Abstract
The membrane protein ETHYLENE INSENSITIVE2 (EIN2), which is supposed to act between the soluble serine/threonine kinase CTR1 and the EIN3/EIL family of transcription factors, is a central and most critical element of the ethylene signaling pathway in Arabidopsis. In a recent study, we have identified that EIN2 interacts tightly with all members of the Arabidopsis ethylene receptor family— proteins that mark the starting point of the signaling pathway. Our studies show consistently that the kinase domain of the receptors is essential for the formation of the EIN2-receptor complex. Furthermore, mutational analysis demonstrates that phosphorylation is a key mechanism in controlling the interaction of EIN2 and the ethylene receptors. Interaction studies in the presence of the ethylene agonist cyanide revealed a causal link between hormone binding and complex formation. In the presence of the plant hormone agonist the auto-kinase activity of the receptors is inhibited and the non-phosphorylated kinase domain of the receptors binds tightly to the carboxyl-terminal domain of EIN2. In the absence of cyanide inhibition of the auto-kinase activity is relieved and complex formation with the phosphorylated kinase domain of the receptors is reduced. Our data suggest a novel model on the integration of EIN2 in the ethylene signaling pathway.
Key words: plant hormone signaling, ethylene receptor family, ETR1, EIN2, protein phosphorylation, protein-protein interaction, signal transduction
The plant hormone ethylene regulates a diverse and complex range of developmental processes in higher plants and is also involved in mediating responses to various abiotic and biotic stresses.1,2 Reverse genetics have identified receptors and downstream components involved in ethylene signaling.3,4 In these studies EIN2, an integral membrane protein of the ER network has been recognized as the central component of the signaling pathway.5 Sequence analysis suggests that EIN2 consists of a membrane-intrinsic amino-terminal domain (aa 1–461) and a membrane-extrinsic carboxyl-terminal domain (aa 462–1,294). Expression of the carboxyl-terminal domain on its own in an ein2 loss-of-function background is sufficient to constitutively activate ethylene responses suggesting that the membrane extrinsic part of EIN2 is vital for ethylene-induced gene expression.5 Based on epistasis analysis, EIN2 is thought to operate downstream of the ethylene receptor family, and the soluble protein kinase CTR1,6 but upstream of the EIN3/EIL transcription factor family. Still the mechanism by which the signal is transferred from the ethylene receptors—a family consisting of five members in Arabidopsis thaliana named ETR1, ETR2, ERS1, ERS2 and EIN4—to EIN2 has not been resolved yet. Recent in vivo and in vitro fluorescence studies from our lab have shown that EIN2 interacts at the ER membrane with the ethylene receptor protein ETR1.7
To clarify whether other members of the ethylene receptor family also form complexes with the membrane-extrinsic domain of EIN2, we have analyzed complex formation of EIN2 with ERS1, ETR2, ERS2 and EIN4 by Fluorescence Resonance Energy Transfer (FRET). Transfer efficiencies obtained from Nicotiana benthamina cells co-expressing EIN2-GFP and fluorescent fusion proteins of the different receptor isoforms verified that EIN2 indeed associates with all members of the Arabidopsis ethylene receptor family.8 Moreover, the observed interaction of EIN2 with ERS1 and ERS2—receptor proteins lacking the carboxyl-terminal response regulator domain—suggests that the kinase domain of the receptors is involved in complex formation. This conclusion is further supported by in vitro fluorescence studies using purified recombinant ETR1 and a recombinant tryptophan-less EIN2 mutant. In these studies tryptophan fluorescence of the purified ETR1 protein was quenched upon addition of increasing concentrations of tryptophan-less EIN2. Removal of the carboxyl-terminal response regulator domain (aa 610–738) had no effect on EIN2 binding. Both forms of the receptor showed essentially the same apparent dissociation constant of the EIN2-receptor complex (Fig. 1).
Figure 1.
