Abstract
We recently tested that plant response to cold stress includes a rapid formation of nitric oxide (NO) that participates in the control of cold-responsive gene expression. Unexpectedly we also shed light on a novel downstream element of NO signaling that is phosphosphingolipid (PS) metabolism. Indeed, two phosphosphingolipid species, phytosphingosine phosphate (PHS-P) and a ceramide phosphate (Cer-P) are specifically synthesized upon cold exposure. Manipulating NO levels by pharmacological or genetic means dramatically modified the cold-triggered synthesis of PHS-P and Cer-P, but did not affect the cold-responsive formation of phosphatidic acid (PtdOH), a ubiquitous lipid signal derived from phospholipid degradation. So far no crosstalk between NO and PS signaling had been reported in plants. How NO might modulate PS formation and whether this regulation might be extended to other physiological processes are further discussed.
Key words: nitric oxide, sphingolipid, long chain bases, ceramides, lipid phosphorylation, lipid signaling, cold stress
Nitric Oxide (NO) is a pleiotropic signaling molecule involved in the regulation of plant development and response to abiotic and biotic stresses.1 A set of recent publications brought interactions between NO and lipid signaling out in the open in physiological responses such as elicitor responses, adventious root formation and stomatal movements.2–7 From these studies it is now clear that NO is a potent regulator of phosphatidic acid (PtdOH) signaling in plants. PtdOH is transiently synthesized from membrane phospholipids upon plant stimulation, either by the sole activity of phospholipase D (PLD) or by the sequential action of phospholipase C (PLC) and diacylglycerol kinase (DGK). Depending on the physiological context NO triggers PtdOH production by enhancing PLD, PLC/DGK or both activities.2–5 Not only PtdOH formation is stimulated by NO. Indeed, the synthesis of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate, two PtdOH precursors that act as signaling molecules in plants, is increased in NO-treated plants. Conversely, PtdOH production is required for NO formation stimulated by extracellular ATP.7 This ability for lipid signals to induce NO production has also been observed in alkamide-treated Arabidopsis roots and in Taxus yunnanensis cells treated with fungal cerebrosides.8,9
Our recent experiments add to the complexity of NO-lipid signaling interplay. As evidenced, Arabidopsis exposure to chilling led to the synthesis of PtdOH and NO, but also to the transient formation of two phosphosphingolipid species, i.e., the long chain base (LCB) phytosphingosine phosphate (PHS-P) and a ceramide phosphate (Cer-P). Unexpectedly, whereas PtdOH synthesis was not affected by modulating NO concentration, the formation of PHS-P and Cer-P was dramatically modified depending on NO levels. Indeed, decreasing NO by pharmacological treatments or by genetic tools led to an over-accumulation of PHS-P and Cer-P whereas enhancing NO level strongly impaired sphingolipid phosphorylation. In this context, NO appears as a negative regulator of sphingolipid signaling and may participate in the fine-tuning of phospho- versus unphosphorylated sphingolipid ratio. This aspect is crucial when considering that this ratio orientates mammalian cells towards proliferation or death.10 Our data therefore expend the crosstalk between NO and lipid signaling to sphingolipids, through a control of sphingolipid phosphorylation unreported in plants so far.
As for PtdOH synthesis, the mechanisms by which NO regulates sphingolipid phosphorylation is currently unknown. In this view, a crucial step forward will be to investigate how NO might inhibit phosphosphingolipid formation in chilled plants. A simple model would imply a direct regulation of key enzymes of the phosphosphingolipid pathway. The metabolism of phosphorylated sphingolipids relies on the activity of specific kinases and phosphatases (Fig. 1). In Arabidopsis thaliana, three LCB kinases have been identified: SPHK1, SPHK2 and LCBK1.11,12 In contrast, a single Cer kinase, ACD5, has been characterized.13 Less is known about sphingolipid dephosphorylation. A LCB phosphate phosphatase designated SPPASE has been recently isolated.11 Finally it has been proposed that Cer dephosphorylation would imply unspecific lipid phosphatases. It is also noteworthy that LCB-P can get recycled through the activity of the LCB-P lyase DPL1, which degrades LCB-P into long chain aldehydes and ethanolamine phosphate.14 A direct effect of NO on sphingolipid phosphorylation would therefore imply the inhibition or activation of sphingolipid kinase or phosphatase activities by direct NO-based post-translational modifications (PTM). As reported for a set of plant proteins, the main NO-based PTM are Cysteine nitrosylation, Tyrosine nitration and metal nitrosylation.15 As sphingolipid kinases/phosphatases are not metal-dependent enzymes, only the two first regulatory mechanisms are conceivable. Two lines of evidence suggest that such PTM might occur. Firstly DPL1 has been isolated in a screen for Tyr nitrated proteins.16 Secondly ACD5 presents two sequence motifs meeting the requirements for protein S-nitrosylation.17 Whether these modifications effectively occur and regulate enzyme activity has to be elucidated. Even more important would be the demonstration that such modification effectively occurs in planta and participates in protein regulation in cold stressed plants. Nevertheless, it would constitute the first direct evidence for sphingolipid kinase/phosphatase regulation by NO-based PTM.
