Abstract
Insect pests that attempt to feed on the caterpillar-resistant maize genotype Mp708 encounter a potent, multipronged defense system that thwarts their invasion. First, these plants are on “constant alert” due to constitutively elevated levels of the phytohormone jasmonic acid that signals the plant to activate its defenses. The higher jasmonic acid levels trigger the expression of defense genes prior to herbivore attack so the plants are “primed” and respond with a faster and stronger defense. The second defense is the rapid accumulation of a toxic cysteine protease called Mir1-CP in the maize whorl in response to caterpillar feeding. When caterpillars ingest Mir1-CP, it damages the insect's midgut and retards their growth. In this article, we discuss a third possible defense strategy employed by Mp708. We have shown that foliar caterpillar feeding causes Mir1-CP and defense gene transcripts to accumulate in its roots. We propose that caterpillar feeding aboveground sends a signal belowground via the phloem that results in Mir1-CP accumulation in the roots. We also postulate that the roots serve as a reservoir of Mir1-CP that can be mobilized to the whorl in response to caterpillar assault.
Key words: signaling, maize, herbivory, aboveground, belowground, communication, defense, roots, foliar
Since the beginning of agriculture approximately 10,000 years ago, farmers have struggled to protect their crops from insect pests. This is particularly true for maize, an important agricultural foodstuff that is vulnerable to attack by wide variety of herbivores. Plant breeding is one strategy successfully employed to develop genotypes with improved resistance to these pests. One example is the maize inbred Mp708 that has resistance to several caterpillar species that feed in the whorls and stalks.1 Mp708 was developed from exotic germplasm that originated in Antigua by selecting for plants resistant to feeding by fall armyworm (Spodoptera frugiperda) and southwestern cornborer (Diatraea grandiosella).2,3 Research to understand the mechanism of caterpillar resistance in Mp708 has revealed that it is a multigene trait regulated by several quantitative trait loci (QTL).4,5
Among these traits, there are at least two, apparently unique, resistance characteristics. The first is that these plants are “genetically primed” to withstand caterpillar feeding due to constitutively elevated levels of jasmonic acid (JA) and defense gene transcripts.6 The second is the rapid accumulation of the insecticidal cysteine protease Mir1-CP in the whorl in response to caterpillar feeding.7–9 In addition to these two traits, we have uncovered another possible component of Mp708's resistance mechanism: aboveground to belowground defense signaling. Prior research has shown that there is communication between roots and foliage that enables plants to resist herbivory in these organs.10–19 In fact, it has been shown that belowground feeding by western corn rootworm (Diabrotica virgifera) enhances aboveground defenses to Spodoptera littoralis via hydraulic changes in the foliar physiology that reduce herbivore performance.16 However in Mp708, this signaling pathway appears to function in the opposite direction—caterpillar feeding in the whorl appears to trigger the induction of herbivore defenses in the roots. We propose that aboveground herbivory in Mp708 sends a signal to the roots that results in the belowground expression of defense genes and Mir1-CP accumulation (Fig. 1). We also speculate that the roots serve as a source of Mir1-CP that is transported to whorl during the early stages of foliar feeding. Published and preliminary evidence that supports this model is discussed below.
Figure 1.
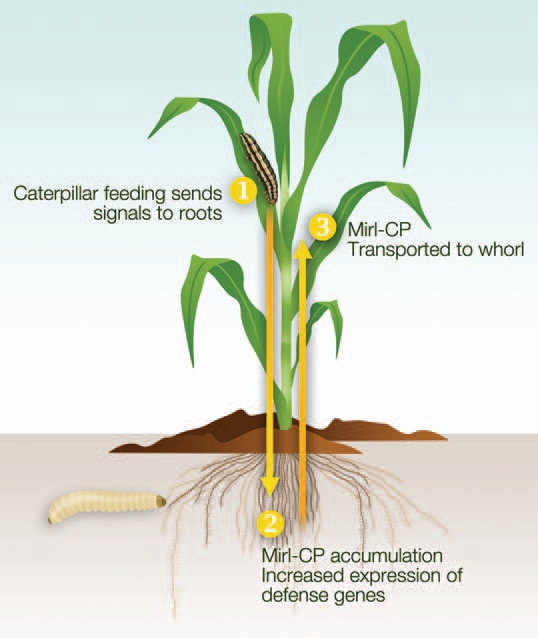
The proposed model of aboveground to belowground defense signaling in the maize inbred Mp708. Caterpillar feeding in the whorl (1) sends a currently unidentified signal through the vascular system to the roots, where Mir1-CP and possibly other defense gene products accumulate (2) and protect plants against root feeding herbivores. The roots (3) serve as a potential source of Mir1-CP that can be translocated back to the whorl in response to caterpillar feeding. Drawing by Nick Sloff.
