Abstract
Arabidopsis has three cytokinin receptors genes: CRE1, AHK2 and AHK3. Availability of plants that are homozygous mutant for these three genes indicates that cytokinin receptors in the haploid cells are dispensable for the development of male and female gametophytes. The triple mutants form a few flowers but never set seed, indicating that reproductive growth is impaired. We investigated which reproductive processes are affected in the triple mutants. Anthers of mutant plants contained fewer pollen grains and did not dehisce. Pollen in the anthers completed the formation of the one vegetative nucleus and the two sperm nuclei, as seen in wild type. The majority of the ovules were abnormal: 78% lacked the embryo sac, 10% carried a female gametophyte that terminated its development before completing three rounds of nuclear division, and about 12% completed three rounds of nuclear division but the gametophytes were smaller than those of the wild type. Reciprocal crosses between the wild type and the triple mutants indicated that pollen from mutant plants did not germinate on wild-type stigmas, and wild-type pollen did not germinate on mutant stigmas. These results suggest that cytokinin receptors in the sporophyte are indispensable for anther dehiscence, pollen maturation, induction of pollen germination by the stigma and female gametophyte formation and maturation.
Key words: cytokinin, cytokinin receptor, female gametophyte, male gametophyte, stigma
Introduction
Cytokinins control a wide range of developmental and physiological processes, including cell proliferation, apical dominance, nutrient mobilization, seed germination, vascular patterning and cambial activity.1,2 However, their functions in sexual reproduction have not been examined in detail.
The female gametophyte is generated from a megaspore, which is formed after meiosis. The megaspore undergoes three rounds of karyokinesis to form an 8-nucleus syncytial cell. This cell, the embryo sac, is then cellularized to form seven cells: an egg, two synergid cells, three antipodal cells and a central cell. In Arabidopsis thaliana, the central cell initially has two nuclei. Female gametophyte formation finishes when the two nuclei of the central cell are fused to form a diploid nucleus.3
A number of genes in female gametophytes are known to be required for their development. SPOROCYTELESS/NOZZLE (SPL/NZZ) is necessary to form cells that undergo meiosis in both carpel and stamen.4,5 Mutations in SWITCH1/DYAD (SWI1/DYAD), which is required for meiosis, impair megaspore formation.6,7 Several mutations are also known to cause developmental arrest during karyokinesis. These include a mutation in genes for anaphase-promoting complex (APC) components NOMEGA8 and APC2,9 double mutations in RPT5a and RPT5b, which code for components of the 26S proteasome,10 and double mutations in RHF1a and RHF1b, which code for components of the E3 ubiquitin ligase.11 The slow walker 1 (swa1) mutation delays the mitotic division cycle,12 and the retinoblastoma-related (rbr) mutation causes extra mitotic divisions.13 The ovules of the cytokinin independent1 (cki1) mutants abort after the 4-nucleus stage. As CKI1 is a histidine kinase, phosphorelay is required for female gametophyte development.14,15 GEMINI2/MOR1, which codes for a microtubule-associated protein,16 and TWO IN ONE (TIO), which codes for a phragmoplast-associated protein kinase, are required for the cellularization process.17 An asymmetric auxin gradient in the embryo sac plays a key role in gametic cell specification.18 Finally, LACHESIS (LIS) and CLOTHO/GFA1 (CLO/GFA1) play a central role in gametic cell fate specification.19,20
Several genes in the sporophytes are also known to be indispensable for female gametophyte development. For example, mutants with defective integument initiation and outgrowth, such as ainteguments (ant), inner no outer (ino), bell1 (bel), tousled (tsl) and short integuments1/dicer like1 (sin1/dcl1), are associated with aborted embryo sac development.21 ANT, INO, BEL1, TSL and SIN1/DEL1 code for an AP2-class transcription factor, a YABBY-class transcription factor, a homeodomain-containing transcription factor, a serine/threonine protein kinase and a dicer-like protein, respectively.22–26 These observations suggest that the sporophytic tissue surrounding the female gametophyte plays a role in controlling female gametophyte development.
