Abstract
The endo-β-mannanase (MAN) family is represented in the Arabidopsis genome by eight members, all with canonical signal peptides and only half of them being expressed in germinating seeds. The transcripts of these genes were localized in the radicle and micropylar endosperm (ME) before radicle protrusion and this expression disappears as soon as the endosperm is broken by the emerging radicle tip. However, only three of these MAN genes, AtMAN5, AtMAN7 and especially AtMAN6 influence the germination time (t50) as assessed by the analysis of the corresponding knock-out lines. The data suggest a possible interaction between embryo and ME regarding the role of MAN during the Arabidopsis germination process.
Key words: arabidopsis, endo-β-mannanases, germination, in situ hybridization, micropylar endosperm, T-DNA insertion mutants
The Endosperm-limited Seed Germination
The seed phase is the most important stage of the plant life cycle, ensuring species survival. The seed is a viable and autonomous organism which will germinate when internal and environment conditions are suitable.1 In endospermic seeds, the diploid embryo is surrounded by two covering layers: the triploid endosperm (nutritive tissue, living cells) and the diploid testa (the seed coat, maternal tissue, dead cells). The endosperm of Arabidopsis is constituted by a single thin layer and originated by a cellularization process that begins in the micropylar region and during seed development spreads to the central and chalazal regions.2
Seed germination is initiated with water uptake by the dry seed and terminates with the elongation and emergence of the embryonic axis. Physically, germination in Arabidopsis is a two-stage sequential process, where testa rupture is followed by endosperm rupture. Following rupture of the micropylar endosperm (ME) by the elongating radicle, germination is completed.1,3 This physiological process is tightly controlled by environmental conditions, as well as, by the developmental program of the seed, in which abscisic acid (ABA) and gibberellins (GA) are some of the main hormones involved. Ethylene (ET), nitric oxide (NO), auxins, etc., are also part of the complex network of interacting signals that together with the transcriptional network involved in germination are now being actively investigated.3–9
Mannans, Endo-β-mannanases and Seed Germination
Two major forces play antagonistic roles in radicle emergence: the radicle growth potential (i.e., primarily cell elongation),10 and the mechanical resistance of the covering layers which is likely to be diminished before radicle protrusion by cell-wall (CW) hydrolytic enzymes.1,4,5 In order for the radicle apex to emerge, the growth potential must overcome the mechanical resistance of the endosperm. ABA inhibits endosperm rupture, but not testa rupture, and interacts with ET during Arabidopsis and Lepidium germination.9,11 Since the weakening of endosperm CW is an important factor for radicle protrusion in seeds of Arabidopsis, it is of great interest to study the genes involved in the dismantling of the endosperm CW structure, which, unlike radicle CW, is rich in mannans,12 conferring to this tissue a remarkable mechanical resistance. Endo-β-mannanase (MAN) activity has been reported in seeds of several endospermic species before and following radicle emergence.13 However, although the degradation of mannans is initiated by MAN,1,14 the role of this enzyme in endosperm weakening is still controversial, to the point that some authors maintain that MAN is not involved, while others conclude that MAN is the main enzyme component of the CW dismantling process.5,15,16 Taken together all these observations, the consensus appears to be that while MAN is required for endosperm weakening, itself is not enough to allow completion of germination.5
Is there a Cross-talk between Radicle and ME in Relation to Endo-β-mannanases during Seed Germination?
