Abstract
The development of Casparian strips (CSs) on the endo- and exodermis and their chemical components in roots of three cultivars of rice (Oryza sativa) with different salt tolerance were compared using histochemistry and Fourier transform infrared (FTIR) spectroscopy. The development and deposition of suberin lamellae of CSs on the endo- and exodermis in the salt-tolerant cultivar Liaohan 109 was earlier than in the moderately tolerant cultivar Tianfeng 202 and the sensitive cultivar Nipponbare. The detection of chemical components indicated major contributions to the structure of the outer part from aliphatic suberin, lignin and cell wall proteins and carbohydrates to the rhizodermis, exodermis, sclerenchyma and one layer of cortical cells in series (OPR) and the endodermal Casparian strip. Moreover, the amounts of these major chemical components in the outer part of the Liaohan 109 root were higher than in Tianfeng 202 and Nipponbare, but there was no distinct difference in endodermal CSs among the three rice cultivars. The results suggest that the exodermis of the salt-tolerant cultivar Liaohan 109 functions as a barrier for resisting salt stress.
Key words: casparian strip, chemical components, development, rice, root
Plant roots are in direct contact with the soil environment and thus particularly affected by unfavorable conditions. To withstand the surrounding environment, roots have developed anatomical and physiological adaptations. The development of Casparian strips (CSs) in the root endo- and exodermis is one such strategy.1–3 In roots of most species, the sequence of development of the endo- and exodermis is roughly the same and involves two consecutive developmental stages: (1) formation of CSs in radial and transverse walls impregnating the primary cell wall pores with lipophilic and aromatic substances and (2) deposition of suberin lamellae to the inner surface of anticlinal and tangential cell walls.4–6
A major function of the CS is to block the non-selective apoplastic bypass flow of water and ions into the stele.3 Therefore, the structure,7–9 chemical nature,10–12 and physiological function13,14 of endo- and exdodermal CSs in roots have been the focus of many investigations. Although oxygen loss, drought and salinity can influence the development and chemical nature of CSs in different rice cultivars,15–19 few investigations have considered the development and formation of endo- and exdodermal CSs in the roots of rice cultivars with different salt tolerance under normal growing conditions.
In the present paper, light microscopy and Fourier transform infrared (FTIR) spectroscopy were used to examine the cytochemistry and root anatomy of isolated CSs. The aim was to compare anatomical development and chemical characteristics of the endoand exdodermal CSs of three rice (Oryza sativa L.) cultivars having different salt tolerance in north China: the salt-tolerant Liaohan 109 and two widely grown cultivars, Tianfeng 202 and Nipponbare.
Results
Comparison of salt tolerance of Liaohan 109, Tianfeng 202 and Nipponbare.
To compare the salt tolerance among Liaohan 109, Tianfeng 202 and Nipponbare, 2 week-old rice seedlings were given 200 mM NaCl solution for 2 weeks. Most of the plants of Liaohan 109 and Tianfeng 202 remained green, but Nipponbare plants became yellow. Both the salt-tolerant cultivar Liaohan 109 and the moderately tolerant cultivar Tianfeng 202 had stronger salt tolerance than the salt-sensitive Nipponbare (data not shown).
Development of Casparian strips and suberin lamellae on the endo- and exodermis.
Casparian strips in the endo- and exodermis were stained with berberine-aniline blue and shown by green fluorescence under UV illumination. The differences among three cultivars were striking (Table 1, Figs. 1A–I and 2A–F).
Table 1.