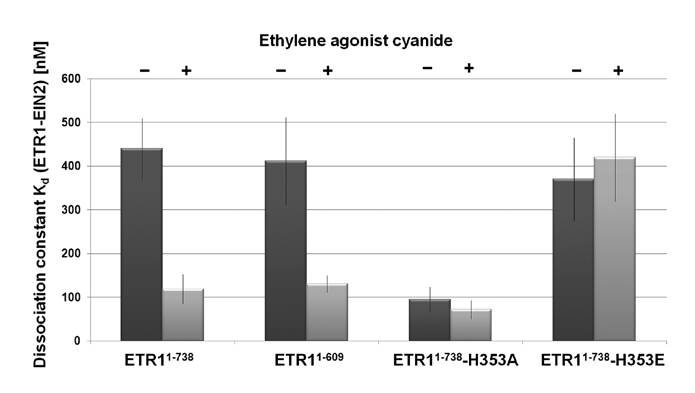
Kinase domain of ethylene receptor ETR1 mediates interaction with EIN2.
To get a more detailed picture on the role of the histidine kinase domain on the formation of the EIN2-receptor complex we employed two phosphorylation mutants of ETR1 mimicking a permanently phosphorylated or a non-phosphorylable kinase domain, respectively. The non-phosphorylable ETR1 mutant H353A where phosphorylation at the predicted histidine-353 phosphorylation site is abolished9 showed a four-fold increased affinity towards EIN2 in these studies (Kd ∼ 100 nM). In contrast substitution of the conserved histidine by a negatively charged glutamate to mimic permanent phosphorylation of the receptor had no effect on the formation of the EIN2-ETR1 complex and showed a Kd similar to ETR1 wild type (Kd ∼ 400 nM, see Fig. 1).
To correlate binding of the plant hormone with the EIN2-ETR1 interaction we added the ethylene agonist cyanide that was shown in previous studies to reduce autophosphorylation of purified recombinant ETR1 to the same level as ethylene in our assays.10 Stability of the EIN2-ETR1 complex was found to be modulated by the ethylene agonist as affinity for EIN2 is increased about four-fold with the ETR1 wild type in the presence of cyanide to almost the same level as obtained with the non-phosphorylable H353A mutant. H353A consequently showed no increased affinity for EIN2 in the presence of cyanide. Likewise, formation of the EIN2-ETR1 complex was not affected by cyanide in the H353E mutant, but showed the reduced affinity towards EIN2 obtained for the ETR1 wild type in the absence of the ethylene agonist (see Fig. 1). These data imply that the plant hormone itself controls the interaction of ETR1 and EIN2 at the ER membrane via the phosphorylation status of the receptor.
Based on our fluorescence data we propose that in the absence of the plant hormone ethylene receptor complexes remain in their phosphorylated state which forms tight interactions with the ER-associated negative regulator CTR1, but not with the ER-anchored EIN2 protein. Separated EIN2 is immediately degraded by a proteasome-dependent pathway.11 Ethylene inhibits the autokinase activity of the receptors switching them to their non-phosphorylated state which triggers inactivation of CTR1 and tight interaction of the receptors with EIN2 at the ER-membrane. Hence, addition of the plant hormone results in an accumulation of EIN2 at the ER membrane.11
A possible scenario following the tight association of EIN2 with the receptor trigged by the binding of the plant hormone and the associated inhibition of the autokinase activity of the receptor is hypothesised in Figure 2. The EIN2-ETR1 receptor complex might recruit a protease or the zymogen of a protease as found in apoptosis with the FLICE protease.12 The activated protease then triggers proteolysis of the carboxyl-terminus of EIN2 which was shown to be sufficient to rescue ethylene responses in an ein2- loss of function background.5 Analysis of the carboxyl-terminal sequence of EIN2 (aa 479–1,294) by PredictNLS13 identified two potential nuclear localization signals—[PL]KxxKRR and [PLV]K[RK] x[RK][RK][RK][PL]-at position 1,261–1,268 and strongly supports the idea that the carboxyl-terminus of EIN2 is translocated to the nucleus in order to trigger ethylene induced gene expression. Both potential NLS in EIN2 have been found in a number of eukaryotic regulatory proteins and transcription factors where nuclear localization is beyond controversy.
