Abstract
We investigated how the pea (Pisum sativum cv. Harunoka) root, upon return to an Al-free condition, recovers from injury caused by exposure to Al. Elongation and re-elongation of the root during the recovery process from Al injury occurred only in the apical 5 mm region of the pea root. With the model system of the pea root for recovery from Al injury, images of the root characterized by zonal staining with Evans blue showed the existence of two regions in the root apex consisting of rupture and zonary stained regions. Ruptures enlarged by increase in their depth but without widening of the intervals between zonary stained regions in the roots treated with Al continuously. On the other hand, intervals of the zonary stained regions were widened due to reelongation of the root and were narrow in the rupture region in the recovery root.
Key words: Al stress, inhibition of elongation, injury, pea, recovery, root apex
Aluminum (Al) is a major growth-inhibiting factor in acid soils distributed worldwide. The primary effect of Al toxicity is inhibition of root elongation. Although there have been many studies on the mechanisms of Al toxicity and tolerance independently,1–4 the process of recovery from Al-induced inhibition of root elongation has received little attention.5–7 Root elongation might not be unilateral under the condition of Al stress. Inhibition and re-elongation (recovery process) are repeated under certain environmental conditions of the root rhizosphere. The root apex accumulates more Al and plays a major role in the Al perception mechanism. Accumulation of coating materials on the epidermis of the root apex is commonly observed concomitant with morphological changes.8–12 The aim of this study was to determine how the root apex of the pea seedling behaves at the recovery stage from Al injury from a morphological point of view.
Induction of Recovery of Elongation of the Pea Root Apex from Al Injury
Pea seedlings (Pisum sativum cv. Harunaka) were placed in holes of plastic boards that were floated on 1,000 ml of a solution containing 50 µM CaCl2 at pH 4.5 in a plastic cabinet. Pea roots grown in a solution containing 40 µM Al (pH 4.5) for 12 h were transferred to an Al-free solution containing 50 µM CaCl2 or left in 40 µM Al solution containing CaCl2 for another 12 h. Root length was measured with a ruler and relative root growth (RRG) was calculated. RRG was about 75% in 40 µM Al solution from 0 to 6 h of Al treatment and it was about 45% from 6 h to 12 h. In the root transferred from 40 µM Al solution to Al-free solution at 12 h, RRG during recovery treatment for 12 h was 75%, while that in the root left in 40 µM Al solution was 20%. In another experiment with the sample pretreated with 100 µM Al, there was almost no recovery after transfer to Al-free solution for another 12 h. As a model system for recovery, pea seedlings were pretreated with 40 µM Al for 12 h followed by transfer to Al-free solution for 12 h throughout this work.
Growing Region of the Root during Recovery from Al Injury
The roots of pea seedlings were marked with India ink at 5 mm intervals from the root tip and treated with 40 µM Al in different ways. At the end of each treatment, position of the India ink mark and elongation rates of both control roots without Al treatment and roots treated with Al followed by recovery treatment were investigated. No region other than the apical 5 mm region elongated in any sample including even recovery roots, suggesting that the re-elongation of Al-treated roots occurred in the apical 5 mm region.
Al-induced Cell Death and the Formation of Ruptures Together with Zonary Regions Stained with Evans Blue
Microscopic observation of several plant roots showed similar Al-induced morphological changes in subapical regions, that is the ruptured region and adjacent zonary region stained with Evans blue were located in the periphery of the Al-treated root. Both formation of ruptures and staining with Evans blue were intensified with increase in the duration of exposure to Al (Fig. 1). Control roots not treated with Al showed almost no Evans blue staining and the root periphery was smooth (data not shown).
Figure 1.
