Abstract
Strigolactones (SLs) have been recently identified as a new group of plant hormones or their derivatives thereof, shown to play a role in plant development. Evolutionary forces have driven the development of mechanisms in plants that allow adaptive adjustments to a variety of different habitats by employing plasticity in shoot and root growth and development. The ability of SLs to regulate both shoot and root development suggests a role in the plant's response to its growth environment. To play this role, SL pathways need to be responsive to plant growth conditions, and affect plant growth toward increased adaptive adjustment. Here, the effects of SLs on shoot and root development are presented, and possible feedback loops between SLs and two environmental cues, light and nutrient status, are discussed; these might suggest a role for SLs in plants' adaptive adjustment to growth conditions.
Key words: strigolactones, light, nutrient status, root, shoot, branching, lateral roots, root hairs
Strigolactones (SLs) are carotenoid-derived terpenoid lactones suggested to stem from the carotenoid pathway1 via the activity of various oxygenases.2,3 SLs production has been demonstrated in both monocotyledons and eudicotyledons (reviewed in ref. 4), suggesting their presence in many plant species.5 SLs are synthesized mainly in the roots and in some parts of the stem and then move towards the shoot apex (reviewed ref. 7).6,8,9
SLs were first characterized more than 40 years ago as germination stimulants of the parasitic plants Striga and Orobanche and later, as stimulants of arbuscular mycorrhiza hyphal branching as well (reviewed in ref. 4, 10–13). Recently, SLs or derivatives thereof, have been identified as a new group of plant hormones, shown to play a role in inhibition of shoot branching,2,3,8,9 thereby affecting shoot architecture; more recently they have also been shown to affect root growth by affecting auxin efflux.14
Plants have developed mechanisms that allow adaptive adjustments to a variety of different habitats by employing plasticity in their growth and development.15 Shoot architecture is affected by environmental cues, such as light quality and quantity and nutrient status.16–19 Root-system architecture and development are affected by environmental conditions such as nutrient availability (reviewed in ref. 20, 21). At the same time, plant hormones are known to be involved in the regulation of plant growth, development and architecture (reviewed in ref. 22–24) and to be mediators of the effects of environmental cues on plant development; one classic example is auxin's role in the plant's shade-avoidance response (reviewed in ref. 25).
The ability of SLs to regulate shoot and root development suggests that these phytohormones also have a role in the plant's growth response to its environment. To play this putative role, SL pathways need to be responsive to plant growth conditions, and affect plant growth toward enhancing its adaptive adjustment. The present review examines the SLs' possible role in adaptive adjustment of the plant's response to growth conditions, by discussing their effect on plant development and the possible associations and feedback loops between SLs and two environmental cues: light and nutrient status.
Strigolactones Affect Shoot Development
SLs or derivatives thereof are newly identified phytohormones that play a role in suppressing the growth of pre-formed axillary buds, acting as long-distance branching inhibitors.2,3 SL pathways have so far been identified in several plant species, including Arabidopsis thaliana, rice (Oryza sativa), petunia (Petunia hybrid), pea (Pisum sativum), tomato (Solanum lycopersicum) and chrysanthemum (Dendranthema grandiflorum)2,3,26–28 (reviewed in ref. 7 and 29). Based on mutant analysis, within the SL pathways, both SL synthesis and SL signaling components have been identified (reviewed in ref. 7 and 29).