Comparison of CSs development among the three rice cultivars
| Distance to root tip | Nipponbare | Tianfeng 202 | Liaohan 109 | |
| Endodermis | 10 mm | A few endodermal cells had dotlike CSs in radial walls (Fig. 1A) | Some cells had dot-like CSs in radial walls (Fig. 1B) | Dot-like CSs were continuous in endodermis (Fig. 1C) |
| 20 mm | Dot-like CSs continuous in endodermis (Fig. 1D) | CSs filled most of the length of the radial walls in some endodermal cells (Fig. 1E) | CSs filled most of the length of the radial walls in all the endodermal cells (Fig. 1F) | |
| 30 mm | CSs filled most of the length of the radial walls in most endodermal cells (Fig. 1G) | CSs more prominent (Fig. 1H) | CSs more prominent (Fig. 1I) | |
| >40 mm | Well-developed CSs | Well-developed CSs | Well-developed CSs | |
| Exodermis | 40 mm | Few exodermal cells had CSs in radial walls (Fig. 2A) | Some exodermal cells had CSs in radial walls (Fig. 2B) | All exodermal cells had CSs in radial walls (Fig. 2C) |
| 50 mm | Complete exodermal CSs in all cells (Fig. 2D) | Complete exodermal CSs in all cells (Fig. 2E) | Complete exodermal CSs in all cells (Fig. 2F) |
Figure 1.

Development of Casparian strips and suberin lamellae on the root endodermis of three rice cultivars. (A–I) Cross-sections were made at 10 mm (A–C), 20 mm (D–F) and 30 mm (G, H and I) from the root tip in Nipponbare, Tianfeng 202 and Liaohan 109. Casparian strips showed green fluorescence under UV illumination after staining with berberine-aniline blue. Arrowheads: Casparian strips on endodermis. Nipponbare 10 mm (A), 20 mm (D), 30 mm (G); Tianfeng 202 10 mm (B), 20 mm (E), 30 mm (H); Liaohan 109 10 mm (C), 20 mm (F), 30 mm (I). (J–O) Cross-sections were made at 20 mm (J–L) and 50 mm (M, N and O) from the root tip in Nipponbare, Tianfeng 202 and Liaohan 109; suberin lamellae showed yellow-green fluorescence under UV illumination after staining with FY088. Arrowheads: deposition of suberin lamella. Nipponbare 20 mm (J), 50 mm (M); Tianfeng 202 20 mm (K), 50 mm (N); Liaohan 109 20 mm (L) and 50 mm (O). Bar = 40 µm.
Figure 2.
Development of Casparian strips and suberin lamellae on the exodermis of roots of the three rice cultivars. (A–F) Cross-sections at 40 mm (A–C) and 50 mm (D–F) from the root tip in Nipponbare, Tianfeng 202 and Liaohan 109. Casparian strips showed green fluorescence under UV illumination after staining with berberine-aniline blue. Arrowheads: Casparian strips in the exodermis. Nipponbare 40 mm (A), 50 mm (D); Tianfeng 202 40 mm (B), 50 mm (E); Liaohan 109 40 mm (C), 50 mm (F). (G–L) Cross-sections at 40 mm (G–I) and 50 mm (J–L) from the root tip in Nipponbare, Tianfeng 202 and Liaohan 109; the suberin lamellae showed yellow-green fluorescence under UV illumination after stained with FY088. Arrowheads: deposition of suberin lamellae. Nipponbare 40 mm (G), 50 mm (J); Tianfeng 202 40 mm (H), 50 mm (K); Liaohan 109 40 mm (I), 50 mm (L). Bar = 30 µm.
The lipophilic fluorochrome Fluorol yellow 088 was used to stain suberin lamellae, giving intense yellow-green fluorescence under UV illumination. A comparison of root cross-sections of the three cultivars showed that there were differences in the deposition of suberin lamellae among cultivars according to the distance from the root tip (Table 2, Figs. 1J–O and 2G–L).
Table 2.
