Abstract
The ability to regulate the rates of metabolic processes in response to changes in the internal and/or external environment is a fundamental feature which is inherent in all organisms. This adaptability is necessary for conserving the stability of the intercellular environment (homeostasis) which is essential for maintaining an efficient functional state in the organism. Symbiotic nitrogen fixation in legumes is an important process which establishes from the complex interaction between the host plant and microorganism. This process is widely believed to be regulated by the host plant nitrogen demand through a whole plant N feedback mechanism in particular under unfavorable conditions. This mechanism is probably triggered by the impact of shoot-borne, phloem-delivered substances. The precise mechanism of the potential signal is under debate, however, the whole phenomenon is probably related to a constant amino acid cycling within the plant, thereby signaling the shoot nitrogen status. Recent work indicating that there may be a flow of nitrogen to bacteroids is discussed in light of hypothesis that such a flow may be important to nodule function. Large amount of γ-aminobutyric acid (GABA) are cycled through the root nodules of the symbiotic plants. In this paper some recent evidence concerning the possible role of GABA in whole-plant-based upregulation of symbiotic nitrogen fixation will be reviewed.
Key words: γ-aminobutyric acid, nitrogen fixation, nodule, symbiosis, translocation, signaling
Nitrogen (N) is major limiting nutrient for the growth of most plant species in different ecosystems. Acquisition and assimilation of N is second in importance only to photosynthesis for plant growth and development. Elemental N is a key constituent of protein, nucleic acids and other vital cellular components. Most plants acquire N from the soil solution either as nitrate or ammonium ions. In addition, some plants can utilize the atmospheric gaseous nitrogen pool through symbiotic associations with species of bacteria, cyanobacteria or actinomycetes that contain the N2 fixing enzyme, nitrogenase. Clearly, the crucial role that symbiotic plants play in plant growth requires that physiologists understand the biochemical and molecular events that regulate fixation and subsequent metabolism of nitrogen.
Symbiotic N2 fixation is an important process for increasing the plant available N and thereby the growth capacity of legumes. This process results from the complex interaction between the host plant and microorganism.1 The host plant provides the microorganism with carbon and a source of energy for growth and functions while the microorganism fixes atmospheric N2 and provides the plant with a source of reduced nitrogen in the form of ammonium. An adequate supply of carbohydrates is an essential requirement of nodule functioning as N2 fixation is expensive in terms both of energy and carbon for the synthesis of N-products. Sucrose synthesized in photosynthesis and exported to the nodules via the phloem, is the primary fuel for N2 fixation.2 Sucrose can be metabolized in the cytoplasm of infected, uninfected or interstitial cells with organic acids as the end products. Malate is strongly believed to be the major respiratory substrate for bacteroids.3 This dicarboxylic acid is the major energy source for the bacteroids and plant mitochondria, and is used for NH4+ assimilation as carbon skeleton in the glutamine synthetase/glutamate synthase (GS/GOGAT) pathway.4 The products of symbiotic N2 fixation are exported from the nodules to the rest of the host plant where they are incorporated into essential macro-molecules such as amino acids, proteins that drive plant growth, development and yields. According to the fixation products, root nodules are generally divided into two major groupings:1 (1) indeterminate nodules that are elongate-cylindrical activity that transport fixed N as amides such as alfalfa, pea and clover; and (2) determinate nodules that are spherical with determinate internal meristematic activity that transport fixed N as ureides, such as soybean and common bean. The complex series of events leading to the formation and functioning of the fixation machinery required controlled coordinated expression of both bacterial and host plant genes.