Among the SLs synthesis components two CAROTENOID CLEAVAGE DIOXYGENASEs (CCD) enzymes, CCD7 and CCD8 were identified (reviewed in ref. 3, 7, 26–29), and were suggested to sequentially catalyze carotenoid cleavage reactions.30,31 Also, a cytochrome P450 monooxygenase, MAX1, was suggested to be involved in a later biosynthetic step,2,32 whereas additional steps in SL synthesis are anticipated yet unknown.1
As for the SLs signaling components, although the SLs receptor was not identified yet, MAX2, an F-box protein, was suggested to be a component of SL signaling and function in a ubiquitine-mediated degradation of yet unknown protein targets.3,33
However, other plant hormones also play a role in shoot branching (such as auxin and cytokinin, reviewed in ref. 22). Accordingly, cross-talks between SLs and other regulators of shoot branching were discovered (reviewed in ref. 7 and 29). Cytokinin biosynthesis was suggested to be involved in bud outgrowth following decapitation.34 However, the best described cross-talk is that between SLs and auxin. It was suggested that SLs serve as auxin-promoted secondary messengers that move up into the buds to repress their outgrowth (reviewed in ref. 7, 8 and 34), and that both auxin and SLs can change each other's levels and distribution in a dynamic feedback loop that is required for the coordinated control of axillary branching.35 Alternatively, it was suggested that SLs inhibit polar auxin transport from the buds by reducing the capacity for such transport from the apical meristem, resulting in restrained bud outgrowth (reviewed in ref. 29, 36–38). However, to support this last notion it still remains to be demonstrated that the timing of auxin transport out of a bud is causal to the bud outgrowth, rather than its result.7 This is especially important since SLs application to growing buds were shown to reduced their growth, with no effect on auxin transport from the bud.8
Strigolactones may Affect Root Development
The effect of SLs on root development is only now being revealed, and more research is needed for its full characterization. Based on analyses of mutants flawed in SL biosynthesis or signaling and the treatment of seedlings with GR24 (a bioactive, synthetic SL39), evidence has emerged for the involvement of SLs in root development. These results suggested that SLs repress lateral root initiation. In addition, GR24 treatment led to an increase in root-hair length, which might be mediated via the MAX2 F-box.40
Similar involvement in root-hair elongation was recorded for the compound D'orenone, mediated via an increase in auxin transport caused by an increment in PIN2 protein abundance and alterations in its localization;41 D'orenone is suggested to be structurally identical to the proposed precursor of SL synthesis.41 Accordingly, in tomato roots, SLs were suggested to interfere with the inhibitory effect of exogenously applied auxin on root elongation via an increase in root-cell length. Auxin-efflux carriers were involved in this SL effect on root growth and root-hair elongation.14 Together, these results point to possible cross-talk junctures between SLs and auxin for the control of root development, which operate through SLs' interference with an auxin efflux carrier.
Strigolactones and Environmental Cues
Light.
Light quality and intensity were shown to affect multiple processes in plants, including determination of shoot architecture16,18 and shade-avoidance response; the latter is comprised of enhanced elongation of hypocotyls petioles and stems, leaves movement to a vertical position and enhanced apical dominance.25,43
Several lines of evidence suggest a connection between SL pathways and light (reviewed in ref. 44). max2 mutant seedlings (pps) were found to be hyposensitive to red (R) and far red (FR) light. Moreover, several genes, including Rubisco small subunit and chlorophyll a/b-binding protein precursors, were found to exhibit a slower rate of induction upon R light exposure in max2 mutants relative to the WT.45 Since light quality, i.e., a low R:FR ratio, has been found to suppress shoot branching as part of the shade-avoidance response (reviewed in ref. 25, 29 and 46), it might be that SLs, which have been identified as shoot-branching inhibitors, are one of the mediators of that response. However, SL mutants in pea retained or even enhanced their sensitivity to day length in terms of their pattern of shoot branching, in comparison to the WT.47
SLs have also been suggested to be positive regulators of light-associated processes. Mashiguchi and co-workers found that light-signaling-related genes are induced in Arabidopsis seedlings shortly (90 min) following exposure to GR24.48 Analysis of roots and shoots of WT tomato plants and of the SLs-deficient tomato mutant Sl-ORT149,50 suggested that SLs are positive regulators of plant light harvesting. This was deduced from the list of genes induced by GR24, following 24 to 48 h of GR24 treatment, which was enriched in genes putatively associated with light harvesting. It was also deduced from the fact that light-induced genes in the SL-deficient mutant were transcriptionally downregulated in comparison to the WT, and from the fact that the level of chlorophyll was reduced in leaves of Sl-ORT1 relative to the WT.51 In the shoots, therefore, SLs may regulate shoot branching in response to light quality on the one hand and light harvesting on the one.
Interestingly, direct illumination on roots was suggested to markedly inhibit lateral root initiation in pea.52 Moreover, light was shown to positively regulate root-hair formation in Arabidopsis.53 SLs are suggested to be associated with both inhibition of lateral root initiation and root hair elongation.40 Therefore, it might be that SLs are mediators of root-light responses. However, more research is needed to confer or rebut this hypothesis, since in many cases similar developmental responses may be triggered by different signaling mechanisms.24
To conclude, further studies are clearly needed to determine the junction points of the co-regulation of SLs and light in light-regulated processes, in both shoots and roots. Moreover, the cross-talk between SLs and light-associated pathways might follow a feedback loop, because carotenoid biosynthesis has been shown to be light-dependent (reviewed in ref. 54) and SLs are thought to be derived from this pathway.1 This feedback loop may be required for the plant's coordinated growth and development under different light conditions.