The first suggestion that Mir1-CP could be a mobile protein came from immunolocalization studies showing its presence in the maize vascular system.20 Specifically, it is found in the thick-walled sieve elements (TSE) in the phloem of the minor leaf veins and xylem elements in the roots. After 24 hours of caterpillar feeding in the whorl, the abundance of Mir1-CP in the TSE dramatically increased. In situ hybridization indicated that mir1 transcripts were most abundant in the phloem parenchyma cells, which suggests that Mir1-CP is made in these cells and translocated to the TSE. According to Evert et al.21 there are numerous plasmadesmata connecting the TSE and phloem parenchyma cells, which could facilitate the movement of Mir1-CP into the phloem elements. We believe that transport via phloem also may account for short-distance movement of Mir1-CP, since previous results indicated that Mir1-CP could rapidly accumulate ∼1 cm from the caterpillar feeding site.9
Surprisingly, the immunolocalization analysis also indicated that Mir1-CP abundance in the root increased after foliar caterpillar feeding.20 Prior to herbivory, a small amount of Mir1-CP was detected in root vascular parenchyma adjacent to the metaxylem elements, but after feeding the amount of Mir1-CP increased and it appeared to be in the lumen of the metaxylem elements.20 These results are supported by an immunoblot (Fig. 2A), showing that Mir1-CP levels in the root tips increased after caterpillar feeding in the whorl.
Figure 2.

Immunoblot analysis of Mir1-CP accumulation in Mp708 organs. (A) Mir1-CP accumulation in the whorl after 24 hr of caterpillar feeding (W-Fed); control whorl (W-unfed); roots after 24 hr of foliar feeding (R-fed) and control roots (R-unfed). (B) Mir1-CP accumulation in the whorl of intact plants after 24 hr of caterpillar feeding (W-intact) or in the whorl of plants with the roots removed prior to caterpillar infestation (W-no roots). (C) Mir1-CP accumulation in the xylem sap of whorls infested for 0, 3 and 12 hr. (D) Lack of Mir1-CP accumulation in the xylem sap of the susceptible inbred Tx601 for 0, 3 and 12 hr. Arrow on the right indicate the position of Mir1-CP on the blots.
To test if roots could be a potential source of Mir1-CP, they were excised from Mp708 prior to caterpillar feeding. The results indicated that Mir1-CP accumulation was significantly suppressed by root removal20 (Fig. 2B). In addition, another experiment has shown the presence of Mir1-CP in xylem exudate collected from Mp708, but not the susceptible genotype Tx601, after 12 hr of foliar herbivory (Fig. 2C).
Two experimental techniques have shown that aboveground feeding influences the expression of herbivore defense genes belowground. First, microarray analysis (Ankala A, unpublished data) showed that foliar caterpillar feeding increased the expression of 135 genes in the whorl and 173 genes in the roots. The upregulated root genes encoded transcription factors, JA biosynthesis enzymes and proteins involved in direct defense such as chitinase.22 This study has been complemented by targeted analysis of specific defense gene expression in roots in response to foliar feeding using qRT-PCR. Figure 3 shows that expression of several genes believed to be involved in direct defense against herbivores was upregulated in the roots in response to caterpillar feeding in the whorl. These included transcripts for Mir1-CP, maize protease inhibitor23 and chitinase.22 Another study reported that root defense gene expression increased in response to foliar treatment with JA in the closely related C4 monocot sorghum and corroborates our preliminary findings in maize.24
Figure 3.

Relative expression of maize defense gene transcripts in maize whorl (leaf) and root tissues after 24 of caterpillar feeding in the whorl. (A) Mir1-CP (mir1) transcript levels in fed and non-fed plants. (B) Maize protease inhibitor (MPI) transcript levels in fed and non-fed plants. (C) Chitinase (Chi1) transcript levels in fed and non-fed plants. Relative transcript levels were determined by quantitative real-time PCR.