Comparison of suberin lamellae (SL) development among the three rice cultivars
| Distance to root tip | Nipponbare | Tianfeng 202 | Liaohan 109 | |
| Endodermis | 20 mm | Deposition of SL in a few endodermal cells (Fig. 1J) | Deposition of SL in some endodermal cells (Fig. 1K) | Deposition of SL in most endodermal cells (Fig. 1L) |
| 50 mm | SL along the length of radial walls in a few endodermal cells (Fig. 1M) | SL along the length of radial walls in some endodermal cells (Fig. 1N) | SL filled most of the length of radial walls in most endodermal cells (Fig. 1O) | |
| Exodermis | 40 mm | Deposition of SL in a few exodermal cells (Fig. 2G) | Deposition of SL in some exodermal cells (Fig. 2H) | Deposition of SL in most exodermal cells (Fig. 2I) |
| 50 mm | SL filled most of the length of radial walls in a few exodermal cells (Fig. 2J) | SL filled most of the length of radial walls in some exodermal cells (Fig. 2K) | SL filled most of the length of radial walls in most exodermal cells (Fig. 2L) |
Fourier transform infrared spectra of isolated Casparian strips and the outer root parts.
The outer root parts and endodermal CSs were isolated from the three rice cultivars by enzymatic digestion and then freeze-dried and scanned with an FTIR spectrometer. Figure 3A and B show differences in FTIR spectra of OPR and endodermal CSs from roots of the three cultivars. According to Xie et al.3 and assignment to the main IR absorption peaks, absorption of asymmetric and symmetric stretching vibration of methylene groups (CH2) appeared at 2,924, 2,855; 2,922, 2,854 and 2,922, 2,854 cm−1 in the spectra of OPR, and 2,924, 2,854; 2,923, 2,854 and 2,924, 2,855 cm−1 in spectra of CSs in Liaohan 109, Tianfeng 202 and Nipponbare, respectively, which contain suberin.24
Figure 3.
Comparison of FTIR spectra and second-derivative IR spectra of OPR and endodermal CSs from Liaohan 109, Tianfeng 202 and Nipponbare. (A) Comparison of FTIR spectra of OPR. (B) Comparison of FTIR spectra of endodermal CSs. (C) Comparison of second-derivative IR spectra of OPR. (D) Comparison of second-derivative IR spectra of endodermal CSs.
As shown in Figure 3A and B, many absorption peaks significantly overlapped from 1,800 cm−1 to 1,000 cm−1. The second-derivative IR spectra present slope-change characteristics of overlapped peaks, from which more information on the component compounds can be obtained. For the FTIR spectra from 1,800 cm−1 to 1,000 cm−1 (Fig. 3C and D), and based on reports23 and assignment to the main IR absorption bands (Table 3), absorption bands at 1,734–1,745 cm−1 could be attributed to C = O stretching vibrations of suberin. Absorption at 1,639–1,650 cm−1 was attributed to amide I, produced by C = O stretching vibrations and N-H bending vibration in protein; that at 1,541–1,551 cm−1 was attributed to amide II caused by N-H bending vibration; that at 1,508–1,520 cm−1 was attributed to bending vibration of aromatic or heterocyclic skeletons; and bands 1,371–1,389 and 1,462–1,469 cm−1 were assigned to -CH2- cut angle-shift rocking. The 1,246–1,271 cm−1 band contained character peaks of amide III including C-N stretching vibrations and N-H bending vibrations; C-O-C and P-O stretching vibration peaks in aromatic compounds or lipids could also play a role in this band. The absorption band at 1,163–1,167 cm−1 was attributed to the C-O-stretching vibration peak of lipids or cellulose, while that at 1,029–1,036 cm−1 was attributed to C-OH bending vibrations in carbohydrates. In addition, it has been reported that IR absorption corresponding to the vibrations associated with unsaturated bonds or aromatic compounds may be related to the occurrence of lignin and ester-linked aromatics in suberin.24 From the above analyses, the IR data indicate major contributions of aliphatic suberin, lignin, cell wall carbohydrates and cell wall proteins to the structure of the outer part and endodermal CSs in all three rice cultivars.
Table 3.