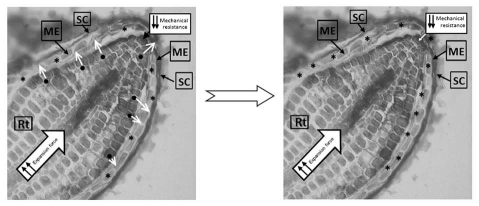
In our recent study with T-DNA insertion mutants in AtMAN genes, we concluded that the expression of AtMAN5, AtMAN6 and AtMAN7 in imbibed seeds is important for A. thaliana germination, probably by decreasing the mechanical resistance of the ME, thus facilitating the radicle emergence.18 Germination time course is negatively affected in knock-out (K.O.) mutants of AtMAN7 and AtMAN5 and strongly inhibited in K.O. AtMAN6 (Fig. 1). In parallel, we have detected transcripts of AtMAN5, AtMAN6 and AtMAN7 genes not only in the ME but also in the radicle, thus not ruling out the possibility that the AtMAN gene products could be also involved in the radicle cell growth through a transglycosylase activity. This conclusion is a consequence of the fact that the role of MAN as a hydrolase may not be the dominant role in CW of the radicle itself, where mannans are only minor, although important components.12 MAN expression is high in the vascular elements of the radicle, probably indicating a role in emptying the nascent conducting vessels that will ultimately develop into the vascular systems of xylem and phloem. Another point to be considered is that all the annotated MAN genes in Arabidopsis have predicted signal peptides, an indication that their corresponding proteins will be probably exported into the apoplastic space and could reach the CWs of the ME. Thus, radicle and endosperm MAN synthesis will contribute together to the weakening of the micropylar zones of the endosperm (Fig. 2). Remarkably, hormonal cooperation between the embryo and endosperm have been demonstrated in other physiological processes.19,20 As far as we know, since the radicle of Arabidopsis is also a production site of MAN that can be allocated to the periplasmic space, through the secretion pathway, it is reasonable to postulate a cooperation between embryo and endosperm MAN enzymes in the dismantling of the ME CW thus facilitating radicle protrusion. Our work18 would be the first data to support such a cross-talk regarding the synthesis of MAN upon germination.
Figure 1.
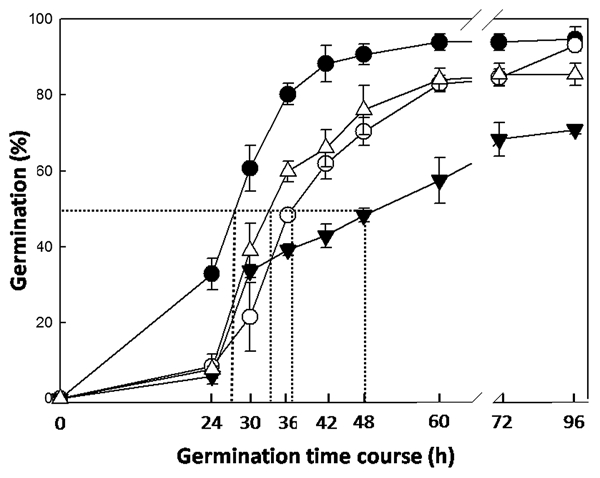
Germination time course of Arabidopsis thaliana Wt and T-DNA insertion mutant seeds. Wt: Closed circles (●; t50 = 25); K.O. MAN7 (GABI_747H02): Opened triangles (Δ; t50 = 34.4); K.O. MAN5 (GABI_707G06): Opened circles (○; t50 = 37.5); K.O. MAN6 (Salk_122701): Closed triangles (▼; t50 = 48). Data are means ± standard error (SE) of three independent experiments.
Figure 2.
Proposed model of MAN enzyme traffic in the germinating seed. MAN proteins with their signal peptides (SP; ●) are secreted ■ to the apoplastic space where they become active after proteolysis of the SP (*). Root, Rt; micropylar endosperm, ME; and seed coat SC.
Acknowledgements
This work was financially supported by grants from Ministerio de Ciencia e Innovación (MICINN, Spain) (CGL2009-11425). R. Iglesias-Fernández is supported by a post-doctoral Juan de la Cierva contract in the CBGP (Centro de Biotecnología y Genómica de Plantas, Madrid, Spain).