Regulation of Symbiotic N2 Fixation
The marketed influence of the environment on symbiotic N2 fixation has been known for a long time. The delicate balance existing between the host plant and microsymbiont is disturbed even by mildly adverse conditions while plant growth depending on combined nitrogen remains usually unaffected under such conditions. The response of symbiosis to the environment is mainly determined by the genetical and physiological constitution of the host plant, although the recent findings have indicated that the response to stress is modified even by the microsymbiont.5 There are numerous environmental effects causing a decrease in symbiotic N2 fixation activity. In many cases it has been assumed that such decrease in N2 fixation could induce growth retardation of the legume plant as well as the plant demand for symbiotically fixed N.6 There is a growing body of evidence indicating that symbiotic N2 fixation of legumes is tightly regulated by the host N-demand which is mediated through N-feedback mechanism.6,7 The functioning of the feedback control requires specific N metabolites (amino acids and/or amides) circulating in the phloem circuit and arriving at the nodule. The phloem mobile N metabolites could thus be involved in detecting the nitrogen status of the plant, transmitting a signal which is perceived, decoded and modulate the nitrogenase activity. The existence of the systemic signaling suggests that legumes optimize the fixing capacity probably in concert with endogenous and environmental inputs. The involvement of phloem-borne amino acids in regulation of N2 fixation of nodulated Medicago truncatula plants was recently advocated by Sulieman et al.6 when they increased the amino acid content of phloem sap by supplying amino acids externally and observed an inhibition of nitrogenase activity. The greatest inhibitory effects were obtained with asparagine and even less with other amino acids. The exact mechanisms, specificities and site of any downregulation of nitrogenase activity by phloem-borne amino acids remain to be elucidated. By using 15N labeling (ammonium) of common bean leaves in hydroponic experiment, it has been demonstrated that there is a rapid cycling of N and subsequent nodule labeling.8 At present, numerous molecules have been envisaged as potential signals involved in the regulation of nitrogenase activity at the whole-plant level in different legume species. The possible role of asparagine,6,7 glutamine,9 ureides10 and polyamines11 have been reported. This does not preclude the possibility that other compounds may take part in the regulation of nitrogenase activity. Such hypothesis is so far supported by many physiological data, thus looks very plausible. However, the link between such a N-feedback mechanism and nodulation still awaits further biochemical and/or physical elucidation. Overall, while there are a large number of studies on the relation of stress, reduced nodule activity and a phloem mediated N-feedback regulation, no investigations of upregulated nodules and a possible connection with shoot signaling have been made. More recently, our lab is able to establish a simple precise system for studying the upregulation of nitrogenase activity.12 An exact understanding of such feed-forward processes will provide insights that are necessary to increase N2 fixation by modern genetic engineering techniques.
Role of γ-Aminobutyric Acid (GABA) in Plants
γ-aminobutyric acid (GABA) is a ubiquitous four-carbon, nonprotein amino acid with a molecular weight of 103.1 daltons. It appears ubiquitously in living organisms and comprises a significant proportion of the free amino acid pool in plant cells.13 GABA is found virtually in all prokaryotic and eukaryotic organisms. In contrast to vertebrates, the physiological role of GABA in plants remains still unclear. Contrary to the characterization as main inhibitory neurotransmitter in animals, most investigation of GABA in plants has been focused mainly on the metabolic functions, although the recent findings have indicated that this amino acid has a significant signaling nature in response to stressful conditions and C/N metabolism.14 It is widely believed that GABA is strongly involved in the communications between plants, animals, microorganisms (bacteria and fungi) and other plants, although the mechanism behind such function differs greatly. The remarkable rapid accumulation of GABA, especially shoot tissues, in response to a variety of stresses (biotic and abiotic) has been well documented, although the real reasons behind that accumulation is not well defined. Different reports has indicated that GABA accumulation has been localized within various plant tissues such as shoots, roots, nodules, cultured plant cells, tubers, flowers and fruits (Table 1).12,15,16 A wide range of stimuli that have been reported to induce GABA accumulation include nitrogen deficiency, hypoxia, drought, cold, UV stress, heat shock, CO2 enrichment, mechanical stimulation, mechanical damage, cytosolic acidification and plant phytohormones (Table 1) (Shelp et al.16 and reference therein). The most recent findings in connection with such stress stimulations have revealed that GABA readily accumulates by a combination of biochemical and transcriptional processes.16 The significant remarkable accumulation of GABA has been associated with the appearance of extracellular GABA, either in the apoplast or external medium.17 The fast observed increase in the cellular GABA concentration was first discussed as a regulatory mechanism for homeostasis of the cytosolic pH, since GABA synthesis is consuming protons, and the increasing cytosolic [H+] will consequently lead to GABA accumulation.18 Later, an increase of the cytosolic Ca2+-concentration preceding the elevated GABA synthesis rate was reported, linking GABA synthesis to stress signaling.19
Table 1.