Nutrient status.
Nutrient status has been shown to affect shoot branching. For example, boron (B) deficiency in pea reduced shoot apical dominance.19 Nitrogen (N) availability in peach trees affected shoot architecture: secondary axes responded to N limitation by decreasing their growth according to their position along the main axis.17 However, nutrient supply to pea did not prevent the outgrowth of buds, although it did affect branch length.9
It is possible that SLs are involved in regulation of shoot branching in response to nutrient status. This is since, (1) SLs inhibit shoot branching, (2) they are suggested to modulate auxin transport (auxin transport is involved in the shoot's response to B deficiency19) and (3) their biosynthesis is responsive to nutrient (Phosphate [Pi] and N) levels.55–58 Hence, it is possible that SLs provide a way for the plant to coordinate shoot development with nutritional conditions.
The effects of nutrition level on root development are well documented. Lateral root initiation, primary root elongation and root-hair formation are largely affected by the levels of several nutrients, including N, Pi, iron (Fe), aluminum (Al), calcium (Ca) and sulfur (S). For example, lateral root formation is induced under low Pi and S conditions; primary root elongation is inhibited under low Pi conditions (reviewed in ref. 20 and 59). Root-hair formation is induced by, for example, low Pi, low Fe and low N conditions (reviewed in refs. 59 and 60).
Root hairs development was suggested to be affected by SLs and their putative precursor, D'orenone,14,40,41 and low Pi and low N conditions have been shown to induce SLs production.55–58 Hence, it is tempting to speculate that SLs are mediators of the root response to low nutrient conditions, but this still remains to be demonstrated.
Other Phytohormones and Plant Responses to Environmental Cues
Numerous studies have suggested a connection between phytohormones and plant growth plasticity in response to environmental cues. For example, with respect to the light response, auxin has been shown in a large number of studies to be responsible for the plant's shade-avoidance response (reviewed in ref. 25). Cytokinin has been shown to co-regulate, along with light, many plant processes (reviewed in ref. 61), whereas both gibberellins and brassinosteroids have been shown to be involved in dark-light transition and photomorphogenesis (i.e., influence of light on plant morphogenesis), demonstrated for hypocotyl growth and elongation.62,63
With respect to nutrient response, auxin, cytokinin, ethylene, gibberellins and abscisic acid have been shown to affect root and shoot development in response to nutrient conditions; conversely, mineral nutrient conditions have been shown to influence hormone biosynthesis, transport and signaling (reviewed in ref. 20, 24 and 64). For example, under low Pi conditions, the changes in root-system architecture and root-hair formation in Arabidopsis were suggested to be a result of increased auxin sensitivity.65 Nitrate application was also shown to increase cytokinin biosynthesis and expression of its signaling components, leading to morphological effects on both roots and shoots (reviewed in ref. 24), whereas the reduction in shoot apical dominance in pea was suggested to be a result of reduced auxin transport out of the apex under conditions of B deficiency.19
Notably, many of the phytohormones are coordinated with other phytohormones in their effects on plant development, and have multiple cross-talk junctions between them, forming a complex network of coordinated effects (reviewed in ref. 66).
Conclusions and Perspectives
SLs may play a role in plant development plasticity in both roots and shoots in response to environmental cues, such as light and nutrient status (Fig. 1). On the one hand, SLs are suggested to increase the plant's light-harvesting ability; on the other, they inhibit shoot branching. It is tempting to speculate that the SL-induced increase in light-harvesting capability compensates for the reduction in total leaf area resulting from reduced shoot branching.
Figure 1.