Formation of the male gametophyte (i.e., the pollen) is initiated by periclinal divisions that form archesporial cells in the anther primordium. Mitotic divisions of the archesporial cells then occur to form the different cell layers: the inner primary sporogenous cells and the outer primary parietal cells. The primary sporogenous cells undergo a small number of divisions to form pollen mother cells, which go through meiotic divisions to form tetrads consisting of four microspores. The microspore undergoes an asymmetric cell division to form the larger vegetative cell and the smaller generative cell. The smaller generative cell again divides to form two sperm cells, which are engulfed in the vegetative cell. The primary parietal cells go through a series of further divisions to form endothecial cells and secondary parietal cells; the secondary parietal cells then divide to form the middle cell layer and the tapetum. After pollen maturation, anthers dehisce to release pollen.27
Mutations that affect each step of pollen development are also known. For example, the spl/nzz mutant fails to form the pollen mother cells and the surrounding cell layers.4,5 EXTRA SPOROGENOUS CELLS/EXCESS MICROSPOROCYTES1 (EXS/EMS1), which encodes a leucine-rich repeat receptor kinase and TAPETAL DETERMINANT1 (TPD1), which encodes for a small secreted protein, may regulate archesporial cell number in the anther.28–32 The sidecar pollen (scp) mutant affects microspore asymmetric division and cellular pattern.33 Mutations in genes for the A-type cyclin-dependent kinase (CDKA;1) and F-box-Like 17 (FBL17) are also known to cause arrest during generative cell division.34,35 DUO POLLEN1 (DUO1), which encodes a R2R3 MYB protein, may function as a generative cell fate determinant, linking cell division and gamete specification.36,37 DYSFUNCTIONAL TAPETUM1 (DYT1), coding for a bHLH transcription factor; ABORTED MICROSPORE (AMS), coding for a bHLH transcriotion factor; and MALE STERILITY 1 (MS1), coding for a PHD-finger transcription factor are required for normal tapetal function and viable pollen production.38
There is some evidence that cytokinins are involved in male reproductive development. For example, anthers of several male-sterile mutants, including the stamenless-2 (sl-2) mutant of tomato (Solanum lycopersicum)39 and a genetic male-sterile line of rapeseed (Brassica napus),40 have lower endogenous cytokinin levels. Cytokinins have also been shown to reverse cytoplasmic male sterility in barley (Hordeum vulgare).41 Accumulation of CKX (cytokinin oxidase/dehydrogenase) in male reproductive tissues of transgenic maize (Zea mays) resulted in male-sterile plants.42 Fertility of Arabidopsis overexpressing AtCKX1 was greatly diminished.43 In rice, the trans-zeatin-type cytokinins were slightly higher in the anther than in the leaf blade and pistil.44
To clarify the role of sporophytic cytokinins in reproductive growth, we carefully examined the phenotypes of a cytokinin-receptor triple mutant (cre1-12 ahk2-2tk ahk3-3), indicating that cytokinin receptors in the sporophyte are required for female gametophyte development and function of pollen and pistil.
Results
Male and female functions are impaired in the cytokinin-receptor triple mutants.
Although a triple mutant containing weaker alleles (ahk2-5 ahk3-7 cre1-2) set a few seeds,45 two other cytokinin-receptor triple mutants (cre1-12 ahk2-2tk ahk3-3, ahk2-1 ahk3-1 ahk4-1) with a stronger phenotype do not produce seeds.46,47 We first examined segregation ratio of the triple-mutant phenotype in a population generated by selfing cre1-12/cre1-12 ahk2-2tk/ahk2-2tk AHK3/ahk3-3 plants (i.e., heterozygous for one of the three cytokinin receptor genes). The triple-mutant phenotype appeared in 24.5% of the plants (147 mutant phenotype: 451 normal phenotype; χ2 = 0.055741, p > 0.05, based on an expected ratio of 1 small: 3 normal), indicating that the presence of the triple-mutant genotype in either male or female gametophytes does not distort the segregation ratio.
The triple mutant plants (cre1-12 ahk2-2tk ahk3-3) were small but occasionally produced an inflorescence with a few flowers. The flowers were smaller than the wild type and looked normal, but did not produce seeds (Fig. 1). This indicates that cytokinin receptors in sporophytic tissue are required for reproductive functions, because the segregation experiment demonstrated that cytokinin receptor genes in gametophytes are dispensable.
Figure 1.