References
- 1.Nonogaki H, Bassel GW, Bewley JD. Germination—still a mystery. Plant Sci. 2010;179:574–581. [Google Scholar]
- 2.Linkies A, Graeber K, Knight Ch, Leubner-Metzger G. The evolution of seeds. New Phytol. 2010;186:817–831. doi: 10.1111/j.1469-8137.2010.03249.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu PP, Koizuka N, Homrichhausen TM, Hewitt JR, Martin RC, Nonogaki H. Large-scale screening of Arabidopsis enhancer-trap lines for seed germination-associated genes. Plant J. 2005;41:936–944. doi: 10.1111/j.1365-313X.2005.02347.x. [DOI] [PubMed] [Google Scholar]
- 4.Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytol. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- 5.Holdsworth MJ, Bentsink L, Soppe WJ. Molecular networks regulating Arabidopsis seed maturation, after-ripening, dormancy and germination. New Phytol. 2008;179:33–54. doi: 10.1111/j.1469-8137.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 6.Rodríguez-Gacio MC, Matilla-Vázquez MA, Matilla AJ. Seed dormancy and ABA signaling. The breakthrough goes on. Plant Signal Behav. 2009;4:1035–1048. doi: 10.4161/psb.4.11.9902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matilla AJ, Matilla-Vázquez MA. Involvement of ethylene in seed physiology. Plant Sci. 2008;175:87–97. [Google Scholar]
- 8.Vicente-Carbajosa JV, Carbonero P. Seed maturation: developing an intrusive phase to accomplish a quiescent state. Int J Dev Biol. 2005;49:645–651. doi: 10.1387/ijdb.052046jc. [DOI] [PubMed] [Google Scholar]
- 9.Linkies A, Müller K, Morris K, Turecková V, Wenk M, Cadman CSC, et al. Ethylene interacts with abscisic acid to regulate endosperm rupture during germination: a comparative approach using Lepidium sativum and Arabidopsis thaliana. Plant Cell. 2009;21:3803–3822. doi: 10.1105/tpc.109.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sliwinska E, Bassel GW, Bewley JD. Germination of Arabidopsis thaliana seeds is not completed as a result of elongation of the radicle but of the adjacent transition zone and lower hypocoty. J Exp Bot. 2009;60:3587–3594. doi: 10.1093/jxb/erp203. [DOI] [PubMed] [Google Scholar]
- 11.Müller K, Tintelnot S, Leubner-Metzger G. Endosperm-limited Brassicaceae seed germination: abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell Physiol. 2006;47:864–877. doi: 10.1093/pcp/pcj059. [DOI] [PubMed] [Google Scholar]
- 12.Marcus SE, Blake AW, Benians TAS, Lee KJD, Poyser C, et al. Restricted access of proteins to mannan polysaccharides in intact plant cell walls. Plant J. 2010;64:191–203. doi: 10.1111/j.1365-313X.2010.04319.x. [DOI] [PubMed] [Google Scholar]
- 13.Yuan JS, Yang X, Lai J, Lin H, Cheng ZM, Nonogaki N, et al. The endo-β-mannanase gene families in Arabidopsis, rice and poplar. Funct Integr Genomics. 2007;7:1–16. doi: 10.1007/s10142-006-0034-3. [DOI] [PubMed] [Google Scholar]
- 14.Moreira LRS, Filho EXF. An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol. 2008;79:165–178. doi: 10.1007/s00253-008-1423-4. [DOI] [PubMed] [Google Scholar]
- 15.Gong XM, Bassel GW, Wang A, Greewood JS, Bewley JD. The emergence of embryos from hard seeds is related to the structure of the cell walls of the micropylar endosperm, and not to endo-β-mannanase activity. Ann Bot. 2005;96:1–9. doi: 10.1093/aob/mci269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nonogaki H, Chen F, Bradford KJ. Mechanisms and genes involved in germination “senso stricto”. In: Bradford K, Nonogaki H, editors. Seed development, dormancy and germination. Blackwell: Oxford; 2007. pp. 264–304. [Google Scholar]
- 17.Schröder R, Atkinson RG, Redgwell RJ. Re-interpreting the role of endo-β-mannanases as mannan endotransglycosylase/hydrolases in the plant cell wall. Ann Bot. 2009;104:197–204. doi: 10.1093/aob/mcp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iglesias-Fernández R, Rodríguez-Gacio MC, Barrero-Sicilia C, Carbonero P, Matilla AJ. Three endo-β-mannanase genes expressed in the micropylar endosperm and in the radicle influence germination of Arabidopsis thaliana seeds. Planta. 2011;233:25–36. doi: 10.1007/s00425-010-1257-z. [DOI] [PubMed] [Google Scholar]
- 19.Arana MV, de Miguel LC, Sánchez RA. A phytochrome dependent embryonic factor modulates gibberellin responses in the embryo and micropylar endosperm of Datura ferox seeds. Planta. 2006;223:847–857. doi: 10.1007/s00425-005-0134-7. [DOI] [PubMed] [Google Scholar]
- 20.Arana MV, Burgin MJ, de Miguel LC, Sánchez RA. The very-low-fluence and high-irradiance responses of the phytochromes have antagonistic effects on germination, mannan-degrading activities and DfGA3ox transcript levels in Datura ferox seeds. J Exp Bot. 2007;58:3997–4004. doi: 10.1093/jxb/erm256. [DOI] [PubMed] [Google Scholar]