Figure 2.
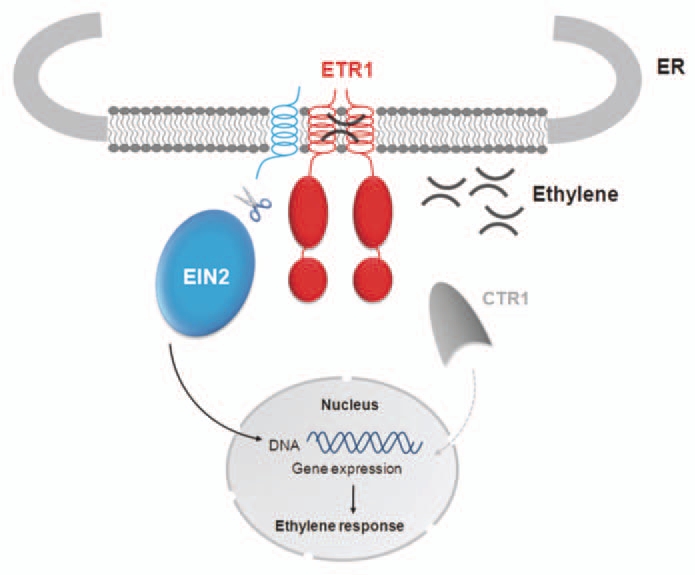
Model of complex formation at the ER membrane and downstream signal transfer in ethylene signaling.
To elaborate this hypothesis, further studies have to elucidate the role of the predicted NLS domain in EIN2 and to identify the putative cleavage site and protease involved in the processing of EIN2.
References
- 1.Kende H. Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1993;44:283–307. [Google Scholar]
- 2.Johnson PR, Ecker JR. The ethylene gas signal transduction pathway: a molecular perspective. Annu Rev Genet. 1998;32:227–254. doi: 10.1146/annurev.genet.32.1.227. [DOI] [PubMed] [Google Scholar]
- 3.Bleecker AB, Kende H. Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol. 2000;16:1–18. doi: 10.1146/annurev.cellbio.16.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Stepanova AN, Ecker JR. Ethylene signaling: from mutants to molecules. Curr Opin Plant Biol. 2000;3:353–360. doi: 10.1016/s1369-5266(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 5.Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR. EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science. 1999;284:2148–2152. doi: 10.1126/science.284.5423.2148. [DOI] [PubMed] [Google Scholar]
- 6.Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell. 1993;72:427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- 7.Bisson MM, Bleckmann A, Allekotte S, Groth G. EIN2, the central regulator of ethylene signalling, is localized at the ER membrane where it interacts with the ethylene receptor ETR1. Biochem J. 2009;424:1–6. doi: 10.1042/BJ20091102. [DOI] [PubMed] [Google Scholar]
- 8.Bisson MM, Groth G. New insight in ethylene signaling: autokinase activity of ETR1 modulates the interaction of receptors and EIN2. Mol Plant. 2010;5:882–889. doi: 10.1093/mp/ssq036. [DOI] [PubMed] [Google Scholar]
- 9.Gamble RL, Coonfield ML, Schaller GE. Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis. Proc Natl Acad Sci USA. 1998;95:7825–7829. doi: 10.1073/pnas.95.13.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voet van Vormizeele J, Groth G. Ethylene controls autophosphorylation of the histidine kinase domain in ethylene receptor ETR1. Mol Plant. 2008;1:380–387. doi: 10.1093/mp/ssn004. [DOI] [PubMed] [Google Scholar]
- 11.Qiao H, Chang KN, Yazaki J, Ecker JR. Interplay between ethylene, ETP1/ETP2 F-box proteins and degradation of EIN2 triggers ethylene responses in Arabidopsis. Genes Dev. 2009;23:512–521. doi: 10.1101/gad.1765709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 13.Cokol M, Nair R, Rost B. Finding nuclear localization signals. EMBO Rep. 2000;1:411–415. doi: 10.1093/embo-reports/kvd092. [DOI] [PMC free article] [PubMed] [Google Scholar]