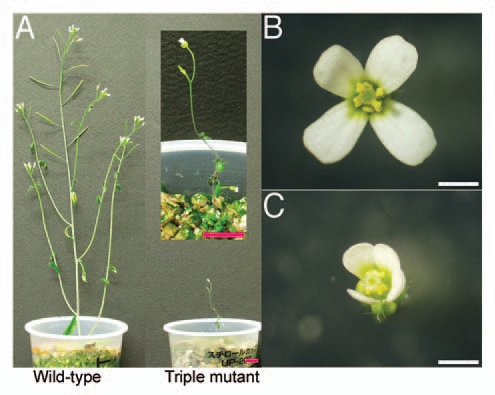
Phenotype of the triple mutant (cre1-12 ahk2-2tk ahk3-3). The triple mutant formed fewer and smaller flowers than wild type. (A) 6-week-old plants of wild type (left) and triple mutant (right). Bars = 1 cm. (B) Wild-type and (C) triple-mutant flowers immediately after opening. Bars = 1 mm.
To know which male or female functions of the triple mutants are impaired, we performed reciprocal crosses between triple-mutant and wild-type plants. Both male and female functions were impaired in these crosses (Table 1). Although the triple mutant female set a few seeds when pollinated with wild-type pollen, those seeds did not germinate. This suggests that female gametophyte functions or pistil functions necessary to support fertilization and embryogenesis are also impaired.
Table 1.
Reciprocal crosses between wild-type and triple mutants indicated that male and female functions were impaired
| Female parent | Male parent | Ratio* | N |
| Wild type | Wild type | 0.86 | 15 |
| Wild type | Triple mutant | 0 | 98 |
| Triple mutant | Wild type | 0.03 | 60 |
Ratio of the number of siliques containing at least one seed to total number of pistils pollinated. N, number of pistils pollinated.
Mutant anthers do not dehisce, and pollen germination is decreased.
To clarify what male processes are impaired, we observed both pollen and anthers in detail. During anther development in Arabidopsis, the male sporophyte and gametophyte tissues undergo unique biological processes, including specific cell divisions (meiosis and division of the haploid nuclei), cell differentiation of the male gametophyte, cell-cell communication between the tapetum and the microspore/pollen, and death of tapetum cells. The process of dehiscence, which involves opening the anther wall to release the mature pollen grains, requires the degeneration of specific anther tissues called the septum and the stomium.48
At anthesis, the stamen filaments of the triple mutants elongated normally (Fig. 1B and C), but the anthers were slightly smaller than the wild type and failed to dehisce (Fig. 2A and B). To analyze the developmental defects in the triple mutant, we compared the terminal phenotypes of anthers in transverse section (Fig. 2C and D). The mutant anthers showed several defects. First, only two- or three-lobed structures were formed in the mutant anthers, whereas four-lobed structures were formed in wild-type anthers. Second, the number of pollen grains was less than that in wild type. Third, degeneration of the tapetum and the break of the septum and stomium were incomplete. Finally, the vascular tissues within the center of the anther failed to form normally.
Figure 2.
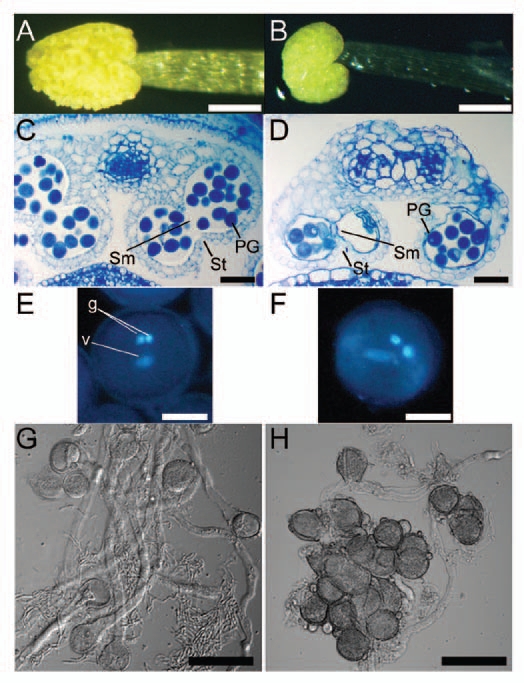
Triple mutant anthers did not dehisce. Pollen of the triple mutant was morphologically normal, but less fraction of pollen, when compared to wild-type, germinated in vitro. (A and B) Anthers removed from open flowers. (A) Wild-type anther. (B) Triple-mutant anther. Bars = 200 µm. (C and D) Transverse sections of anthers. (C) A wild-type anther from a flower immediately before opening. (D) A triple-mutant anther at a similar stage as in (C). In wild-type anthers, the septum (sm) and stomium (st) degenerated, which allow release of pollen grains, whereas in mutant anthers, the septum and stomium did not degenerate. PG, pollen grain. Bars = 50 µm. (E and F) Pollen grains stained with 4′,6-diamidino-2-phenylindole. One vegetative (v) and two generative (g) nuclei were observed in both wild-type (E) and triple-mutant (F) pollen grains. Bars = 10 µm. (G and H) In vitro germination of pollen. (G) Wild type. (H) Triple mutant. Triple-mutant grains germinated less frequently than wild-type, and germinating pollen tubes were shorter than those of wild type. Bars = 50 µm.
