Abstract
Sieve element occlusion (SEO) genes encoding forisome subunits have been identified in Medicago truncatula and other legumes. Forisomes are structural phloem proteins uniquely found in Fabaceae sieve elements. They undergo a reversible conformational change after wounding, from a condensed to a dispersed state, thereby blocking sieve tube translocation and preventing the loss of photoassimilates. Recently, we identified SEO genes in several non-Fabaceae plants (lacking forisomes) and concluded that they most probably encode conventional non-forisome P-proteins. Molecular and phylogenetic analysis of the SEO gene family has identified domains that are characteristic for SEO proteins. Here, we extended our phylogenetic analysis by including additional SEO genes from several diverse species based on recently published genomic data. Our results strengthen the original assumption that SEO genes seem to be widespread in dicotyledonous angiosperms, and further underline the divergent evolution of SEO genes within the Fabaceae.
Key words: forisome, P-protein, sieve element occlusion, phloem, wound sealing, gene family, Fabacea
SEO Genes in Fabaceae and Non-Fabaceae Plants
Structural P-proteins involved in sieve tube sealing after wounding are characteristic components of all dicotyledonous and some monocotyledonous angiosperms.1,2 Comprehensive molecular studies with forisomes, a special type of structural P-proteins with unique characteristics,3,4 led to the identification of SEO (sieve element occlusion) genes,5,6 initially thought to be restricted to the Fabaceae. However, we recently reported the identification of numerous SEO genes from non-Fabaceae plants and proposed that they might encode conventional P-proteins, which are widely distributed among angiosperms and ultrastructurally and functionally similar to forisomes.7
Members of the SEO gene family share several characteristics: they are predominantly expressed in phloem tissue, probably restricted to the sieve elements (based on the data available so far),5,7,8 and have a conserved intron-exon structure. The deduced proteins all feature three characteristic domains, namely the SEO N-terminal domain (SEO-NTD), a potential thioredoxin fold and the SEO C-terminal domain (SEO-CTD), which specify proteins as family members when present in combination. Phylogenetic analysis has shown that SEO proteins cluster in different subgroups, three of them presumably specific to the Fabaceae and comprising the SEO-F genes which encode forisome subunits.7,9
Classification of New SEO Gene Family Members
To gain further insight into the evolution of the SEO gene family, we extended our analysis to include diverse additional species with recently published genome data. We were able to identify SEO proteins in Cucumis sativus (Cucurbitaceae), Prunus persica (Rosaceae), Manihot esculenta (Euphorbiaceae), Ricinus communis (Euphorbiaceae), Populus trichocarpa (Saliaceae), Arabidopsis lyrata (Brassicaceae), Carica papaya (Caricaceae), Solanum lycopersicum (Solanaceae), Mimulus guttatus (Phrymaceae) and Aquilegia coerulea (Ranunculaceae) by performing BLASTP searches and applying the domain-based assignment criteria described above using Hidden Markov Models (Table 1). Since SEO genes were identified in the family Ranunculaceae, the basal-most eudicot clade,10 the SEO family appears to have evolved prior to or congruent with the appearance of dicotyledonous plants. This strengthens our assumption that SEO genes are widespread in dicotyledonous angiosperms.
Table 1.