The accumulation of defense gene transcripts and proteins in the roots does not prove that there is enhanced resistance to belowground pests. Because prior reports suggested that Mp708 exhibited resistance to Southern and peanut root-knot nematodes,1,25 we examined its native resistance to the western corn rootworm. Pots containing Mp708 or the susceptible inbred Tx601 were infested with 250 rootworm larvae and after 14 days only 6 surviving rootworm larvae were recovered from Mp708 whereas 25 were found in Tx601.26 This effect was enhanced by concurrent aboveground caterpillar infestation that significantly reduced the survivorship of rootworm larvae in several of the maize genotypes.26 Furthermore, belowground feeding improved aboveground resistance to fall armyworm and supports finding of others.15
Although our model must be rigorously tested, the current data suggest that whorl to root signaling could be another component of Mp708's unique herbivore defense phenotype. Alternatively, it could be a universal mechanism used by all maize genotypes15–19 and other plant species10,11,13,14 to withstand assault by foliar and root-feeding herbivores.
Acknowledgments
The authors thank G.W. Felton for his constructive review of the manuscript and Nick Sloff, Department of Entomology, Penn. State University for the wonderful corn and insect drawing. National Science Foundation Award IOS-0641219 to D.S. Luthe funded this research.
References
- 1.Davis F, Williams WP, Mihm A, Barry BE, Overman LJ, Wiseman BR, et al. Resistance to multiple lepidopterous species in tropical derived corn germplasm. Miss Agric For Exp Stn Tech Bull. 1988;157:1–6. [Google Scholar]
- 2.Williams W, Davis FM, Windham GL. Registration of Mp708 germplasm line of maize. Crop Sci. 1990;30:757. [Google Scholar]
- 3.Williams W, Davis F. Registration of Mp704 germplasm line of maize. Crop Sci. 1982;22:1269–1270. [Google Scholar]
- 4.Brooks TD, Bushman BS, Williams WP, McMullen MD, Buckley PM. Genetic basis of resistance to fall armyworm (Lepidoptera: Noctuidae) and southwestern corn borer (Lepidoptera: Crambidae) leaf-feeding damage in maize. J Econ Entomol. 2007;100:1470–1475. doi: 10.1603/0022-0493(2007)100[1470:gbortf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Brooks TD, Willcox MC, Williams WP, Buckley PM. Quantitative trait loci conferring resistance to fall armyworm and southwestern corn borer leaf feeding damage. Crop Sci. 2005;45:2430–2434. [Google Scholar]
- 6.Shivaji R, Camas A, Ankala A, Engelberth J, Tumlinson JH, Williams WP, et al. Plants on constant alert: Elevated levels of jasmonic acid and jasmonate-induced transcripts in caterpillar-resistant maize. J Chem Ecol. 2010;36:179–191. doi: 10.1007/s10886-010-9752-z. [DOI] [PubMed] [Google Scholar]
- 7.Mohan S, Ma PWK, Pechan T, Bassford ER, Williams WP, Luthe DS. Degradation of the S. frugiperda peritrophic matrix by an inducible maize cysteine protease. J Insect Physiol. 2006;52:21–28. doi: 10.1016/j.jinsphys.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Pechan T, Cohen A, Williams WP, Luthe DS. Insect feeding mobilizes a unique plant defense protease that disrupts the peritrophic matrix of caterpillars. Proc Natl Acad Sci USA. 2002;99:13319–13323. doi: 10.1073/pnas.202224899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pechan T, Ye LJ, Chang YM, Mitra A, Lin L, Davis FM, et al. A unique 33 kD cysteine proteinase accumulates in response to larval feeding in maize genotypes resistant to fall armyworm and other lepidoptera. Plant Cell. 2000;12:1031–1040. doi: 10.1105/tpc.12.7.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bezemer TM, van Dam NM. Linking aboveground and belowground interactions via induced plant defenses. Trends Ecol Evol. 2005;20:617–624. doi: 10.1016/j.tree.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Dodd IC. Root-to-shoot signalling: Assessing the roles of ‘up’ in the up and down world of long-distance signalling in planta. Plant Soil. 2005;274:251–270. [Google Scholar]