Despite these defects in anther development, the mutant pollen grains from undehisced anthers were indistinguishable in size and shape from wild type. Both wild-type and mutant pollen grains contained a single vegetative nucleus and two generative nuclei (Fig. 2E and F). To test whether the viability of mutant pollen grains differs from that of wild type, we put both wild-type and mutant pollen grains on artificial germination medium. Pollen from the triple mutant germinated, but at a lower frequency than the wild type, and the pollen tubes were shorter than those of the wild type (Fig. 2G and H).
Female gametophyte development is abnormal in the triple mutant.
To determine the steps of female gametophyte development that were affected by the lack of cytokinin receptors, we observed the megagametophyte terminal phenotypes by using confocal laser scanning microscopy (CLSM).49,50 In Arabidopsis, the megaspore mother cell undergoes meiosis to produce four megaspores in the ovule. Only one functional megaspore out of the four survives, and it then undergoes three rounds of mitotic divisions and subsequent cellularization to produce a seven-celled mature female gametophyte.51,52
All female gametophytes of opened wild-type flowers were at the terminal developmental stage (Fig. 3B). We observed 73 ovules of triple-mutant flowers, and the phenotypes of the mutant female gametophytes fell into three categories. In the first and most frequent category (57/73 ovules), the female gametophytes lacked the embryo sac (Fig. 3C). In the second category (7/73 ovules), development was terminated before completion (Fig. 3D) and most female gametophytes had either 2 or 4 nuclei. In the third category (9/73 ovules), the female gametophytes appeared to be morphologically normal (Fig. 3E), but the ovules were slightly smaller than the wild type. In the triple mutant, both inner and outer integuments were formed normally, suggesting that the integuments can develop without female gametogenesis.
Figure 3.

CLSM (confocal laser scanning microscopy) images of mutant and wild-type female gametophytes. The majority of triple-mutant ovules were abnormal. (A) Schematic diagram of ovule, with female gametophyte encircled by red line. (B) Wild-type ovule. (C–E) Triple-mutant ovules. Of the 73 triple-mutant ovules observed, 57 lacked the embryo sac (C), 7 carried a female gametophyte that terminated its development before completion (D), and 9 appeared to be morphologically normal (E). ecn, egg cell nucleus; scn, synergid cell nucleus; sen, secondary nucleus. Bars = 20 µm.
Pollen-pistil interaction is impaired in the cytokinin receptor triple mutants.
Although a fraction of the female gametophytes appeared to be morphologically normal, the triple mutants were female sterile and produced very few seeds upon pollination with wild-type pollen. Also, although some of the triple-mutant pollen grains germinated in vitro, triple mutant pollen did not produce seed when applied to wild-type pistils (Table 1). To investigate whether the sterility is caused by defects in pollen-pistil interaction, we placed pollen from the triple mutant onto wild-type pistils, and wild-type pollen on mutant pistils, and checked for pollen germination. In both cases, the pollen grains did not germinate (Fig. 4), indicating that cytokinin receptors in the sporophyte are required for pollen germination, which depends on pollen-pistil interaction.
Figure 4.
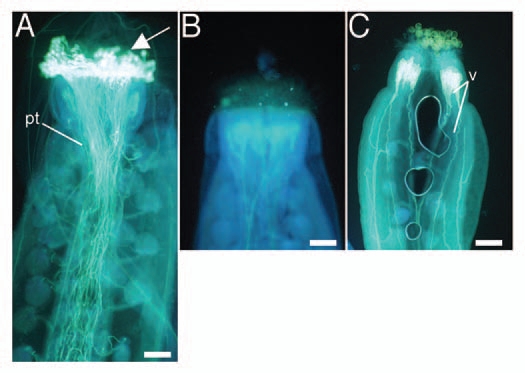
Aniline blue-stained pistils 24 h after reciprocal crosses between wild-type and triple-mutant plants. (A) Wild-type stigma pollinated with wild-type pollen. (B) Wild-type stigma pollinated with triple-mutant pollen. (C) Triple-mutant stigma pollinated with wild-type pollen. White on the stigma (arrow, A) indicates pollen germination. In contrast, no signal was observed in (B and C). pt, pollen tubes; v, vasculature. Bars = 50 µm.