Assignments of FTIR absorption bands of the three rice cultivars from 1,800 to 1,000 cm−1
| Sample | v(C = O) | δ(N-H) | |||||
| amide band I | amide band II | vrf (ar) | δ(C-H) | δ(C-O-C) | v(C-OH) | ||
| Liaohan 109 OPR | 1744 | 1641 | 1549 | 1512 | 1373, 1462 | 1246, 1165 | 1029 |
| Nipponbare OPR | 1745 | 1641 | 1551 | 1510 | 1389, 1464 | 1246, 1167 | 1032 |
| Tianfeng 202 OPR | 1744 | 1641 | 1541 | 1512 | 1373, 1464 | 1248, 1163 | 1029 |
| Liaohan 109 SCs | 1744 | 1639 | 1551 | 1512 | 1373, 1462 | 1247, 1167 | 1035 |
| Nipponbare SCs | 1734 | 1650 | 1541 | 1520 | 1385, 1469 | 1246, 1165 | 1036 |
| Tianfeng 202 SCs | 1745 | 1641 | 1551 | 1508 | 1371, 1462 | 1271, 1167 | 1034 |
Note: v, stretching or skeletal vibration; δ, bending vibration; ar, aromatic compound; rf, ring skeleton.
Subtraction spectral analyses were made with ORIGIN 6.0. The relative intensities of OPR from Liaohan 109 to those from both Tianfeng 202 and Nipponbare were positive (Fig. 4A), with maxima of 0.109 and 0.094, respectively, indicating that the content of all components (including suberin) in OPR from Liaohan 109 was higher than in Tianfeng 202 and Nipponbare. Moreover, the relative intensities of endodermal CSs from Liaohan 109 to those of Nipponbare were positive (Fig. 4B), but were below 0.012, indicating that the content of all components (including suberin) in endodermal CSs from Liaohan 109 was slightly higher than in Nipponbare. While most of the relative intensities of endodermal CSs from Liaohan 109 to those of Tianfeng 202 were positive, they only ranged from −0.02–0.02, which indicates that the content of all components (including suberin) in endodermal CSs from Liaohan 109 was little different from that of Tianfeng 202.
Figure 4.
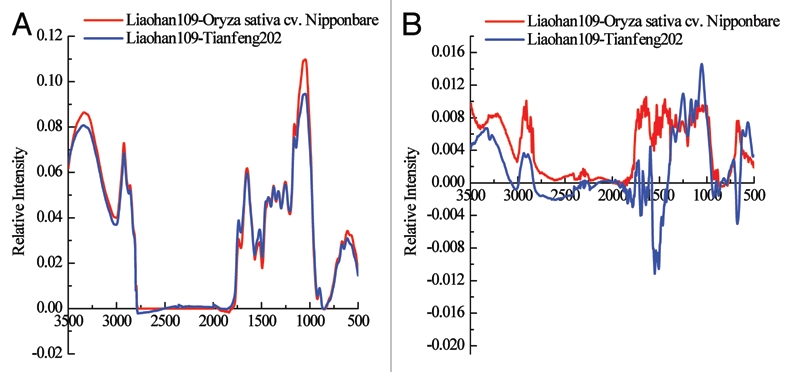
Subtraction spectral comparisons between the root outer part and endodermal Casparian strips of three rice cultivars. (A) Subtraction spectral comparison of the root outer part; (B) subtraction spectral comparison of root endodermal Casparian strips.