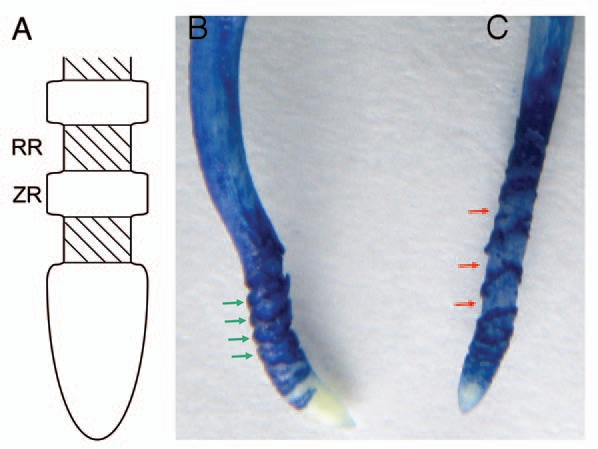
Changes in morphology of the pea root apex during and after recovery from Al treatment. (A) Schematic diagram of the root apex structure induced by Al toxicity. RR: rupture region, ZR: zonary region stained with Evans blue and other reagents.7,12 (B) Pea root treated with 40 µM Al for 24 h. (C) Pea root treated with 40 µM Al for 12 h and then in Al-free solution for 12h. Both (B and C) show the image of Evans blue staining. Green arrows indicate the zones stained markedly with Evans blue corresponding to the ZR in (A). Red arrows indicate the rupture zone corresponding to the RR in (A).
A clear difference in the structure of the subapical region (more than 1 mm from the tip) was observed between recovery roots and continuously Al-treated roots (Fig. 1B and C). In Figure 1B, the ruptures are deep but narrow between the Evans blue-stained zonary regions. Recently, Kopittke et al.8 investigated ruptures in cowpea roots exposed to Al by electron microscopy. They found no transverse rupture closer than ca. 1 mm to the root apex, which coincides with our results. Ruptures increase in width and are often several hundred micrometers in width after long exposure to Al. Ruptures are formed not by separation between intact cells but by breaking and tearing of individual cells.8 Evans blue staining of the zonary regions of the roots continuously treated with Al for 24 h was denser than that of recovery roots, suggesting that the number of seriously injured or dead cells in continuously Al-treated roots was larger than that in recovery roots. On the other hand, ruptures between the zonary regions stained with Evans blue were shallow and intervals of the zonary stained regions widened in the root after recovery from Al-induced growth inhibition (Fig. 1C). The interval regions were weakly stained by Evans blue. This suggests that these regions elongated during the recovery process.
The question arises as to whether the elongation of cells occurs only in the intervals. In other words, did the cells located under the zonary region stained with Evans blue elongate or not? It is generally thought that Al injury occurs more intensively in cells located closer to region of Al invation.13 It is possible that cells in the inner layer of the cortex and stele in the whole elongation zone could elongate during the recovery process, because those cell were not seriously injured by treatment with Al for 12 h. It is interesting that two different regions, rupture region and zonary region stained with Evans blue, are induced at the apical portion of the root as a general syndrome of Al toxicity. Assuming that the two regions behave differently in terms of their resistance and/or sensitivity to Al toxicity, two explanations for the difference can be possible. One is the finding by Ciamporova9 that two different structural changes of Zea mays L., cv. TO360 appeared within the root epidermis just behind the root cap. Within the root cortex, individual cells or cell files contain severely damaged cytoplasm in contrast to the almost undisturbed cytoplasm of adjacent cells. Their structural similarity to cells that are observed after a hypersensitive response (HP) in infected plant tissues suggests a role in accumulation of Al, in order to allow the surrounding tissue to survive the stress. However, such extremely sensitive cells appear irregularly within the root apex, which seems to be contradictory to the relatively regular distribution of rupture and zonary regions in the pea root. Other explanation is based on the proposal of Delisle et al.14 on a programmed cell death (PCD)-like idea in Triticum aestivum L.: accelerated epidermal cell turnover caused by a high level of H2O2 production through oxalate oxidase may repress a new detoxification mechanism, helping to protect deeper cell layers of the meristematic and elongation zones essential for root growth. In relation to this line, Sasaki et al.15 reported an interesting finding that outer cells of the cortex of wheat root (Triticum aestivum) accumulated lignin concomitant with decrease in cell length but increase in cell diameter at the elongation zone exposed to Al. Several researchers have suggested that rigidity of the cell wall caused by lignin deposition reduces the cell elongation under Al stress.8,10 Sasaki et al.15 also found that lignin accumulates more in the longitudinal cell wall than in the horizontal cell wall. Therefore, the distorted cell elongation due to the rigidity induced by lignin may cause tearing of cells, resulting in the formation of the rupture region. Both rupture and zonary regions are differentially stained with Evans blue, with aniline blue for callose, and with Schiff 's reagent for lipid peroxidation.7,12 Why are these zones induced by Al toxicity, with a marked changes occurring in the rupture region during the recovery process, is important question which may be explained by physiological research on Al-induced oxidative stress. These studies are is underway in our laboratory.
