Abstract
Under Fe deficiency, strategy I (non-graminaceous) plants upregulate the expression of many Fe acquisition genes and develop morphological changes in their roots. The regulation of these responses is not completely known, but since the 1980s different results suggest a role for auxin, ethylene and, more recently, nitric oxide. The upregulation of the Fe acquisition genes does not depend solely on these hormones that would act as activators, but also on some other signals, probably phloem Fe, that would act as inhibitors. It is not known which of the hormones considered is the last activator of the Fe acquisition genes, but some results suggest that auxin acts upstream of ethylene and NO and that, perhaps, ethylene is the last activator.
Key words: auxin, ethylene, FIT, iron, nitric oxide, phloem Fe, strategy I
To acquire iron (Fe) from soils plants have developed different strategies aimed to facilitate the acquisition of this abundant but usually unavailable element. So far, two different strategies have been described: the strategy I, present in non graminaceous plants, such as dicots, and the strategy II, present in graminaceous plants.1 The present review is devoted to strategy I plants. The main characteristic of these plants is that they need to reduce Fe(III), the most abundant form of Fe found in soils, to Fe(II), prior to uptake. The Fe(III) reduction is mediated by a ferric reductase encoded by the FRO gene, and the Fe(II) uptake is mediated by a transporter encoded by the IRT1 gene.1 Both genes are upregulated under Fe deficiency and are activated by some bHLH transcription factors, which are also upregulated by Fe deficiency. Some of these bHLH transcription factors are AtFIT, AtbHLH38 and AtbHLH39 in Arabidopsis;2 SlFER is the FIT homolog in tomato.3 Besides these genes, there are other important Fe-related genes also upregulated under Fe deficiency, such as AtAHA7 and CsHA1, encoding H+-ATPases;4 AtNAS1 and AtNAS2, encoding nicotianamine synthases, involved in the synthesis of the Fe(II) chelating agent nicotianamine;5 and AtFRD3, encoding a protein of the MATE (multidrug and toxin efflux) family responsible for the xylem loading of citrate, an Fe chelator, which is essential for the correct distribution of Fe throughout the plant tissues.6 In addition to the upregulation of the above-cited genes, and of others, strategy I plants also develop morphological changes in their roots under Fe deficiency, such as subapical root hairs and transfer cells.7 The regulation of the expression of the Fe-related genes and of the morphological changes is not completely known, but since the early 1980s Landsberg suggested a role for auxin in the regulation of Fe deficiency stress responses by strategy I plants.8 Since then, several studies have been published suggesting a role for auxin and for other hormones in such a regulation.7
In 2007, we published a broad review on the influence of plant hormones on iron uptake by plants.7 That review was mainly devoted to strategy I plants, since at that time (and even today) almost no research has been done about the role of hormones on the regulation of Fe deficiency responses by strategy II plants. In that work, we reviewed a role for two hormones, auxin and ethylene, on the regulation of Fe deficiency responses by Strategy I plants. In 2006, Lucena et al. published a work showing that ethylene could upregulate Fe acquisition genes in several Strategy I plants.9 Later on, Waters, et al. published a work extending the role of ethylene to more Fe-related genes;10 and Graziano and Lamattina published a work showing that NO could also upregulate Fe acquisition genes in tomato.11 All these works suggested that hormones, like ethylene and NO, can affect the regulation of Fe acquisition genes. NO was previously involved on internal iron availability by Lamattina's group12 but was not related to Fe acquisition genes until the above-cited work of 2007. Very recently, a work by Chen et al. has also shown that auxin can be involved in the upregulation of some Fe acquisition genes in Arabidopsis.13 In the present review we will try to put into context the possible interactions of auxin, ethylene and NO on the regulation of Fe deficiency responses by Strategy I plants.
Interactions Among Auxin, Ethylene, Nitric Oxide and Fe Deficiency
Under Fe deficiency, the production of auxin,13–15 ethylene7,16 and NO11,13 increase in roots of several Strategy I plants. Very recently, García et al. have presented evidence that Fe deficiency upregulates the expression of many genes involved in ethylene synthesis and signaling.17 Although different researchers have assigned the regulation of Fe deficiency responses to the individual action of one of these hormones, every day it is more difficult to maintain these assignments. Firstly because some hormones affect the biosynthesis of others, and secondly because hormones act through complex signalling pathways, in which there is cross-talk.18,19 Auxin, ethylene and NO can interact at different levels (Fig. 1). Auxin can enhance ethylene production by affecting the ACC synthase activity.19–21 On the other hand, ethylene can affect auxin accumulation and polar auxin transport.20 Although ethylene and NO are negatively related in ripe fruits, where ethylene increases and NO decreases upon ripening, there are several works showing that NO can enhance ethylene production.22–24 Garcia et al. have found that NO upregulates the expression of many genes involved in ethylene synthesis, coding for SAM synthetases, ACC synthases, and ACC oxidases, in the strategy I plants Arabidopsis and cucumber.25 On the other hand, it was published that ACC (ethylene precursor) enhanced NO emission in senescing pea leaves22 and recently, García et al. have found that ACC can enhance NO production in the subapical regions of the roots of several Strategy I plants (Fig. 2).25 Furthermore, auxin can enhance nitrate reductase-associated NO production26 and NO can increase auxin content by reducing auxin degradation mediated by IAA oxidase.27 So, each of the hormones considered, auxin, ethylene and NO, can influence the production (or accumulation) of the other two (Fig. 1).