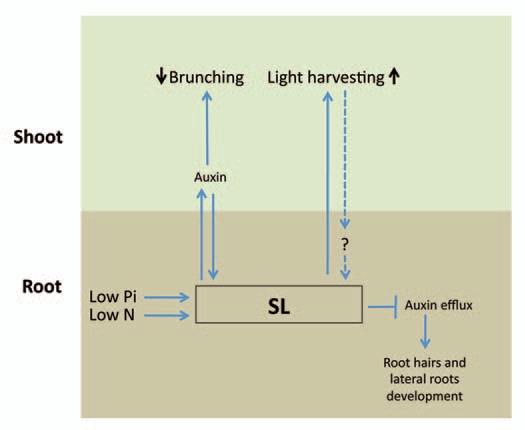
Strigolactones (SLs) may have a role in plant developmental plasticity in both roots and shoots, in response to environmental cues such as light and nutrient status. SLs have been shown to inhibit shoot branching, mediated by either auxin transport or auxin signaling, whereas both auxin and SLs have the ability to change each other's levels and distribution. SLs have been shown to increase plant light-harvesting capability, but the effects of light harvesting on SL production or sensitivity are not clear (dashed arrow). Low levels of nutrients (e.g., Pi, N) might increase SL production in roots, whereas SLs or their putative precursor may lead to alteration of root development, which may be mediated via auxin transport.
Low levels of nutrients (Pi and N) increase root SLs production, and there are evidences suggesting that levels of SLs and its putative precursor lead to alterations in root-hair length; the latter is thought to be directly associated with enhancement of the plant's ability to absorb nutrients (reviewed in ref. 67 and 68). It might be that SLs affect root-hair elongation, resulting in modulation of nutrient uptake by the plant.
To conclude, although more research is needed on the subject, it might be that SLs are players in a carefully controlled network that coordinates shoot and root development in response to environmental conditions. SLs' effects are probably coordinated with those of other phytohormones, conferring plasticity on plant growth and development for adaptive responses to growth conditions.
References
- 1.Matusova R, Rani K, Verstappen FW, Franssen MC, Beale MH, Bouwmeester HJ. The strigolactone germination stimulants of the plant-parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiol. 2005;139:920–934. doi: 10.1104/pp.105.061382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- 3.Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- 4.Xie X, Yoneyama K, Yoneyama K. The strigolactone story. Annu Rev Phytopathol. 2010;48:93–117. doi: 10.1146/annurev-phyto-073009-114453. [DOI] [PubMed] [Google Scholar]
- 5.Klee H. Plant biology: Hormones branch out. Nature. 2008;455:176–177. doi: 10.1038/455176a. [DOI] [PubMed] [Google Scholar]
- 6.Foo E, Turnbull CG, Beveridge CA. Long-distance signaling and the control of branching in the rms1 mutant of pea. Plant Physiol. 2001;126:203–209. doi: 10.1104/pp.126.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dun EA, Brewer PB, Beveridge CA. Strigolactones: discovery of the elusive shoot branching hormone. Trends Plant Sci. 2009;14:364–372. doi: 10.1016/j.tplants.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 2009;150:482–493. doi: 10.1104/pp.108.134783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferguson BJ, Beveridge CA. Roles for auxin, cytokinin and strigolactone in regulating shoot branching. Plant Physiol. 2009;149:1929–1944. doi: 10.1104/pp.109.135475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akiyama K, Matsuzaki K, Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- 11.Cook CE, Whichard LP, Wall ME, Egley GH, Coggon P, Luhan PA, et al. Germination stimulants. 2. The structure of strigol—a potent seed germination stimulant for witchweed (Striga lutea Lour.) J Am Chem Soc. 1972;94:6198–6199. [Google Scholar]
- 12.Yoneyama K, Xie X, Sekimoto H, Takeuchi Y, Ogasawara S, Akiyama K, et al. Strigolactones, host recognition signals for root parasitic plants and arbuscular mycorrhizal fungi, from Fabaceae plants. New Phytol. 2008;179:484–494. doi: 10.1111/j.1469-8137.2008.02462.x. [DOI] [PubMed] [Google Scholar]
- 13.García-Garrido JM, Lendzemo V, Castellanos-Morales V, Steinkellner S, Vierheilig H. Strigolactones, signals for parasitic plants and arbuscular mycorrhizal fungi. Mycorrhiza. 2009;19:449–459. doi: 10.1007/s00572-009-0265-y. [DOI] [PubMed] [Google Scholar]
- 14.Koltai H, Dor E, Hershenhorn J, Joel DM, Wininger S, Lekalla S, et al. Strigolactones' effect on root growth and root-hair elongation may be mediated by auxin-efflux carriers. J Plant Growth Regul. 2010;29:129–136. [Google Scholar]