Newly identified SEO genes in plants with recently published genome data
| Species | SEO gene accesions in genome project | Genome reference |
| Cucumis sativus | Cucsa.156170, Cucsa.196320, Cucsa.383870, Cucsa.383880, Cucsa.383910, Cucsa.386570, Cucsa.386580, Cucsa.386590, Cucsa.386600, Cucsa.394600 | Huang, et al. 200912 |
| Prunus persica | ppa002624m, ppa002363m, ppa002391m, ppa015869m, ppa026573m, ppa023840m, ppa023699m, ppa002007m, ppa001579m, ppa026484m, ppb018052m, ppa021991m | International Peach Genome Initiative; v1.0 |
| Manihot esculenta | cassava12436, cassava12437, cassava29192, cassava32300, cassava35774, cassava3695, cassava911, cassava912 | Cassava Genome Project 2009; gene set 1.1 |
| Ricinus communis | 29633.m000920, 29633.m000921, 29633.m000922, 29633.m000923, 29633.m000925, 29633.m000926, 29633.m000927, 29929.m004788, 29929.m004789 | Chan, et al. 2010; v0.113 |
| Populus trichocarpa | POPTR_0001s35850, POPTR_0001s35860, POPTR_0001s35870, POPTR_0001s35880, POPTR_0001s35900, POPTR_0008s18360, POPTR_0010s06000, POPTR_0010s06010, POPTR_0010s06030, POPTR_0010s06050, POPTR_0010s06060, POPTR_0012s09220, POPTR_0017s10720 | Tuskan, et al. 2006; v2.014 |
| Arabidopsis lyrata | 315794, 477347, 477348 | http://genome.jgi-psf.org/Araly1/Araly1.info.html; v.1.0 |
| Carica papaya | evm.model.supercontig_130.60, evm.model.supercontig_33.71, evm.model.supercontig_33.72, evm.model.supercontig_4.9 | Ming, et al. 2008.15 |
| Solanum lycopersicum | SL1.00sc03823_12.1.1, SL1.00sc03823_13.1.1, SL1.00sc00633_146.1.1, SL1.00sc00463_191.1.1, SL1.00sc00463_192.1.1, SL1.00sc00463_193.1.1 | The International Tomato Genome Sequencing Consortium; ITAG1 |
| Mimulus guttatus | mgf003278m, mgf013341m, mgf016796m | Mimulus Genome Project, DoE Joint Genome Institute; v1.0 |
| Aquilegia coerulea | AcoGoldSmith_v1.017183m, AcoGoldSmith_v1.019931m, AcoGoldSmith_v1.001517m, AcoGoldSmith_v1.025934m, AcoGoldSmith_v1.001960m | US Department of Energy Joint Genome Institute |
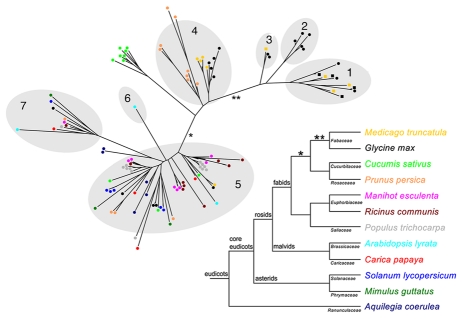
The phylogenetic relationship between the newly identified SEO proteins with known SEO and SEO-F proteins from Glycine max and M. truncatula was analyzed by creating a new phylogenetic tree and clustering the proteins into the established subgroups of the SEO family using OrthoMCL7,11 (Fig. 1). Most of the new SEO proteins cluster in these subgroups although several proteins from different species exhibit a higher number of substitutions but could be shown to originate from the corresponding subgroup, as is clear from the confident tree topology.
Figure 1.
Phylogenetic tree of SEO proteins from different plants and the corresponding species tree. The phylogenetic tree was constructed using FastTree2,16 on a T-Coffee17 alignment of the SEO protein sequences. Branch lengths are proportional to the number of amino acid substitutions. The shaded parts of the tree represent the subgroups previously identified with OrthoMCL.7,11 SEO proteins are represented as colored dots (color indicates the species). SEO-F proteins that have been confirmed as forisome components are shown as colored squares. The species tree, including all analyzed plants, was prepared according to the Angiosperm Phylogeny Group.10
In agreement with our recent study,7 newly identified SEO proteins from all the analyzed species cluster in the most representative subgroup 5, which strengthens the assumption that the widely-distributed SEO gene family originated from similar ancestral SEO genes. The composition of subgroup 4 is worthy of special attention. As reported in the original study,7 this subgroup contains proteins of the Fabaceae species G. max and M. truncatula as well as two SEO proteins from Malus domestica, a member of the closely-related family Rosaceae. This exclusivity is confirmed in the updated tree since only SEO proteins from the Rosaceae species P. persica and the Cucurbitaceae species C. sativus cluster together with or close to the previously described Fabaceae SEO proteins of this subgroup. Thus, a duplication event probably occurred prior to the divergence of these plant families (* in Fig. 1). Another distinctive feature is the relatively large branch length towards subgroup 4 (*), reflecting a large number of substitutions, which indicates the accelerated evolution of these genes.
Despite the inclusion of numerous new SEO proteins, subgroups 1, 2 and 3 remained Fabaceae-specific and therefore confirm the special status of Fabaceae SEO and SEO-F genes clustering in these subgroups. The reconstructed phylogenetic tree (Fig. 1) indicates accelerated evolution (**) in the Fabaceae resulting in subgroups containing SEO-F genes, which encode forisome subunits. Forisomes are thought to be unique to Fabaceae plants, which agrees with the phylogenetic analysis of the SEO family presented here. However, not all SEO proteins belonging to subgroups 1, 2 and 3 necessarily encode forisome components. Regarding the hypothesis that SEO genes also encode conventional structural P-proteins in Fabaceae and non-Fabaceae plants, further molecular studies concerning the function of both forisomes and conventional P-proteins are required to enhance our understanding of the unique properties of forisomes.
