Mattioli R, Costantino P, Trovato M. Proline accumulation in plants. Not only stress. Plant Sign Behav. 2009;4:1016–1018. doi: 10.4161/psb.4.11.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattioli R, Marchese D, D'Angeli S, Altamura MM, Costantino P, Trovato M. Modulation of intracellular proline levels affects flowering time and inflorescence architecture in Arabidopsis. Plant Mol Biol. 2008;66:277–288. doi: 10.1007/s11103-007-9269-1. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann S, Gumy C, Blatter E, Boeffel S, Fricke W, Rentsch D. In planta function of compatible solute transporters of the AtProT family. J Exp Bot. 2010;62:787–796. doi: 10.1093/jxb/erq320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Udomprasert N, Kijjanon J, Thiraporn R, Machuay A. Effects of water deficit at tasseling on proline and abscisic acid levels and yield of corn. Kasetsart J Nat Sci. 1999;33:310–316. [Google Scholar]
- 22.Suwa R, Hakata H, Hara H, El-Shemy HA, Adu-Gyamfi JJ, Nguyen NT, et al. High temperature effects on photosynthate partitioning and sugar metabolism during ear expansion in maize (Zea mays L.) genotypes. Plant Physiol Biochem. 2010;48:124–130. doi: 10.1016/j.plaphy.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Morison JIL, Baker NR, Mullineaux PM, Davies WJ. Improving water use in crop production. Philos T Roy Soc B. 2008;363:639–658. doi: 10.1098/rstb.2007.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruce WB, Edmeades GO, Barker TC. Molecular and physiological approaches to maize improvement for drought tolerance. J Exp Bot. 2002;53:13–25. [PubMed] [Google Scholar]
- 25.Valentovič P, Luxová M, Kolarovič L, Gašparíková O. Effect of osmotic stress on compatible solutes content, membrane stability and water relations in two maize cultivars. Plant Soil Environ. 2006;52:186–191. [Google Scholar]
- 26.Raymond MJ, Smirnoff N. Proline metabolism and transport in maize seedlings at low water potential. Ann Bot. 2002;89:813–823. doi: 10.1093/aob/mcf082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schafleitner R, Gaudin A, Gutierrez Rosales RO, Alvarado Aliaga CA, Bonierbale M. Proline accumulation and real time PCR expression analysis of genes encoding enzymes of proline metabolism in relation to drought tolerance in Andean potato. Acta Physiol Plant. 2007;29:19–26. [Google Scholar]
- 28.Chandra A, Dubey A. Effect of ploidy levels on the activities of Δ1-pyrroline-5-carboxylate synthetase, superoxide dismutase and peroxidase in Cenchrus species grown under water stress. Plant Physiol Biochem. 2010;48:27–34. doi: 10.1016/j.plaphy.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Marino R, Ponnaiah M, Krajewski P, Frova C, Gianfranceschi L, Pe ME, et al. Addressing drought tolerance in maize by transcriptional profiling and mapping. Mol Genet Genomics. 2009;281:163–179. doi: 10.1007/s00438-008-0401-y. [DOI] [PubMed] [Google Scholar]
- 30.Luna S, Figueroa VJ, Baltazar MB, Gomez MR, Townsend LR, Schoper JB. Maize pollen longevity and distance isolation requirements for effective pollen control. Crop Sci. 2001;41:1551–1557. [Google Scholar]
- 31.Bates LS. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- 32.Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Hayzer DJ, Leisinger T. The gene-enzyme relationships of proline biosynthesis in Escherichia coli. J Gen Microbiol. 1980;118:287–293. doi: 10.1099/00221287-118-2-287. [DOI] [PubMed] [Google Scholar]
- 34.Rena AB, Splittstoesser WE. Proline dehydrogenase and pyrroline-5-carboxylate reductase from pumpkin cotyledons. Phytochemistry. 1975;14:657–661. [Google Scholar]