- 15.Sultan SE. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci. 2000;5:537–542. doi: 10.1016/s1360-1385(00)01797-0. [DOI] [PubMed] [Google Scholar]
- 16.Horvath DP, Anderson JV, Chao WS, Foley ME. Knowing when to grow: signals regulating bud dormancy. Trends Plant Sci. 2003;8:534–540. doi: 10.1016/j.tplants.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Mediene S, Pages L, Jordan MO, Le Bot J, Adamowicz S. Influence of nitrogen availability on shoot development in young peach trees [Prunus persica (L.) Batsch] Trees Struct Funct. 2002;16:547–554. [Google Scholar]
- 18.Nishimura E, Suzaki E, Irie M, Nagashima H, Hirose T. Architecture and growth of an annual plant Chenopodium album in different light climates. Ecol Res. 2010;25:383–393. [Google Scholar]
- 19.Wang G, Romheld V, Li C, Bangerth F. Involvement of auxin and CKs in boron deficiency induced changes in apical dominance of pea plants (Pisum sativum L.) J Plant Physiol. 2006;163:591–600. doi: 10.1016/j.jplph.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Osmont KS, Sibout R, Hardtke CS. Hidden branches: developments in root system architecture. Annu Rev Plant Biol. 2007;58:93–113. doi: 10.1146/annurev.arplant.58.032806.104006. [DOI] [PubMed] [Google Scholar]
- 21.Müller M, Schmidt W. Environmentally induced plasticity of root hair development in Arabidopsis. Plant Physiol. 2004;134:409–419. doi: 10.1104/pp.103.029066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srivastava LM. Plant Growth and Development: Hormones and Environment. San Diego: Elsevier Science; 2002. [Google Scholar]
- 23.Gray WM. Hormonal regulation of plant growth and development. PLoS Biol. 2004;2:311. doi: 10.1371/journal.pbio.0020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubio V, Bustos R, Irigoyen ML, Cardona-López X, Rojas-Triana M, Paz-Ares J. Plant hormones and nutrient signaling. Plant Mol Biol. 2009;69:361–373. doi: 10.1007/s11103-008-9380-y. [DOI] [PubMed] [Google Scholar]
- 25.Franklin KA. Shade avoidance. New Phytol. 2008;179:930–944. doi: 10.1111/j.1469-8137.2008.02507.x. [DOI] [PubMed] [Google Scholar]
- 26.Liang J, Zhao L, Challis R, Leyser O. Strigolactone regulation of shoot branching in chrysanthemum (Dendranthema grandiflorum) J Exp Bot. 2010;61:3069–3078. doi: 10.1093/jxb/erq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vogel JT, Walter MH, Giavalisco P, Lytovchenko A, Kohlen W, Charnikhova T, et al. SlCCD7 controls strigolactone biosynthesis, shoot branching and mycorrhiza-induced apocarotenoid formation in tomato. Plant J. 2010;61:300–311. doi: 10.1111/j.1365-313X.2009.04056.x. [DOI] [PubMed] [Google Scholar]
- 28.Drummond RS, Martínez-Sánchez NM, Janssen BJ, Templeton KR, Simons JL, Quinn BD, et al. Petunia hybrida CAROTENOID CLEAVAGE DIOXYGENASE7 is involved in the production of negative and positive branching signals in petunia. Plant Physiol. 2009;151:1867–1877. doi: 10.1104/pp.109.146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leyser O. The control of shoot branching: an example of plant information processing. Plant Cell Environ. 2009;32:694–703. doi: 10.1111/j.1365-3040.2009.01930.x. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz SH, Qin X, Loewen MC. The biochemical characterization of two carotenoid cleavage enzymes from Arabidopsis indicates that a carotenoid-derived compound inhibits lateral branching. J Biol Chem. 2004;279:46940–46945. doi: 10.1074/jbc.M409004200. [DOI] [PubMed] [Google Scholar]
- 31.Auldridge ME, Block A, Vogel JT, Dabney-Smith C, Mila I, Bouzayen M, et al. Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J. 2006;45:982–993. doi: 10.1111/j.1365-313X.2006.02666.x. [DOI] [PubMed] [Google Scholar]