Discussion
We have shown that cytokinin receptor genes within the male and female gametophytes are not required for the development of the gametophytes in Arabidopsis. But those in the sporophyte are required for the production of functional gametophytes.
Pollen produced by the triple mutant deficient in the three cytokinin receptors had the usual two sperm nuclei and one vegetative nucleus, and germinated on an artificial medium, albeit with a reduced efficiency. However, these pollen grains did not germinate on wild-type stigmas. The inability to germinate might have been caused by incomplete maturation, because tapetum degeneration was incomplete and anthers did not dehisce. Tapetum degeneration and anther dehiscence normally require jasmonic acid,53 but application of methyl jasmonate (MeJA) to bud clusters had no effect on pollen maturation or anther dehiscence of the cytokinin receptor triple mutant (data not shown). Thus, jasmonic acid and cytokinin independently regulate anther maturation and dehiscence.
In the cytokinin-receptor triple mutant, the integuments, which are sporophytic tissue surrounding the female gametophytes, looked normal. However, a large majority of ovules either lacked female gametophytes altogether or possessed abnormal ones, indicating that cytokinin receptor genes in the sporophyte are required for normal development of the female gametophyte. It is yet to be determined what cytokinin-mediated processes in the sporophyte are required for gametophyte development.
In the triple mutant, the pistil function required to induce pollen germination was impaired. In wild-type plants, stigmatic papillae provide water to pollen in response to stimulus by pollen of the same species; this water then induces pollen germination. It is possible that the triple mutant is deficient in these processes.
Materials and Methods
Plant materials and growth conditions.
Arabidopsis ecotype Columbia was used in all experiments. Plants were grown on plates containing GM medium (MS salts, 1% sucrose, 1/100 vol. of 2.5% MES-KOH at pH 5.7, 0.3% Phytagel) under continuous light at 22°C. The triple-mutant genotype used was cre1-12 ahk2-2tk ahk3-3.46
Microscopy.
Anther structure was examined as described previously in reference 29, with the following modifications. Dissected floral buds and inflorescences were fixed in 2.8% (vol/vol) glutaraldehyde in 0.1 M HEPES (N-2-hydroxyethyl piperazine-N′-2-ethanesulfonic acid) buffer (pH 7.2) and 0.02% Triton X-100 overnight at 4°C. Samples were washed twice for 15 min each in 0.1 M HEPES buffer (pH 7.2) and then fixed in 1% OsO4 overnight. Samples were then dehydrated in a graded acetone series and embedded in Spurr's resin. Thin (0.5 µm) sections were made and stained with 1% Toluidine Blue O in 1% H3BO3-NaOH (pH 9.5). For examination of pollen, pollen grains were immersed in a solution of 2 µg/mL 4′,6-diamidino-2-phenylindole and 7% sucrose and viewed by fluorescence microscopy under UV light excitation. For female gametophyte analysis, tissue preparation and microscopy were performed as previously described in reference 49 and 50, with a slight modification: the pistils fixed in 4% glutaraldehyde, 12.5 mM cacodylate (pH 6.9) and 0.005% Silwet L77 for several hours on ice.
Pollen germination and staining.
In vitro pollen germination and aniline blue staining of pollinated pistils was performed as previously described in reference 53.
Acknowledgements
We thank Masayuki Higuchi for plant materials. This work was supported by Grants-in-Aid for Scientific Research (KAKENHI) (Grant No. 19060005).