Accumulation of γ-aminobutyric acid (GABA) in different organs of soybean under various environmental stresses
| Stress/treatment | Organ/tissue | Reference |
| Water deficit | Nodules, xylem | Serraj, et al.15 |
| Water logging | Xylem, root, nodules | Puiatti and Sodek41 |
| Hypoxia (N2 exposure) | Leaves, nodules, root | Serraj, et al.15 |
| Salt stress | Roots | Xing, et al.42 |
| Cold stress | Leaves | Wallace, et al.43 |
| Mechanical damage | Leaves | Wallace, et al.43 |
| N stress | Xylem, root, nodules | Lima and Sodek44 |
| Xylem | Antunes, et al.45 |
Several papers have demonstrated the possible metabolic functions of GABA in various processes which might include cytosolic pH regulation,20 protection against oxygen deficiency,21 scavenging against reactive oxygen species (ROS),22 temporary nitrogen storage23 or plant defense against pests.24 Additionally, in the recent years, the possibility that GABA might be involved in regulating plant growth and development has been raised. Several plant development processes has been suggested including pollen tube growth,25 senescence,26 reproduction,25 fertilization27 and regulation of nitrate uptake by increasing expression of nitrate transporters.28
Role of GABA in Nodule Functioning
Among the detected amino acids, GABA is relatively abundant in nodules of various leguminous plants.5,12,20 By the aid of 15N nuclear magnetic resonance (15N NMR), GABA was determined as the second most abundant amino acid of detached pea nodules.29 High [GABA] have been reported in bacteroids isolated from different bacterial strains including Bradyrhizobium japonicum,30 Sinorhizobium meliloti31 and Rhizobium leguminosarum.32 More recently, Prell and co-workers were able to confirm these findings for pea plants. They incubated the nodulated roots of the plant in 15N2 for 30 min and detected a rapid labeling level of GABA in the cytosol and bacteroids fraction of R. leguminosarum.33 The high expression levels of the main enzymes that involve in the GABA metabolism in the bacteroids are consistent with these findings. Despite its relative abundance in nodules, no apparent function for GABA has indicated for a long time.
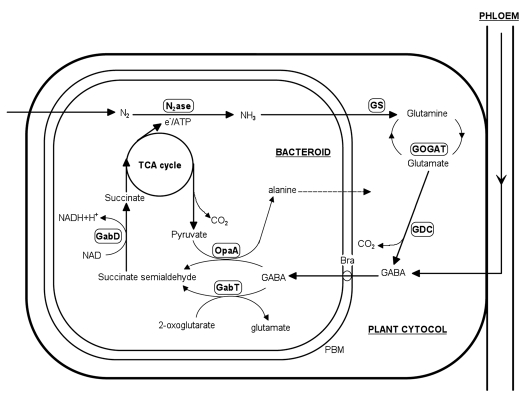
GABA is found in virtually all prokaryotic and eukaryotic organisms. This non-protein amino acid can be made by many bacteria from glutamate by glutamate decarboxylase in what is known as the GABA shunt pathway.32 This pathway is a theoretical bypass of the 2-oxoglutarate dehydrogenase complex, in which glutamate is decarboxylated to GABA by glutamate decarboxylase (GAD, EC 4.1.1.15). The amino residue of GABA is removed by GABA aminotransferase (EC 2.6.1.19) leading to succinate semialdehyde, which is further oxidized to succinate by succinate-semialdehyde dehydrogenase (EC 1.2.1.16). The existence of a GABA shunt pathway has been often proposed in different rhizobial strains. Nevertheless, it has never been confirmed to operate during symbiosis.31 The recent findings have reported that the rhizobial bacteroid contain a non classical GABA shunt.32 The major modification represents the absence of the crucial enzyme glutamate decarboxylase in the rhizoba which is obligatory for the conversion of glutamate to GABA. This finding was supported by the low activity and oxygen dependency in nodules of soybean and snake bean.31,34 This provides a strong indication that the GABA present in bacteroids is likely to be of plant origin (Fig. 1).12,32 Accordingly, a dual role for GABA (i.e., signaling and metabolic) has been reported by many scientists in connection with nodule functioning. As a putative signaling substance, GABA was found circulating in varying concentrations in the phloem of several leguminous species such as alfalfa, lupin, soybean and cowpea (reviewed in ref. 12). This fact has been recently confirmed in our lab for the model legume M. truncatula.12 Higher phloem concentration for this amino acid has been observed under normal conditions with a strong tendency for significant increment (phloem and nodules) under partial nodule detachment (designed to upregulate the nodule activity). Partial nodule excision is a treatment which is known to affect the source-sink balance of the symbiotic plant resulting in a nitrogen deprivation condition. The apparent significant role of GABA in whole-plant-based upregulatory mechanisms was further supported in our studies by external GABA feeding into the phloem sap of the plant which proportionally followed by a short term increase in nodule activity.12
Figure 1.