Acknowledgements
We thank Hannes Luz for helpful discussion and the Phytozome Team for their auxiliary website. This work was supported by grants of the VolkswagenStiftung and the Fraunhofer Vintage Class program.
References
- 1.Cronshaw J. Phloem structure and function. Annu Rev Plant Physiol. 1981;32:465–484. [Google Scholar]
- 2.Knoblauch M, van Bel AJE. Sieve Tubes in Action. Plant Cell. 1998;10:35–50. [Google Scholar]
- 3.Knoblauch M, Peters W, Ehlers K, van Bel AJE. Reversible calcium-regulated stopcocks in legume sieve tubes. Plant Cell. 2001:131221–131230. doi: 10.1105/tpc.13.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knoblauch M, Noll G, Müller T, Prüfer D, Schneider-Hüther I, Scharner D, et al. ATP-independent contractile proteins from plants. Nat Mater. 2003;2:600–603. doi: 10.1038/nmat960. [DOI] [PubMed] [Google Scholar]
- 5.Noll G, Fontanellaz M, Rüping B, Ashoub A, van Bel AJE, Fischer R, et al. Spatial and temporal regulation of the forisome gene for1 in the phloem during plant development. Plant Mol Biol. 2007;65:285–294. doi: 10.1007/s11103-007-9217-0. [DOI] [PubMed] [Google Scholar]
- 6.Pélissier HC, Peters WS, Collier R, van Bel AJE, Knoblauch M. GFP Tagging of Sieve Element Occlusion (SEO) Proteins Results in Green Fluorescent Forisomes. Plant Cell Physiol. 2008;49:1699–1710. doi: 10.1093/pcp/pcn141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rüping B, Ernst AM, Jekat SB, Nordzieke S, Reineke AR, Müller B, et al. Molecular and phylogenetic characterization of the sieve element occlusion gene family in Fabaceae and non-Fabaceae plants. BMC Plant Biol. 2010;10:219. doi: 10.1186/1471-2229-10-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noll GA, Rüping B, Ernst AM, Bucsenez M, Twyman RM, Fischer R, et al. The promoters of forisome genes MtSEO2 and MtSEO3 direct gene expression to immature sieve elements in Medicago truncatula and Nicotiana tabacum. Plant Mol Biol Rep. 2009;27:526–533. [Google Scholar]
- 9.Müller B, Noll GA, Ernst AM, Rüping B, Groscurth S, Twyman RM, et al. Recombinant artificial forisomes provide ample quantities of smart biomaterials for use in technical devices. Appl Microbiol Biotechnol. 2010;88:689–698. doi: 10.1007/s00253-010-2771-4. [DOI] [PubMed] [Google Scholar]
- 10.APG III, author. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc. 2009:161105–161121. [Google Scholar]
- 11.Li L, Stoeckert CJ, Roos DS. OrthoMCL: Identification of ortholog groups for eukaryotic genomes. Genome Res. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang S, Li R, Zhang Z, Li L, Gu X, Fan W, et al. The genome of the cucumber, Cucumis sativus L. Nat Genet. 2009;41:1275–1281. doi: 10.1038/ng.475. [DOI] [PubMed] [Google Scholar]
- 13.Chan AP, Crabtree J, Zhao Q, Lorenzi H, Orvis J, Puiu D, et al. Draft genome sequence of the oilseed species Ricinus communis. Nat Biotechnol. 2010;28:951–956. doi: 10.1038/nbt.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuskan G, DiFazio S, Jansson S, Bohlmann J, Grigoriev I, Hellsten U, et al. The Genome of Black Cottonwood, Populus trichocarpa (Torr & Gray) Science. 2006;313:1596–1604. doi: 10.1126/science.1128691. [DOI] [PubMed] [Google Scholar]
- 15.Ming R, Hou S, Feng Y, Yu Q, Dionne-Laporte A, Saw J, et al. The draft genome of the transgenic tropical fruit tree papaya (Carica papaya Linnaeus) Nature. 2008;452:991–996. doi: 10.1038/nature06856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Price M, Dehal P, Arkin A. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS ONE. 2010;5:9490. doi: 10.1371/journal.pone.0009490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Notredame C, Higgins DG, Heringa J. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J Mol Biol. 2000;302:205. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]