- 32.Booker J, Sieberer T, Wright W, Williamson L, Willett B, Stirnberg P, et al. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev Cell. 2005;8:443–449. doi: 10.1016/j.devcel.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Stirnberg P, Furner IJ, Leyser O. MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J. 2007;50:80–94. doi: 10.1111/j.1365-313X.2007.03032.x. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson BJ, Beveridge CA. Roles for auxin, cytokinin and strigolactone in regulating shoot branching. Plant Physiol. 2009;149:1929–1944. doi: 10.1104/pp.109.135475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayward A, Stirnberg P, Beveridge C, Leyser O. Interactions between auxin and strigolactone in shoot branching control. Plant Physiol. 2009;151:400–412. doi: 10.1104/pp.109.137646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bennett T, Sieberer T, Willett B, Booker J, Luschnig C, Leyser O. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr Biol. 2006;16:553–563. doi: 10.1016/j.cub.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 37.Mouchel CF, Leyser O. Novel phytohormones involved in long-range signaling. Curr Opin Plant Biol. 2007;10:473–476. doi: 10.1016/j.pbi.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Ongaro V, Leyser O. Hormonal control of shoot branching. J Exp Bot. 2008;59:67–74. doi: 10.1093/jxb/erm134. [DOI] [PubMed] [Google Scholar]
- 39.Johnson AW, Gowda G, Hassanali A, Knox J, Monaco S, Razavi Z, et al. The preparation of synthetic analogues of strigol. J Chem Soc Perkin Trans. 1981;1:1734–1743. [Google Scholar]
- 40.Kapulnik Y, Delaux P-M, Resnick N, Mayzlish-Gati E, Wininger S, Bhattacharya C, Séjalon-Delmas N, Combier J-P, Bécard G, Belausov E, Beeckman T, Dor E, Hershenhorn J, Koltai H. Strigolactones affect lateral root formation and root hair elongation in Arabidopsis. Planta. 2010;233:209–216. doi: 10.1007/s00425-010-1310-y. [DOI] [PubMed] [Google Scholar]
- 41.Schlicht M, Samajová O, Schachtschabel D, Mancuso S, Menzel D, Boland W, et al. D'orenone blocks polarized tip growth of root hairs by interfering with the PIN2-mediated auxin transport network in the root apex. Plant J. 2008;55:709–717. doi: 10.1111/j.1365-313X.2008.03543.x. [DOI] [PubMed] [Google Scholar]
- 42.Floss DS, Walter MH. Role of carotenoid cleavage dioxygenase 1 (CCD1) in apocarotenoid biogenesis revisited. Plant Signal Behav. 2009;4:172–175. doi: 10.4161/psb.4.3.7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierik R, Keuskamp DH, Sasidharan R, Djakovic-Petrovic T, de Wit M, Voesenek LA. Light quality controls shoot elongation through regulation of multiple hormones. Plant Signal Behav. 2009;4:755–756. doi: 10.4161/psb.4.8.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Waldie T, Hayward A, Beveridge CA. Axillary bud outgrowth in herbaceous shoots: how do strigolactones fit into the picture? Plant Mol Biol. 2010;73:27–36. doi: 10.1007/s11103-010-9599-2. [DOI] [PubMed] [Google Scholar]
- 45.Shen H, Luong P, Huq E. The F-box protein MAX2 functions as a positive regulator of photomorphogenesis in Arabidopsis. Plant Physiol. 2007;145:1471–1483. doi: 10.1104/pp.107.107227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franklin KA, Whitelam GC. Phytochromes and shade-avoidance responses in plants. Ann Bot. 2005;96:169–175. doi: 10.1093/aob/mci165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beveridge CA, Weller JL, Singer SR, Hofer JMI. Axillary meristem development. Budding relationships between networks controlling flowering, branching and photoperiod responsiveness. Plant Physiol. 2003;131:927–934. doi: 10.1104/pp.102.017525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mashiguchi K, Sasaki E, Shimada Y, Nagae M, Ueno K, Nakano T, et al. Feedback-regulation of strigolactone biosynthetic genes and strigolactone-regulated genes in Arabidopsis. Biosci Biotechnol Biochem. 2009;73:2460–2465. doi: 10.1271/bbb.90443. [DOI] [PubMed] [Google Scholar]
- 49.Dor E, Alperin B, Wininger S, Ben-Dor B, Somvanshi VS, Koltai H, et al. Characterization of a novel tomato mutant resistant to Orobanche and Phelipanche spp. weedy parasites. Euphytica. 2010;171:371–380. [Google Scholar]