Discussion
In arid or semi-arid land, water is the key factor limiting plant growth. The root is the major organ for plants to take up water. Water stress will cause a decrease in the growth rate of the root system and an apparent decrease of root length, root number and weight, which would reduce the effective water absorption area of the root system, the speed of water absorption, and the total water absorption capacity, simultaneously inhibiting nutrient and salt absorption. In angiosperms, Casparian strips are bands of tissue impregnated with lignin and suberin on the radial and transverse walls of endo- and exodermal cells. The main function of CSs is to inhibit water and salt transport into the stele by blocking non-selective apoplastic bypass in the root.3
In general, an exodermis is present in most angiosperm roots,1,25 whereas an endodermis is present in all roots so far examined, except for some members of the Lycopodiaceae.26,27 In roots of most species, the endodermis starts to mature before the exodermis, but the sequence of development is roughly the same in cells of both layers. In the endodermis, there is typically a gap between development of CSs and suberin lamellae, whereas in the uniform exodermis, CSs and suberin lamellae are normally deposited simultaneously.28 According to Enstone et al. (2003) and Karahara et al. (2004), the formation of CSs can significantly increase salt stress or decrease nutrient deficiency tolerance in response to environmental stress, which can be assessed by the distance of the first CSs formed close to the root tip.2,17 Using histochemistry and microscopy to compare the development of CSs on the endo- and exodermis in the three rice cultivars, it was found that 10 mm from the root tip, Liaohan 109 had continuous dot-like CSs in the radial walls of the endodermis, while some endodermal cells had dot-like CSs in the radial walls in Tianfeng 202 and few had CSs in Nipponbare. At 40 mm from the root tip, all exodermal cells of Liaohan 109 had CSs in the radial walls, while some exodermal cells had CSs in the radial walls in Tianfeng 202, and few had CSs in Nipponbare. These results suggest that the development of CSs on the endo- and exodermis in the salt- and drought-tolerant Liaohan 109 occurred earlier than in Tianfeng 202 and Nipponbare. Furthermore, even without salt in the nutrient solution, the development of CSs in Liaohan 109 had been brought forward and increased.
The FTIR technique has been widely used to determine chemical components of CSs.11,12 We used this technique to detect compounds in the OPR and endodermal CSs isolated from roots of three rice cultivars after enzyme digestion. The results showed that the OPR and endodermal CSs were mainly composed of aliphatic suberin, lignin and cell wall proteins and carbohydrates, in agreement with previous reports from FTIR, chemical degradation and chromatographic analyses.8,9,11,12,19,34 In addition, we compared subtraction spectra of OPR and endodermal CSs from the three rice cultivars. With regard to the OPR, the relative intensities of Liaohan 109 to those of Tianfeng 202 and Nipponbare (wavelength range from 3,500 to 500 cm−1) were positive, with maxima of 0.109 and 0.094, respectively, indicating that the content of suberin and lignin in OPR from Liaohan 109 was higher than in Tianfeng 202 and Nipponbare. For endodermal CSs, the relative intensities of Liaohan 109 to Nipponbare (wavelength range 3,500 to 500 cm−1) were positive, but the relative intensity of the highest peak was only 0.011; similarly, most Liaohan 109 to Tianfeng 202 gave positive values, indicating little difference in the content of suberin and lignin in endodermal CSs from Liaohan 109, Tianfeng 202 and Nipponbare.
The biopolymer suberin contains both an aliphatic and an aromatic domain and is deposited in primary cell walls.29,30 The aromatic suberin is composed primarily of coumaric and ferulic acids, whereas aliphatic suberin is composed of fatty acids, alcohols, diacids, ω-hydroxy acids, 2-hydroxy acids and hydroxy acids.31,32 In primary roots, suberin is an important component of endo- and exodermal cell walls.3 The development of suberin lamellae and CSs is well known to reduce water loss and control transport of solutions by non-selective apoplastic bypass.14,33,34 Moreover, the early formation of suberin lamellae on the outer part of roots can function as a barrier to gas diffusion.35,36 Histochemical methods revealed that plants respond to environmental stimuli, such as drought, salt stress and oxygen deficiency, with increased suberization of apoplastic barriers in roots.13–15 The exodermis can contribute to the regulation of water uptake into roots in plants grown in soil with low water potentials.15,37 Steudle and Peterson14 reported that the exodermis contributes to the regulation of water uptake into roots, together with the well-known role of the endodermis as a barrier to back-diffusion of ions released into the apoplast of the stele. The endodermis thus confines ions to a zone near the tracheary elements, making delivery of ions to the shoot more efficient, and also allows root pressure to build under conditions of low transpiration. As a result, the hydraulic resistance of the endodermis is rather low, at least for cells that only have CSs. Krishnamurthy et al.18 showed, in plants that had not been subjected to salt stress, that the salt-tolerant Pokkali deposits a complete exodermis (OPR) close to the root tip, while it is only partially formed in the salt-sensitive IR20. However, differences in the endodermis are small between these cultivars, as also found in our rice varieties. The development of CSs on the exodermis in salt- and drought-tolerant Liaohan 109 was earlier than that in Tianfeng 202 and Nipponbare, and content of suberin in OPR was also higher than that in Tianfeng 202 and Nipponbare. However, the CSs and suberin in the endodermis of Liaohan 109 grown without salt were similar to those of Tianfeng 202 and Nipponbare. As a result, the exodermis has prepared the CSs barrier before drought or salt stress in salt- and drought-tolerant Liaohan 109. Further study is required to clarify whether this cultivar also has higher drought or salt tolerance under stress conditions.