Figure 1.
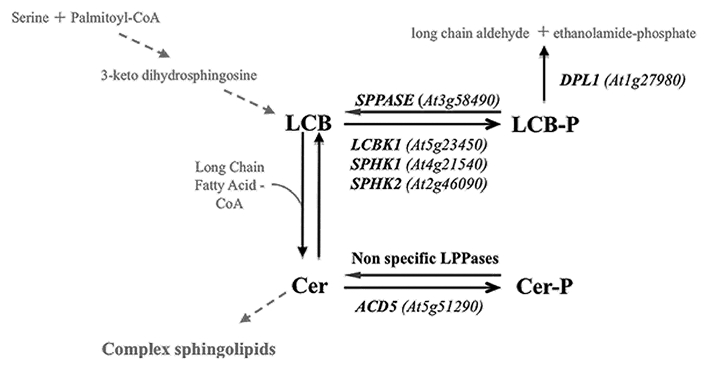
Metabolic pathways for LCB and Cer phosphorylation, dephosphorylation and degradation. ID for Arabidopsis genes encoding the characterized enzymes of the phosphosphingolipid metabolism are indicated.
Alternatively or in parallel to a direct effect on sphingolipid metabolism, NO might affect LCB/LCB-P and Cer/Cer-P ratio indirectly, through the regulation of additional signaling pathways. The multiplicity of the signaling elements acting downstream of NO, together with the lack of data on sphingolipid kinase/phosphatase regulation, make it difficult to designate likely candidates a priori.1,15 A recent report on the indirect regulation of mammalian sphingosine kinase 1 (SK1) by NO brings some hints into possible regulatory mechanisms.18 In this study, NO stimulated SK1 activity, but also enhanced SK1 transcript levels. The regulation of gene transcription and protein activity appeared independent of the cGMP pathway (the major NO-activated signaling pathway in animals). On the other hand, it required the activity of a MAP kinase cascade. So far the activation of MAP kinase cascades by NO in plants remains elusive.19 In response to cold stress two MAP kinases i.e., AtMPK4 and AtMPK6 get transiently activated in Arabidopsis and could therefore constitute intermediates in NO-mediated sphingolipid regulation.20 It would therefore be interesting to evaluate if NO-dependent sphingolipid regulation still occurs in mpk4 and mpk6 plant mutants.
Increasing evidence point out the involvement of NO and sphingolipids in numerous aspects of plant development and response to environmental cues.1,15 For instance, NO and LCB have been reported as key elements of the signaling cascades leading to stomatal movements.21 Similarly, Cer-P and NO are required for plant resistance towards pathogens.13,22 It is therefore tempting to anticipate that interactions between NO and sphingolipid signaling might not be restricted to cold stress response. Future investigations in a range of physiological contexts and organisms will help evaluate how universal such regulation may be.
References
- 1.Besson-Bard A, Pugin A, Wendehenne D. New insights into nitric oxide signaling in plants. Annu Rev Plant Biol. 2008;59:21–39. doi: 10.1146/annurev.arplant.59.032607.092830. [DOI] [PubMed] [Google Scholar]
- 2.Laxalt AM, Raho N, Have AT, Lamattina L. Nitric oxide is critical for inducing phosphatidic acid accumulation in xylanase-elicited tomato cells. J Biol Chem. 2007;282:21160–21168. doi: 10.1074/jbc.M701212200. [DOI] [PubMed] [Google Scholar]
- 3.Distefano AM, Garcia-Mata C, Lamattina L, Laxalt AM. Nitric oxide-induced phosphatidic acid accumulation: a role for phospholipase C and D in stomatal closure. Plant Cell Environm. 2008;31:187–194. doi: 10.1111/j.1365-3040.2007.01756.x. [DOI] [PubMed] [Google Scholar]
- 4.Lanteri ML, Laxalt AM, Lamatinna L. Nitric oxide triggers phosphatidic acid accumulation via phospholipase D during auxin-induced adventious root formation in cucumber. Plant Physiol. 2008;147:188–198. doi: 10.1104/pp.107.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raho N, Ramirez L, Lanteri ML, Gonorazky G, Lamattina L, Ten Have A, et al. Phosphatidic acid production in chitosan-elicited tomato cells via both phospholipase D and phospholipase C/diacylglycerol kinase requires nitric oxide. J Plant Physiol. 2011;168:534–539. doi: 10.1016/j.jplph.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Zhu H, Zhang Q, Li M, Yan M, Wang R, et al. Phospholipase dalpha1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell. 2009;21:2357–2377. doi: 10.1105/tpc.108.062992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sueldo DJ, Foresi NP, Casalongue CA, Lamattina L, Laxalt AM. Phosphatidic acid is required for extracellular ATP-mediated nitric oxide production in suspension-cultured tomato cells. New Phytol. 2010;185:909–916. doi: 10.1111/j.1469-8137.2009.03165.x. [DOI] [PubMed] [Google Scholar]