References
- 1.Kakimoto T. Perception and signal transduction of cytokinins. Annu Rev Plant Biol. 2003;54:605–627. doi: 10.1146/annurev.arplant.54.031902.134802. [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto-Kitano M, Kusumoto T, Tarkowski P, Kinoshita-Tsujimura K, Vaclavikova K, Miyawaki K, et al. Cytokinins are central regulators of cambial activity. Proc Natl Acad Sci USA. 2008;105:20027–20031. doi: 10.1073/pnas.0805619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang WC, Shi DQ, Chen YH. Female gametophyte development in flowering plants. Annu Rev Plant Biol. 2010;61:89–108. doi: 10.1146/annurev-arplant-042809-112203. [DOI] [PubMed] [Google Scholar]
- 4.Yang WC, Ye D, Xu J, Sundaresan V. The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev. 1999;13:2108–2117. doi: 10.1101/gad.13.16.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiefthaler U, Balasubramanian S, Sieber P, Chevalier D, Wisman E, Schneitz K. Molecular analysis of NOZZLE, a gene involved in pattern formation and early sporogenesis during sex organ development in Arabidopsis thaliana. Proc Natl Acad Sci USA. 1999;96:11664–11669. doi: 10.1073/pnas.96.20.11664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravi M, Marimuthu MP, Siddiqi I. Gamete formation without meiosis in Arabidopsis. Nature. 2008;451:1121–1124. doi: 10.1038/nature06557. [DOI] [PubMed] [Google Scholar]
- 7.Mercier R, Vezon D, Bullier E, Motamayor JC, Sellier A, Lefevre F, et al. SWITCH1 (SWI1): a novel protein required for the establishment of sister chromatid cohesion and for bivalent formation at meiosis. Genes Dev. 2001;15:1859–1871. doi: 10.1101/gad.203201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwee HS, Sundaresan V. The NOMEGA gene required for female gametophyte development encodes the putative APC6/CDC16 component of the anaphase promoting complex in Arabidopsis. Plant J. 2003;36:853–866. doi: 10.1046/j.1365-313x.2003.01925.x. [DOI] [PubMed] [Google Scholar]
- 9.Capron A, Serralbo O, Fulop K, Frugier F, Parmentier Y, Dong A, et al. The Arabidopsis anaphase-promoting complex or cyclosome: molecular and genetic characterization of the APC2 subunit. Plant Cell. 2003;15:2370–2382. doi: 10.1105/tpc.013847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallois JL, Guyon-Debast A, Lecureuil A, Vezon D, Carpentier V, Bonhomme S, et al. The Arabidopsis proteasome RPT5 subunits are essential for gametophyte development and show accession-dependent redundancy. Plant Cell. 2009;21:442–459. doi: 10.1105/tpc.108.062372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Zhang Y, Qin G, Tsuge T, Sakaguchi N, Luo G, et al. Targeted degradation of the cyclin-dependent kinase inhibitor ICK4/KRP6 by RING-type E3 ligases is essential for mitotic cell cycle progression during Arabidopsis gametogenesis. Plant Cell. 2008;20:1538–1554. doi: 10.1105/tpc.108.059741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi DQ, Liu J, Xiang YH, Ye D, Sundaresan V, Yang WC. SLOW WALKER1, essential for gametogenesis in Arabidopsis, encodes a WD40 protein involved in 18S ribosomal RNA biogenesis. Plant Cell. 2005;17:2340–2354. doi: 10.1105/tpc.105.033563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebel C, Mariconti L, Gruissem W. Plant retinoblastoma homologues control nuclear proliferation in the female gametophyte. Nature. 2004;429:776–780. doi: 10.1038/nature02637. [DOI] [PubMed] [Google Scholar]
- 14.Hejatko J, Pernisova M, Eneva T, Palme K, Brzobohaty B. The putative sensor histidine kinase CKI1 is involved in female gametophyte development in Arabidopsis. Mol Genet Genomics. 2003;269:443–453. doi: 10.1007/s00438-003-0858-7. [DOI] [PubMed] [Google Scholar]
- 15.Pischke MS, Jones LG, Otsuga D, Fernandez DE, Drews GN, Sussman MR. An Arabidopsis histidine kinase is essential for megagametogenesis. Proc Natl Acad Sci USA. 2002;99:15800–15805. doi: 10.1073/pnas.232580499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glab N, Teste MA, Slonimski PP. MRG1-1, a dominant allele that confers methomyl resistance in yeast expressing the cytoplasmic male sterility T-urf13 gene from maize. Curr Genet. 1994;26:477–485. doi: 10.1007/BF00309937. [DOI] [PubMed] [Google Scholar]