Simplified representations of the proposed metabolic routes of γ-aminobutyric acid (GABA) metabolism and the key enzymes involved in symbiotic nodules. Enzymes involved include: GabD, succinate semialdehyde dehydrogenase; GabT, GABA oxoglutarate aminotransferase; GDC, glutamate decarboxylase; GOGAT, glutamate oxoglutarate amidotransferase; GS, glutamine synthetase; N2ase, nitrogenase; OpaA, omega amino acid pyruvate aminotransferase. Adapted from Prell et al.33
The strong correlation between GABA and nitrogen metabolism has been observed in non leguminous plants. In Brassica napus, shoot derived GABA was found translocating to the root fraction and that NO3-influx during N-shortage is positively correlated with phloem GABA concentration.28 In Arabidopsis thaliana, signaling and specific metabolic functions of GABA have been provided in connection with nitrate metabolism.35 Exogenous GABA application strongly affected the regulation of nitrate uptake and metabolism in Arabidopsis seedlings. Additionally, the activity of nitrate reductase, glutamate synthase, phosphoenolpyruvate carboxylase and other enzymes involved in nitrogen and carbon metabolism were found regulated by exogenous application of GABA.
How could the shoot-derived GABA increase the efficiency of symbiotic fixation? A proposed cycling mechanism of certain amino acids in nodules (between bacteroids and the cytoplasm) could strongly explain such scenario. For a long time it is widely accepted that no amino acid is able to cross the peribacteroid membrane (PBM) which separates the bacteroids and plant cytosol.36 More recently, this fact has widely weakened due to the artifacts concerning the isolation procedure and the subsequent measurements.2 The initial model proposed was provided by Kahn et al.37 and more recently additional evidences strongly supported the amino acid cycling between cytosol and the symbiosome/bacteroid in pea nodules.32,38 For the amino acid candidate, initially glutamate (or one of its metabolites) was proposed and the recent advances identified GABA as one of the strongest possible amino acids that could take part in cycling model.32,33 The above mention rapid detection of GABA in the bacteroid fraction (after 15N2 application) provides strong support for this thesis. Further evidence was provided by Hosie et al.39 who mentioned that branched-chain amino acid transporter (Bra) was be able to transport GABA across the PBM. Branch-chained amino acids were additionally indicated of particular importance for the proposed cycling model.40 As mentioned above, GABA is made from glutamate by the enzyme glutamate decarboxylase within the GABA-shunt. In the model diagrammed in Figure 1, the end product is succinate which delivered to the TCA of bacteroids to supply energy. Succinate was found an excellent substrate that supports the highest level of nitrogen fixation by isolated bacteroids.37 Higher relative levels of glutamate alongside with succinate were recognized in the nodules of M. truncatula in our lab in several experiments.5,12
Conclusions and Future Prospects
GABA is found virtually in higher concentrations in the nodules of various leguminous plants with high mobility nature particularly in the phloem sap. Recent studies have indicated that GABA has a metabolic function and serves as a signaling molecule within leguminous plants. The physiological role of GABA in nodule functioning is not yet clear, but this metabolite is readily accumulate in response to stress as well as conditions that were designed to upregulate the nitrogenase activity. It is concluded that this non-protein amino acid might be involved in upregulating nodule activity, possibly because of its constituting part of a putative amino acid cycle in nodules (between bacteroids and the cytoplasm). A better understanding of the role of GABA in the nodule physiology and biochemistry remains of major interest. Future studies should attempt to address these issues and to uncover further examples and the mechanisms by which GABA is employed to mediate the host plant communication with the microsymbiont.