- 8.Mendez-Bravo A, Raya-Gonzalez J, Herrera-Estrella L, Lopez-Bucio J. Nitric oxide is involved in alkamide-induced leateral root development in Arabidopsis. Plant Cell Physiol. 2010;51:1612–1626. doi: 10.1093/pcp/pcq117. [DOI] [PubMed] [Google Scholar]
- 9.Wang JW, Zheng LP, Tan RX. Involvement of nitric oxide in cerebroside-induced defense responses and taxol production in Taxus yunnanensis suspension cells. Appl Microbiol Biotechnol. 2007;75:1183–1190. doi: 10.1007/s00253-007-0927-7. [DOI] [PubMed] [Google Scholar]
- 10.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758:2016–2026. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Worrall D, Liang YK, Alvarez S, Holroyd GH, Spiegel S, Panagopulos M, et al. Involvement of sphingosine kinase in plant cell signaling. Plant J. 2008;56:64–72. doi: 10.1111/j.1365-313X.2008.03579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai H, Nishiura H. Phosphorylation of sphingoid long-chain bases in Arabidopsis: functional characterization and expression of the first sphingoid longchain base kinase gene in plants. Plant Cell Physiol. 2005;46:375–380. doi: 10.1093/pcp/pci023. [DOI] [PubMed] [Google Scholar]
- 13.Liang H, Yao N, Song JT, Luo S, Lu H, Greenberg JT. Ceramides modulate programmed cell death in plants. Genes Dev. 2003;17:2636–2641. doi: 10.1101/gad.1140503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishikawa M, Hosokawa K, Ishiguro M, Minamioka H, Tamura K, Hara-Nishimura I, et al. Degradation of sphingoid long-chain base 1-phosphate (LCB-1Ps): functional characterization and expression of AtDPL1 encoding LCB-1P lyase involved in the dehydration stress response in Arabidopsis. Plant Cell Physiol. 2008;49:1158–1163. doi: 10.1093/pcp/pcn149. [DOI] [PubMed] [Google Scholar]
- 15.Baudouin E. The language of nitric oxide signaling. Plant Biol. 2010;13:233–242. doi: 10.1111/j.1438-8677.2010.00403. [DOI] [PubMed] [Google Scholar]
- 16.Zhan X, Desiderio DM. Nitroproteins from a human pituitary adenoma tissue discovered with a nitrotyrosine affinity column and tandem mass spectrometry. Anal Biochem. 2006;354:279–289. doi: 10.1016/j.ab.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 17.Lindermayr C, Saalbach G, Bahnweg G, Durner J. Differential inhibition of Arabidopsis methionine adenosyltransferases by protein S-nitrosylation. J Biol Chem. 2006;281:4285–4291. doi: 10.1074/jbc.M511635200. [DOI] [PubMed] [Google Scholar]
- 18.Schwalm S, Pfeilshifter J, Huwiler A. Sphingosine kinase 1 is critically involved in nitric oxide-mediated human endothelial cell migration and tube formation. Br J Pharmacol. 2010;160:1641–1651. doi: 10.1111/j.1476-5381.2010.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar D, Klessig DF. Differential induction of tobacco MAP kinases by the defense signals nitric oxide, salicylic acid, ethylene and jasmonic acid. Mol Plant Microbe Interact. 2000;13:347–351. doi: 10.1094/MPMI.2000.13.3.347. [DOI] [PubMed] [Google Scholar]
- 20.Ichimura K, Mizoguchi T, Yoshidaz R, Yuasa T, Shinozaki K. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant J. 2000;24:655–665. doi: 10.1046/j.1365-313x.2000.00913.x. [DOI] [PubMed] [Google Scholar]
- 21.Acharya BR, Assmann SM. Hormone interactions in stomatal function. Plant Mol Biol. 2009;69:451–462. doi: 10.1007/s11103-008-9427-0. [DOI] [PubMed] [Google Scholar]
- 22.Delledonne M, Xia Y, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]