- 17.Palopoli MF, Wu CI. Genetics of hybrid male sterility between drosophila sibling species: a complex web of epistasis is revealed in interspecific studies. Genetics. 1994;138:329–341. doi: 10.1093/genetics/138.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagnussat GC, Alandete-Saez M, Bowman JL, Sundaresan V. Auxin-dependent patterning and gamete specification in the Arabidopsis female gametophyte. Science. 2009;324:1684–1689. doi: 10.1126/science.1167324. [DOI] [PubMed] [Google Scholar]
- 19.Gross-Hardt R, Kagi C, Baumann N, Moore JM, Baskar R, Gagliano WB, et al. LACHESIS restricts gametic cell fate in the female gametophyte of Arabidopsis. PLoS Biol. 2007;5:47. doi: 10.1371/journal.pbio.0050047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moll C, von Lyncker L, Zimmermann S, Kagi C, Baumann N, Twell D, et al. CLO/GFA1 and ATO are novel regulators of gametic cell fate in plants. Plant J. 2008;56:913–921. doi: 10.1111/j.1365-313X.2008.03650.x. [DOI] [PubMed] [Google Scholar]
- 21.Schneitz K. The molecular and genetic control of ovule development. Curr Opin Plant Biol. 1999;2:13–17. doi: 10.1016/s1369-5266(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 22.Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQ, Gerentes D, et al. AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell. 1996;8:155–168. doi: 10.1105/tpc.8.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villanueva JM, Broadhvest J, Hauser BA, Meister RJ, Schneitz K, Gasser CS. INNER NO OUTER regulates abaxial-adaxial patterning in Arabidopsis ovules. Genes Dev. 1999;13:3160–3169. doi: 10.1101/gad.13.23.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reiser L, Modrusan Z, Margossian L, Samach A, Ohad N, Haughn GW, et al. The BELL1 gene encodes a homeodomain protein involved in pattern formation in the Arabidopsis ovule primordium. Cell. 1995;83:735–742. doi: 10.1016/0092-8674(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 25.Roe JL, Rivin CJ, Sessions RA, Feldmann KA, Zambryski PC. The Tousled gene in A. thaliana encodes a protein kinase homolog that is required for leaf and flower development. Cell. 1993;75:939–950. doi: 10.1016/0092-8674(93)90537-z. [DOI] [PubMed] [Google Scholar]
- 26.Golden TA, Schauer SE, Lang JD, Pien S, Mushegian AR, Grossniklaus U, et al. SHORT INTEGUMENTS1/SUSPENSOR1/CARPEL FACTORY, a Dicer homolog, is a maternal effect gene required for embryo development in Arabidopsis. Plant Physiol. 2002;130:808–822. doi: 10.1104/pp.003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson ZA, Zhang DB. From Arabidopsis to rice: pathways in pollen development. J Exp Bot. 2009;60:1479–1492. doi: 10.1093/jxb/erp095. [DOI] [PubMed] [Google Scholar]
- 28.Canales C, Bhatt AM, Scott R, Dickinson H. EXS, a putative LRR receptor kinase, regulates male germline cell number and tapetal identity and promotes seed development in Arabidopsis. Curr Biol. 2002;12:1718–1727. doi: 10.1016/s0960-9822(02)01151-x. [DOI] [PubMed] [Google Scholar]
- 29.Zhao DZ, Wang GF, Speal B, Ma H. The excess microsporocytes1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsis anther. Genes Dev. 2002;16:2021–2031. doi: 10.1101/gad.997902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang SL, Xie LF, Mao HZ, Puah CS, Yang WC, Jiang L, et al. Tapetum determinant1 is required for cell specialization in the Arabidopsis anther. Plant Cell. 2003;15:2792–2804. doi: 10.1105/tpc.016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang SL, Jiang L, Puah CS, Xie LF, Zhang XQ, Chen LQ, et al. Overexpression of TAPETUM DETERMINANT1 alters the cell fates in the Arabidopsis carpel and tapetum via genetic interaction with excess microsporocytes1/extra sporogenous cells. Plant Physiol. 2005;139:186–191. doi: 10.1104/pp.105.063529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jia G, Liu X, Owen HA, Zhao D. Signaling of cell fate determination by the TPD1 small protein and EMS1 receptor kinase. Proc Natl Acad Sci USA. 2008;105:2220–2225. doi: 10.1073/pnas.0708795105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen YC, McCormick S. sidecar pollen, an Arabidopsis thaliana male gametophytic mutant with aberrant cell divisions during pollen development. Development. 1996;122:3243–3253. doi: 10.1242/dev.122.10.3243. [DOI] [PubMed] [Google Scholar]
- 34.Iwakawa H, Shinmyo A, Sekine M. Arabidopsis CDKA;1, a cdc2 homologue, controls proliferation of generative cells in male gametogenesis. Plant J. 2006;45:819–831. doi: 10.1111/j.1365-313X.2005.02643.x. [DOI] [PubMed] [Google Scholar]