Acknowledgements
This research was supported by the Japanese Ministry of Education, Culture, Sports, Science and Technology (Grand-in-Aid for Scientific Research C <22580072> to H.M.).
References
- 1.Matsumoto H. Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol. 2000;200:1–46. doi: 10.1016/s0074-7696(00)00001-2. [DOI] [PubMed] [Google Scholar]
- 2.Kochian LV, Pineros MA, Hoekenga OA. The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil. 2005;274:175–195. [Google Scholar]
- 3.Matsumoto H, Sivaguru M. Advances in the aluminum toxicity and tolerance of plants for increased productivity in acid soils. In: Dubois AN, editor. Soil Contamination: New Research. New York: Nova Science Publications; 2008. pp. 1–42. [Google Scholar]
- 4.Miyasaka SC, Hue NV, Dunn MA. Aluminum. In: Barker AV, Pilbeam DJ, editors. Handbook of Plant Nutrition. New York: CRC Taylor & Francis; 2007. pp. 39–497. [Google Scholar]
- 5.Kikui S, Sasaki T, Osawa H, Matsumoto H, Yamamoto Y. Malate enhances recovery from aluminum-caused inhibition of root elongation in wheat. Plant Soil. 2007;290:1–15. [Google Scholar]
- 6.Tamás L, Huttová J, Mistrík I. Inhibition of Al-induced root elongation and enhancement of Al-induced peroxidase in Al-sensitive and Al-resistant barley cultivars are positively correlated. Plant Soil. 2003;250:193–200. [Google Scholar]
- 7.Motoda H, Kano Y, Hiragami F, Kawamura K, Matsumoto H. Morphological changes in the apex of pea roots during and after recovery from aluminium treatment. Plant Soil. 2010;333:49–58. [Google Scholar]
- 8.Kopittke PM, Blamey FPC, Menzies NW. Toxicities of soluble Al, Cu and La include ruptures to rhizodermal and root cortical cells of cowpea. Plant Soil. 2008;303:217–227. [Google Scholar]
- 9.Ciamporová M. Morphological and structural response of plant roots to aluminum at organ, tissue and cellular levels. Biologia Plantarum. 2002;45:161–171. [Google Scholar]
- 10.Jones DL, Blancaflor EB, Kochian LV, Gilroy S. Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant Cell Environ. 2006;29:1309–1318. doi: 10.1111/j.1365-3040.2006.01509.x. [DOI] [PubMed] [Google Scholar]
- 11.Ciamporová M. Diverse responses of root cell structure to aluminum stress. Plant Soil. 2000;226:113–116. [Google Scholar]
- 12.Yamamoto Y, Kobayashi Y, Matsumoto H. Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol. 2001;125:199–208. doi: 10.1104/pp.125.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagatsuma T, Ishikawa S, Obata H, Tawaraya K, Katohda S. Plasma membrane of younger and outer cells is the primary specific site for aluminum toxicity in roots. In: Date RA, et al., editors. Plant Soil Interactions at Low pH. Netherlands: Kluwer; 1995. pp. 271–278. [Google Scholar]
- 14.Delisle G, Champoux M, Houde M. Characterization of oxalate oxidase and cell death in Al-sensitive and tolerant wheat roots. Plant Cell Physiol. 2001;42:324–333. doi: 10.1093/pcp/pce041. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki M, Yamamoto Y, Matsumoto H. Lignin deposition induced by aluminum in wheat (Triticum aestivum) roots. Physiol Plant. 1996;96:193–198. [Google Scholar]