- 12.Orians C. Herbivores, vascular pathways and systemic induction: facts and artifacts. J Chem Ecol. 2005;31:2231–2242. doi: 10.1007/s10886-005-7099-7. [DOI] [PubMed] [Google Scholar]
- 13.Rasmann S, Agrawal AA. In defense of roots: A research agenda for studying plant resistance to belowground herbivory. Plant Physiol. 2008;146:875–880. doi: 10.1104/pp.107.112045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Putten WH, Bardgett RD, de Ruiter PC, Hol WH, Meyer KM, Bezemer TM, et al. Empirical and theoretical challenges in aboveground-belowground ecology. Oecologia. 2009;161:1–14. doi: 10.1007/s00442-009-1351-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erb M, Flors V, Karlen D, de Lange E, Planchamp C, D'Alessandro M, et al. Signal signature of aboveground-induced resistance upon belowground herbivory in maize. Plant J. 2009;59:292–302. doi: 10.1111/j.1365-313X.2009.03868.x. [DOI] [PubMed] [Google Scholar]
- 16.Erb M, Kollner TG, Degenhardt J, Zwahlen C, Hibbard BE, Turlings TC. The role of abscisic acid and water stress in root herbivore-induced leaf resistance. New Phytol. 2011;189:308–320. doi: 10.1111/j.1469-8137.2010.03450.x. [DOI] [PubMed] [Google Scholar]
- 17.Erb M, Lenk C, Degenhardt J, Turlings TCJ. The underestimated role of roots in defense against leaf attackers. Trends Plant Sci. 2009;14:653–659. doi: 10.1016/j.tplants.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Erb M, Ton J, Degenhardt J, Turlings TC. Interactions between arthropod-induced aboveground and belowground defenses in plants. Plant Physiol. 2008;146:867–874. doi: 10.1104/pp.107.112169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erb M, Gordon-Weeks R, Flors V, Camanes G, Turlings TC, Ton J. Belowground ABA boosts aboveground production of DIMBOA and primes induction of chlorogenic acid in maize. Plant Signal Behav. 2009;4:636–638. doi: 10.4161/psb.4.7.8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez L, Camas A, Shivaji R, Ankala A, Williams P, Luthe D. Mir1-CP, a novel defense cysteine protease accumulates in maize vascular tissues in response to herbivory. Planta. 2007;226:517–527. doi: 10.1007/s00425-007-0501-7. [DOI] [PubMed] [Google Scholar]
- 21.Evert RF, Eschrich W, Heyser W. Leaf structure in relation to solute transport and phloem loading in Zea mays L. Planta. 1978;138:279–294. doi: 10.1007/BF00386823. [DOI] [PubMed] [Google Scholar]
- 22.Rakwal R, Yang G, Komatsu S. Chitinase induced by jasmonic acid, methyl jasmonate, ethylene and protein phosphatase inhibitors in rice. Mol Biol Rep. 2004;31:113–119. doi: 10.1023/b:mole.0000031407.18708.95. [DOI] [PubMed] [Google Scholar]
- 23.Cordero MJ, Raventos D, San Segundo B. Expression of a maize proteinase-inhibitor gene is induced in response to wounding and fungal Infection—Systemic wound-response of a monocot gene. Plant J. 1994;6:141–150. doi: 10.1046/j.1365-313x.1994.6020141.x. [DOI] [PubMed] [Google Scholar]
- 24.Salzman RA, Brady JA, Finlayson SA, Buchanan CD, Summer EJ, Sun F, et al. Transcriptional profiling of sorghum induced by methyl jasmonate, salicylic acid and aminocyclopropane carboxylic acid reveals cooperative regulation and novel gene responses. Plant Physiol. 2005;138:352–368. doi: 10.1104/pp.104.058206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams W, Buckley PM, Davis FM. Combining ability for resistance in corn to fall armyworm and southwestern corn borer. Crop Sci. 1989;29:913–915. [Google Scholar]
- 26.Gill TA, Sandoya G, Williams WP, Luthe DS. Belowground resistance to western corn rootworm in Lepidopteran-resistant maize genotypes. J Econ Entomol. 2010;104:299–230. doi: 10.1603/ec10117. [DOI] [PubMed] [Google Scholar]