Acknowledgements
This work was supported by the German Academic Exchange Service (DAAD), Germany.
References
- 1.Hirsch AM. Developmental biology of legume nodulation. New Phytol. 1992;122:211–237. doi: 10.1111/j.1469-8137.1992.tb04227.x. [DOI] [PubMed] [Google Scholar]
- 2.White J, Prell J, James EK, Poole P. Nutrient sharing between symbionts. Plant Physiol. 2007;144:604–614. doi: 10.1104/pp.107.097741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schulze J, Tesfaye M, Litjens RHMG, Bucciarelli B, Trepp G, Miller S, et al. Malate plays a central role in plant nutrition. Plant Soil. 2002;247:133–139. [Google Scholar]
- 4.Stitt M, Müller C, Matt P, Gibon Y, Carillo P, Morcuende R, et al. Steps towards an integrated view of nitrogen metabolism. J Exp Bot. 2002;53:959–970. doi: 10.1093/jexbot/53.370.959. [DOI] [PubMed] [Google Scholar]
- 5.Sulieman S, Schulze J. The efficiency of nitrogen fixation of the model legume Medicago truncatula (Jemalong A17) is low compared to Medicago sativa. J Plant Physiol. 2010;167:683–692. doi: 10.1016/j.jplph.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 6.Sulieman S, Fischinger SA, Gresshoff PM, Schulze J. Asparagine as a major factor in the N-feedback regulation of N2 fixation in Medicago truncatula. Physiol Plant. 2010;140:21–31. doi: 10.1111/j.1399-3054.2010.01380.x. [DOI] [PubMed] [Google Scholar]
- 7.Almeida JPF, Hartwig UA, Frehner M, Nösberger J, Lüscher A. Evidence that P deficiency induces N feedback regulation of symbiotic N2 fixation in white clover (Trifolium repens L.) J Exp Bot. 2000;51:1289–1297. [PubMed] [Google Scholar]
- 8.Fischinger SA, Drevon JJ, Claassen N, Schulze J. Nitrogen from senescing lower leaves of common bean is re-translocated to nodules and might be involved in a N-feedback regulation of nitrogen fixation. J Plant Physiol. 2006;163:987–995. doi: 10.1016/j.jplph.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Neo HH, Layzell DB. Phloem glutamine and the regulation of O2 diffusion in legume nodules. Plant Physiol. 1997;113:259–267. doi: 10.1104/pp.113.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vadez V, Sinclair TR, Serraj R. Asparagine and ureide accumulation in nodules and shoots as feedback inhibitors of N2 fixation in soybean. Physiol Plant. 2000;110:215–223. [Google Scholar]
- 11.Whitehead LF, Tyerman SD, Day DA. Polyamines as potential regulators of nutrient exchange across the peribacteroid membrane in soybean root nodules. Aust J Plant Physiol. 2001;28:675–681. [Google Scholar]
- 12.Sulieman S, Schulze J. Phloem derived γ-aminobutyric acid (GABA) is involved in upregulating nodule N2 fixation efficiency in the model legume Medicago truncatula. Plant Cell Environ. 2010;33:2162–2172. doi: 10.1111/j.1365-3040.2010.02214.x. [DOI] [PubMed] [Google Scholar]
- 13.Bown AW, Shelp BJ. The metabolism and functions of γ-aminobutyric acid. Plant Physiol. 1997;115:1–5. doi: 10.1104/pp.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouché N, Fromm H. GABA in plants: just a metabolite? Trends Plant Sci. 2004;9:110–115. doi: 10.1016/j.tplants.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Serraj R, Shelp BJ, Sinclair TR. Accumulation of γ-aminobutyric acid in nodulated soybean in response to drought stress. Physiol Plant. 1998;102:79–86. doi: 10.1034/j.1399-3054.1998.1020111.x. [DOI] [PubMed] [Google Scholar]
- 16.Shelp BJ, Allan WL, Faure D. Role of γ-aminobutyrate and γ-hydroxybutyrate in plant communication. In: BaluŠka F, editor. Plant-Environment Interactions, Signaling and Communication in Plants. Springer: 2009. pp. 73–84. [Google Scholar]