Materials and Methods
Plant materials.
Seeds of three rice cultivars (Liaohan 109, Tianfeng 202 and Nipponbare) were germinated on wet tissue paper for 2–3 days in the dark at 30°C. For further culture, seedlings were transferred to aerated hydroponic ¼ strength Hoagland's nutrient solution for 2 weeks.20 The seedlings were grown at 28°C/25°C (day/night) with a 12 h photoperiod under an irradiance of 350 to 400 µmol/m2/s1 and relative humidity of 60 to 80% in an Illumination incubator. The nutrient solution was replaced each week. Saline stress treatment was applied after 2 weeks by addition of 0 (control) or 200 mM NaC1 solution directly to the nutrient solution. The other culture conditions were maintained as above.
Histochemistry of casparian strips.
Freehand cross-sections were cut at the following distances from the root tip: 5, 10, 20, 30, 40 and 50 mm. To check for CSs, sections were stained with 0.1% berberine hemisulfate for 1 h and counter-stained with 0.5% (w/v) aniline blue for an additional hour.21 Sections were viewed under a Leica DMLB fluorescence microscope with an ultraviolet filter set. To check for suberin lamellae, sections were stained for 1 h with the lipophilic fluorochrome Fluorol Yellow 088.22 The aliphatic component of suberin in the cell walls was detected as yellow-green fluorescence under a Leica DMLB fluorescence microscope using ultraviolet (UV) light.
Isolation of casparian strips from roots.
Roots were cut into pieces 5–8 mm in length and enzymatic isolation of CSs was carried out following the method described by Schreiber et al.10 After several days, root segments were washed three times in deionized water, followed by splitting the root longitudinally and pulling the central cylinder away from the outer parts, i.e., the epidermis, exodermis, sclerenchyma and one layer of cortical cells in series (OPR). Then the networks of CSs were separated from the stele under a binocular microscope using fine-tipped forceps. The resulting OPR and CSs material was dried in a freeze-drying system and stored over silica gel for subsequent chemical analysis.
Fourier transform infrared spectroscopy.
The FTIR spectra of CSs and OPR isolated from roots were recorded using an FTIR spectrometer (BRUKER TENSOR 27, Germany). The FTIR spectra were recorded in the absorbance mode by accumulating 32 scans with a resolution of 4 cm−1 in the spectral range of 4,000 to 400 cm−1. Each sample was scanned five times. All of the data were analyzed using Origin 6.0 software.
Acknowledgements
Firstly, we would like to thank Professor HU YuXi of Institute of Botany, Chinese Academy of Sciences for assistance in preparation of this article, we are very grateful to the funding from the Ministry of Science and Technology of People's Republic of China (2007DFA30770) and an international cooperation project from Sino-German Center (GZ616).