- 50.Koltai H, LekKala SP, Bahattacharya C, Mayzlish-Gati E, Resnick N, Wininger S, et al. A tomato strigolactone-impaired mutant displays aberrant shoot morphology and plant interactions. J Exp Bot. 2010;61:1739–1749. doi: 10.1093/jxb/erq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayzlish-Gati E, LekKala SP, Resnick N, Wininger S, Bhattacharya C, Lemcoff JH, et al. Strigolactones are positive regulators of light-harvesting genes in tomato. J Exp Bot. 2010;61:3129–3136. doi: 10.1093/jxb/erq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torrey JG. Effects of light on elongation and branching in pea roots. Plant Physiol. 1952;27:591–602. doi: 10.1104/pp.27.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Simone S, Oka Y, Inoue Y. Effect of light on root hair formation in Arabidopsis thaliana phytochrome-deficient mutants. J Plant Res. 2000;113:63–69. [Google Scholar]
- 54.Cazzonelli CI, Yin K, Pogson BJ. Potential implications for epigenetic regulation of carotenoid biosynthesis during root and shoot development. Plant Signal Behav. 2009;4:339–341. doi: 10.4161/psb.4.4.8193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoneyama K, Takeuchi Y, Sekimoto H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta. 2007;225:1031–1038. doi: 10.1007/s00425-006-0410-1. [DOI] [PubMed] [Google Scholar]
- 56.Yoneyama K, Xie X, Kusumoto D, Sekimoto H, Sugimoto Y, Takeuchi Y, et al. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta. 2007;227:125–132. doi: 10.1007/s00425-007-0600-5. [DOI] [PubMed] [Google Scholar]
- 57.López-Ráez JA, Bouwmeester H. Fine-tuning regulation of strigolactone biosynthesis under phosphate starvation. Plant Signal Behav. 2008;3:963–965. doi: 10.4161/psb.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.López-Ráez JA, Charnikhova T, Gómez-Roldán V, Matusova R, Kohlen W, De Vos R, et al. Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytol. 2008;178:863–874. doi: 10.1111/j.1469-8137.2008.02406.x. [DOI] [PubMed] [Google Scholar]
- 59.López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Curr Opin Plant Biol. 2003;6:280–287. doi: 10.1016/s1369-5266(03)00035-9. [DOI] [PubMed] [Google Scholar]
- 60.Robinson D, Rorison IH. Relationships between root morphology and nitrogen availability in a recent theoretical model describing nitrogen uptake from soil. Plant Cell Environ. 1983;6:641–647. [Google Scholar]
- 61.Werner T, Schmülling T. Cytokinin action in plant development. Curr Opin Plant Biol. 2009;12:527–538. doi: 10.1016/j.pbi.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Achard P, Liao L, Jiang C, Desnos T, Bartlett J, Fu X, et al. DELLAs contribute to plant photomorphogenesis. Plant Physiol. 2007;143:1163–1172. doi: 10.1104/pp.106.092254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song L, Zhou XY, Li L, Xue LJ, Yang X, Xue HW. Genome-wide analysis revealed the complex regulatory network of brassinosteroid effects in photomorphogenesis. Mol Plant. 2009;2:755–772. doi: 10.1093/mp/ssp039. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt W, Linke B. Nutrients as regulators of root morphology and architecture. In: Pinton R, Varanini Z, Nannipieri P, editors. The Rhizosphere: Biochemistry and Organic Substances at the Soil-Plant Interface. New York: Marcel Dekker Inc; 2007. pp. 135–150. [Google Scholar]
- 65.Pérez-Torres CA, López-Bucio J, Cruz-Ramírez A, Ibarra-Laclette E, Dharmasiri S, Estelle M, et al. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell. 2008;20:3258–3272. doi: 10.1105/tpc.108.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuppusamy KT, Walcher CL, Nemhauser JL. Cross-regulatory mechanisms in hormone signaling. Plant Mol Biol. 2009;69:375–381. doi: 10.1007/s11103-008-9389-2. [DOI] [PubMed] [Google Scholar]
- 67.Clarkson DT. Factors affecting mineral nutrient acquisition by plants. Annu Rev Plant Physiol. 1985;36:77–115. [Google Scholar]
- 68.Gilroy S, Jones DL. Through form to function: root hair development and nutrient uptake. Trends Plant Sci. 2000;5:56–60. doi: 10.1016/s1360-1385(99)01551-4. [DOI] [PubMed] [Google Scholar]