- 17.Bown AW, MacGregor KB, Shelp BJ. Gamma-aminobutyrate: defense against invertebrate pests? Trends Plant Sci. 2006;11:424–427. doi: 10.1016/j.tplants.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Crawford LA, Bown AW, Breitkreuz KE, Guinel F. The synthesis of γ-aminobutyric acid in response to treatments reducing cytosolic pH. Plant Physiol. 1994;104:865–871. doi: 10.1104/pp.104.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cholewa E, Cholewinski AJ, Shelp BJ, Snedden WA, Bown AW. Cold-shock-stimulated γ-aminobutyric acid synthesis is mediated by an increase in cytosolic Ca2+, not by an increase in cytosolic H+ Can J Bot. 1997;75:375–382. [Google Scholar]
- 20.Vance CP, Heichel GH. Carbon in N2 fixation—limitation or exquisite adaptation. Ann Rev Plant Physiol Plant Mol Biol. 1991;42:373–392. [Google Scholar]
- 21.Ott T, Sullivan J, James EK, Flemetakis E, Günther C, Gibon Y, et al. Absence of symbiotic leghemoglobins alters bacteroid and plant cell differentiation during development of Lotus japonicus root nodules. Mol Plant Microbe Interact. 2009;22:800–808. doi: 10.1094/MPMI-22-7-0800. [DOI] [PubMed] [Google Scholar]
- 22.Shi SQ, Shi Z, Jiang ZP, Qi LW, Sun XM, Li CX, et al. Effects of exogenous GABA on gene expression of Caragana intermedia roots under NaCl stress: regulatory roles for H2O2 and ethylene production. Plant Cell Environ. 2010;33:149–162. doi: 10.1111/j.1365-3040.2009.02065.x. [DOI] [PubMed] [Google Scholar]
- 23.Satya Narayan V, Nair PM. Metabolism enzymology and possible roles of 4-aminobutyrate in higher plants. Phytochemistry. 1990;29:367–375. [Google Scholar]
- 24.Ramputh AL, Bown AW. Rapid and γ-aminobutyric acid synthesis and the inhibition of the growth and development of oblique-banded leaf-roller larvae. Plant Physiol. 1996;111:1349–1352. doi: 10.1104/pp.111.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palanivelu R, Brass L, Edlund AF, Preuss D. Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell. 2003;114:47–59. doi: 10.1016/s0092-8674(03)00479-3. [DOI] [PubMed] [Google Scholar]
- 26.Ansari MI, Lee RH, Chen S-CG. A novel senescence-associated gene encoding γ-aminobutyric acid (GABA): pyruvate transaminase is upregulated during rice leaf senescence. Physiol Plant. 2005;123:1–8. [Google Scholar]
- 27.Swanson R, Edlund AF, Preuss D. Species specificity in pollen-pistil interactions. Annu Rev Genet. 2004;38:793–818. doi: 10.1146/annurev.genet.38.072902.092356. [DOI] [PubMed] [Google Scholar]
- 28.Beuve N, Rispail N, Laine P, Cliquet JB, Ourry A, Le Deunff E. Putative role of γ-aminobutyric acid (GABA) as a long-distance signal in upregulation of nitrate uptake in Brassica napus L. Plant Cell Environ. 2004;27:1035–1046. [Google Scholar]
- 29.Scharff AM, Egsgaard H, Hansen PE, Rosendahl L. Exploring symbiotic nitrogen fixation and assimilation in pea root nodules by in vivo 15N nuclear magnetic resonance spectroscopy and liquid chromatographymass spectrometry. Plant Physiol. 2003;131:367–378. doi: 10.1104/pp.015156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Streeter JG. Carbohydrate, organic acid and amino acid composition of bacteroids and cytosol from soybean nodules. Plant Physiol. 1987;85:768–773. doi: 10.1104/pp.85.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller RW, McRae DG, Joy K. Glutamate and γ-aminobutyrate metabolism in isolated Rhizobium meliloti bacteroids. Mol Plant Microbe Interact. 1991;4:37–45. [Google Scholar]