- 35.Nowack MK, Grini PE, Jakoby MJ, Lafos M, Koncz C, Schnittger A. A positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nat Genet. 2006;38:63–67. doi: 10.1038/ng1694. [DOI] [PubMed] [Google Scholar]
- 36.Durbarry A, Vizir I, Twell D. Male germ line development in Arabidopsis. duo pollen mutants reveal gametophytic regulators of generative cell cycle progression. Plant Physiol. 2005;137:297–307. doi: 10.1104/pp.104.053165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rotman N, Durbarry A, Wardle A, Yang WC, Chaboud A, Faure JE, et al. A novel class of MYB factors controls sperm-cell formation in plants. Curr Biol. 2005;15:244–248. doi: 10.1016/j.cub.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Sun Y, Timofejeva L, Chen C, Grossniklaus U, Ma H. Regulation of Arabidopsis tapetum development and function by DYSFUNCTIONAL TAPETUM1 (DYT1) encoding a putative bHLH transcription factor. Development. 2006;133:3085–3095. doi: 10.1242/dev.02463. [DOI] [PubMed] [Google Scholar]
- 39.Sawhney VK, Shukla A. Male Sterility In Flowering Plants: Are Plant Growth Substances Involved? American Journal of Botany. 1994;81:1640–1647. [Google Scholar]
- 40.Shukla A, Sawhney VK. Metabolism of Dihydrozeatin in Floral Buds of Wild-Type and a Genie Male Sterile Line of Rapeseed (Brassica napus L.) J Exper Bot. 1993;44:1497–1505. [Google Scholar]
- 41.Ahokas H. Cytoplasmic male sterility in barley: Evidence for the involvement of cytokinins in fertility restoration. Proc NatL Acad Sci USA. 1982;79:7605–7608. doi: 10.1073/pnas.79.24.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang S, Cerny RE, Qi Y, Bhat D, Aydt CM, Hanson DD, et al. Transgenic studies on the involvement of cytokinin and gibberellin in male development. Plant Physiol. 2003;131:1270–1282. doi: 10.1104/pp.102.018598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmulling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell. 2003;15:2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hirano K, Aya K, Hobo T, Sakakibara H, Kojima M, Shim RA, et al. Comprehensive transcriptome analysis of phytohormone biosynthesis and signaling genes in microspore/pollen and tapetum of rice. Plant Cell Physiol. 2008;49:1429–1450. doi: 10.1093/pcp/pcn123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riefler M, Novak O, Strnad M, Schmulling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development and cytokinin metabolism. Plant Cell. 2006;18:40–54. doi: 10.1105/tpc.105.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higuchi M, Pischke MS, Mahonen AP, Miyawaki K, Hashimoto Y, Seki M, et al. In planta functions of the Arabidopsis cytokinin receptor family. Proc Natl Acad Sci USA. 2004;101:8821–8826. doi: 10.1073/pnas.0402887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishimura C, Ohashi Y, Sato S, Kato T, Tabata S, Ueguchi C. Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell. 2004;16:1365–1377. doi: 10.1105/tpc.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma H. Molecular genetic analyses of microsporogenesis and microgametogenesis in flowering plants. Annu Rev Plant Biol. 2005;56:393–434. doi: 10.1146/annurev.arplant.55.031903.141717. [DOI] [PubMed] [Google Scholar]
- 49.Christensen CA, King EJ, Jordan JR, Drews GN. Megagametogenesis in Arabidopsis wild type and the Gf mutant. Sexual Plant Reproduction. 1997;10:49–64. [Google Scholar]
- 50.Christensen CA, Subramanian S, Drews GN. Identification of gametophytic mutations affecting female gametophyte development in Arabidopsis. Dev Biol. 1998;202:136–151. doi: 10.1006/dbio.1998.8980. [DOI] [PubMed] [Google Scholar]
- 51.Yang WC, Sundaresan V. Genetics of gametophyte biogenesis in Arabidopsis. Curr Opin Plant Biol. 2000;3:53–57. doi: 10.1016/s1369-5266(99)00037-0. [DOI] [PubMed] [Google Scholar]
- 52.Drews GN, Yadegari R. Development and function of the angiosperm female gametophyte. Annu Rev Genet. 2002;36:99–124. doi: 10.1146/annurev.genet.36.040102.131941. [DOI] [PubMed] [Google Scholar]
- 53.Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K. The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence and flower opening in Arabidopsis. Plant Cell. 2001;13:2191–2209. doi: 10.1105/tpc.010192. [DOI] [PMC free article] [PubMed] [Google Scholar]