References
- 1.Perumalla CJ, Peterson CA, Enstone DE. A survey of angiosperm species to detect hypodermal Casparian bands. I. Roots with a uniserate hypodermis and epidermis. Bot J Linn Soc. 1990;103:93–112. [Google Scholar]
- 2.Enstone DE, Peterson CA, Ma F. Root endodermis and exodermis: structure, function and responses to the environment. J Plant Growth Regul. 2003;21:335–351. [Google Scholar]
- 3.Ma F, Peterson CA. Current insights into the development, structure and chemistry of the endodermis and exodermis of roots. Can J Bot. 2003;81:405–421. [Google Scholar]
- 4.Clark LH, Harris WH. Observations of the root anatomy of rice (Oryza sativa L.) Amer J Bot. 1981;68:154–161. [Google Scholar]
- 5.Peterson CA. Exodermal Casparian bands: their significance for ion uptake by roots. Physiol Plant. 1988;72:204–208. [Google Scholar]
- 6.Ranathunge K, Kotula L, Steudle E, Lafitte R. Water permeability and reflection coefficient of the outer part of young rice roots are differently affected by closure of water channels (aquaporins) or blockage of apoplastic pores. J Exp Bot. 2004;55:433–447. doi: 10.1093/jxb/erh041. [DOI] [PubMed] [Google Scholar]
- 7.Wu XQ, Zhu JM, Wang QL, Hu YX, Lin JX. Advances in studies on Casparian strips. Chin Bull Bot. 2002;19:302–309. [Google Scholar]
- 8.Wu XQ, Zhu JM, Huang RZ, Wang QL, Zheng WJ, Hu YX, et al. Evidence of Casparian Strip in the foliar endodermis of Pinus bungeana. Acta Bot Sin. 2001;43:1081–1084. [Google Scholar]
- 9.Wu XQ, Lin JX, Lin QQ, Wang J, Schreiber L. Casparian strips in needles are more solute permeable than endodermal transport barriers in roots of Pinus bungeana. Plant Cell Physiol. 2005;46:1799–1808. doi: 10.1093/pcp/pci194. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber L, Breiner HW, Riederer M, Duggelin M, Guggenheim R. The Casparian band of Clivia miniata Reg. roots: isolation, fine structure and chemical nature. Bot Acta. 1994;107:353–361. [Google Scholar]
- 11.Zeier J, Schreiber L. Comparative investigation of primary and tertiary endodermal cell walls isolated from the roots of five monocotyledonous species: chemical composition in relation to fine structure. Planta. 1998;206:349–361. [Google Scholar]
- 12.Hartmann K, Peiter E, Koch K, Schubert S, Schreiber L. Chemical composition and ultrastructure of broad bean (Vicia faba L.) nodule endodermis in comparison to the root endodermis. Planta. 2002;215:14–25. doi: 10.1007/s00425-001-0715-z. [DOI] [PubMed] [Google Scholar]
- 13.Nagahashi G, Thompson WW, Leonard RT. The Casparian strip as a barrier to the movement of lanthanum in maize roots. Science. 1974;183:670–671. doi: 10.1126/science.183.4125.670. [DOI] [PubMed] [Google Scholar]
- 14.Steudle E, Peterson CA. How does water get through roots? J Expl Bot. 1998;49:775–788. [Google Scholar]
- 15.Stasovsky E, Peterson CA. Effects of drought and subsequent rehydration on the structure, vitality and permeability of Allium cepa adventitious root. Can J Bot. 1993;71:700–707. [Google Scholar]
- 16.Yokoyama M, Karahara I. Radial widening of the Casparian strip follows induced radial expansion of endodermal cells. Planta. 2001;213:474–477. doi: 10.1007/s004250100536. [DOI] [PubMed] [Google Scholar]
- 17.Karahara I, Ikeda A, Kondo T, Uetake Y. Development of the Casparian strip in primary roots of maize under salt stress. Planta. 2004;219:41–47. doi: 10.1007/s00425-004-1208-7. [DOI] [PubMed] [Google Scholar]
- 18.Krishnamurthy P, Ranathunge K, Franke R, Prakash HS, Schreiber L, Mathew MK. The role of root apoplastic transport barriers in salt tolerance of rice (Oryza sativa L.) Planta. 2009;230:119–134. doi: 10.1007/s00425-009-0930-6. [DOI] [PubMed] [Google Scholar]
- 19.Kotula L, Ranathunge K, Schreiber L, Steudle E. Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. J Exp Bot. 2009;60:2155–2167. doi: 10.1093/jxb/erp089. [DOI] [PubMed] [Google Scholar]