- 32.White JP, Prell J, Ramachandran VK, Poole PS. Characterization of a γ-aminobutyric acid transport system of Rhizobium leguminosarum bv. viciae 3841. J Bacteriol. 2009;191:1547–1555. doi: 10.1128/JB.00926-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prell J, Bourdès A, Karunakaran R, Lopez-Gomez M, Poole P. Pathway of γ-aminobutyrate metabolism in Rhizobium leguminosarum 3841 and its role in symbiosis. J Bacteriol. 2009;191:2177–2186. doi: 10.1128/JB.01714-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin HN, Dilworth MJ, Glenn AR. 4-aminobutyrate is not available to bacteroids of cowpea Rhizobium MNF2030 in snake bean nodules. Arch Microbiol. 1990;153:455–462. [Google Scholar]
- 35.Barbosa JM, Singh NK, Cherry JH, Locy RD. Nitrate uptake and utilization is modulated by exogenous γ-aminobutyric acid in Arabidopsis thaliana seedlings. Plant Physiol Biochem. 2010;48:443–450. doi: 10.1016/j.plaphy.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 36.Udvardi MK, Day DA. Metabolite transport across symbiotic membranes of legume nodules. Ann Rev Plant Physiol Plant Mol Biol. 1997;48:493–523. doi: 10.1146/annurev.arplant.48.1.493. [DOI] [PubMed] [Google Scholar]
- 37.Kahn ML, Kraus J, Somerville JE. A model of nutrient exchange in the Rhizobium-legume symbiosis. In: Evans HJ, Bottomley PJ, Newton WE, editors. Nitrogen Fixation Research Progress. Dordrecht, The Netherlands: Martinus Nijhoff; 1985. pp. 193–199. [Google Scholar]
- 38.Lodwig EM, Hosie AHF, Bordes A, Findlay K, Allaway D, Karunakaran R, et al. Amino-acid cycling drives nitrogen fixation in the legume-Rhizobium symbiosis. Nature. 2003;422:722–726. doi: 10.1038/nature01527. [DOI] [PubMed] [Google Scholar]
- 39.Hosie AHF, Allaway D, Galloway CS, Dunsby HA, Poole PS. Rhizobium leguminosarum has a second general amino acid permease with unusually broad substrate specificity and high similarity to branched-chain amino acid transporters (Bra/LIV) of the ABC family. J Bacteriol. 2002;184:4071–4080. doi: 10.1128/JB.184.15.4071-4080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prell J, White JP, Bourdes A, Bunnewell S, Bongaerts RJ, Poole PS. Legumes regulate Rhizobium bacteroid development and persistence by the supply of branched-chain amino acids. Proc Natl Acad Sci USA. 2009;106:12477–12482. doi: 10.1073/pnas.0903653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puiatti M, Sodek L. Waterlogging affects nitrogen transport in the xylem of soybean. Plant Physiol Biochem. 1999;37:767–773. [Google Scholar]
- 42.Xing SG, Jun YB, Hau ZW, Liang LY. Higher accumulation of γ-aminobutyric acid induced by salt stress through stimulating the activity of diamine oxidases in Glycine max (L.) Merr. root. Plant Physiol Biochem. 2007;45:560–566. doi: 10.1016/j.plaphy.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Wallace W, Secor J, Schrader LE. Rapid accumulation of γ-aminobutyric acid and alanine in soybean leaves in response to an abrupt transfer to lower temperature, darkness or mechanical manipulation. Plant Physiol. 1984;75:170–175. doi: 10.1104/pp.75.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lima JD, Sodek L. N-stress alters aspartate and asparagine levels of xylem sap in soybean. Plant Sci. 2003;165:649–656. [Google Scholar]
- 45.Antunes F, Aguilar M, Pineda M, Sodek L. Nitrogen stress and the expression of asparagine synthetase in roots and nodules of soybean (Glycine max) Physiol Plant. 2008;133:736–743. doi: 10.1111/j.1399-3054.2008.01092.x. [DOI] [PubMed] [Google Scholar]