- 20.Ranathunge K, Steudle E, Lafitte R. Control of water uptake by rice (Oryza sativa L.): role of the outer part of the root. Planta. 2003;217:193–205. doi: 10.1007/s00425-003-0984-9. [DOI] [PubMed] [Google Scholar]
- 21.Brundrett MC, Enstone DE, Peterson CA. A berberine-aniline blue fluorescent staining procedure for suberin, lignin and callose in plant tissues. Protoplasma. 1988;146:133–142. [Google Scholar]
- 22.Lux A, Morita S, Abe J, Ito K. An improved method for clearing and staining free-hand sections and whole-mount samples. Ann Bot. 2005;96:989–996. doi: 10.1093/aob/mci266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie JX, Chang JB, Wang XM. Applications of infrared spectra in organic chemistry and pharmaceutical chemist. Beijing: Science Press; 2001. [Google Scholar]
- 24.Lopes MH, Neto P, Barros AS, Rutledge D, Delgadillo I, Gil AM. Quantitation of aliphatic suberin in Quercus suber L. cork by FTIR spectroscopy and solid-state 13C-NMR spectroscopy. Biopolymers. 2000;57:344–351. doi: 10.1002/1097-0282(2000)57:6<344::AID-BIP40>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Peterson CA, Perumalla CJ. A survey of angiosperm species to detect hypodermal casparian bands. II. Roots with a multiseriate hypodermis or epidermis. Bot J Linn Soc. 1990;103:113–125. [Google Scholar]
- 26.Clarkson DT. Root structure and sites of ion uptake. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant Roots: the Hidden Half. 2nd ed. New York, NY: Marcel Dekker; 1996. pp. 483–510. [Google Scholar]
- 27.Damus M, Peterson RL, Enstone DE, Peterson CA. Modifications of cortical cell walls in roots of seedless vascular plants. Bot Acta. 1997;110:190–195. [Google Scholar]
- 28.Meyer CJ, Seago JL, Jr, Peterson CA. Environmental effects on the maturation of the endodermis and exodermis of Iris germanica roots. Ann Bot. 2009;103:687–702. doi: 10.1093/aob/mcn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schreiber L, Hartmann K, Skrabs M, Zeier J. Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. J Exp Bot. 1999;50:1267–1280. [Google Scholar]
- 30.Bernards MA. Demystifying suberin. Can J Bot. 2002;80:227–240. [Google Scholar]
- 31.Zeier J, Schreiber L. Chemical composition of hypodermal and endodermal cell walls and xylem vessels isolated from Clivia miniata (identification of the biopolymers lignin and suberin) Plant Physiol. 1997;113:1223–1231. doi: 10.1104/pp.113.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolattukudy PE. Polyesters in higher plants. Adv Biochem Eng Biot. 2001;71:1–49. doi: 10.1007/3-540-40021-4_1. [DOI] [PubMed] [Google Scholar]
- 33.Zimmermann HM, Steudle E. Apoplastic transport across young maize roots: effect of the exodermis. Planta. 1998;206:7–19. [Google Scholar]
- 34.Wu XQ, Zhang DX, Hu YX, Lin JX. The function of Pinus bungeana foliar endodermis as apoplastic barrier under salt stress. J Trop Subtrop Bot. 2007;15:203–208. [Google Scholar]
- 35.Armstrong W, Cousins D, Armstrong J, Turner DW, Beckett PM. Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Ann Bot. 2000;86:687–703. [Google Scholar]
- 36.Soukup A, Armstrong W, Schreiber L, Franke R, Votrubová O. Apoplastic barriers to radial oxygen loss and solute penetration: a chemical and functional comparison of the exodermis of two wetland species, Phragmites australis and Glyceria maxima. New Phytol. 2007;173:264–278. doi: 10.1111/j.1469-8137.2006.01907.x. [DOI] [PubMed] [Google Scholar]
- 37.Cruz RT, Jordan WR, Drew MC. Structural changes and associated reduction of hydraulic conductance in roots of Sorghum bicolor L. following exposure to water deficit. Plant Physiol. 1992;99:203–212. doi: 10.1104/pp.99.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]