Figure 1.
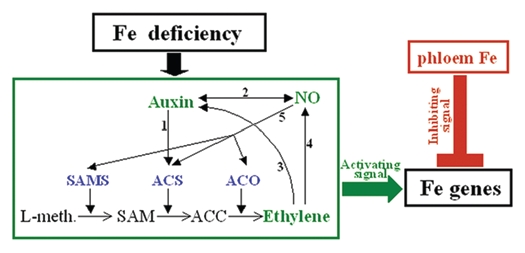
Influence of auxin, ethylene and NO on the expression of Fe acquisition genes. Under Fe deficiency, the production of auxin, ethylene and NO increase in roots of strategy I plants. Fe deficiency upregulates the expression of genes encoding ethylene synthesis enzymes, such as SAM synthetases (SAMS), ACC synthases (ACS) and ACC oxidases (ACO). Auxin can increase ethylene production by affecting ACS (1) and can increase NO production (2). Ethylene can influence auxin by affecting its accumulation and its polar transport (3) and can increase NO production (4). NO can influence auxin by affecting its degradation (2) and can increase ethylene production by affecting ethylene synthesis genes (5). The interplay of auxin, ethylene and NO lead to the amplification of an activating signal, probably directly related to ethylene, that would activate Fe acquisition genes, such as AtFIT and SlFER, only if there is not enough phloem Fe (inhibiting signal) to inhibit them.
Figure 2.
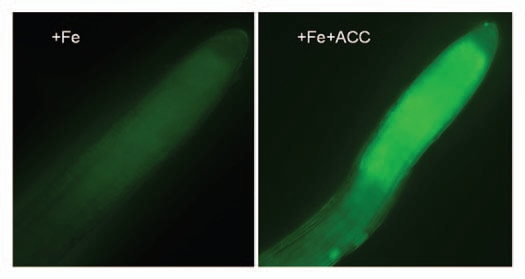
Effect of the ethylene precursor ACC on NO production by Arabidopsis roots. Left: root from a Fe-sufficient plant; Right: root from a Fe-sufficient plant treated with ACC for 2 h. NO was detected with DAF-2 DA.
Role of Auxin, Ethylene and Nitric Oxide on Morphological Responses to Fe Deficiency
In Strategy I plants the roles of auxin and/or ethylene in the regulation of some of their Fe deficiency morphological responses are widely accepted, and include the development of subapical root hairs and transfer cells.16,28,29 The role of both hormones in these morphological responses is based on the fact that their addition to Fe-sufficient plants caused the development of these morphological changes while the addition of either auxin or ethylene inhibitors to Fe-deficient plants inhibits them.7,16 There is evidence that in some of these morphological changes auxin could act through ethylene since auxin did not induce subapical root hairs when applied to ethylene insensitive mutants, such as Arabidopsis etr1 and ein2, or when applied simultaneously with ethylene inhibitors.7,16 Nonetheless, the results of Takahashi et al. suggesting that ethylene promotes root hair initiation by increasing the sensitivity of the hair-forming cells to auxin, implies that both hormones are required for root hair formation.30 So, it is probable that under Fe deficiency both hormones could participate in the development of subapical root hairs.
Recently, Graziano and Lamattina have also shown that NO can cause development of subapical root hairs in the roots of tomato plants.11 In our group, we have done some preliminary experiments by applying simultaneously GSNO (NO donor) and ethylene inhibitors to Fe-sufficient cucumber plants and the results found show that the subapical root hairs are not induced by GSNO in the presence of ethylene inhibitors. These preliminary results suggest that NO could act through ethylene.
Role of Auxin, Ethylene and Nitric Oxide on the Regulation of Physiological Responses to Fe Deficiency
Although a role for auxin and ethylene in the regulation of morphological changes to Fe deficiency has been widely accepted, their involvement in the regulation of physiological responses to Fe deficiency has been more controversial.31–33 Until 2006 the evidence was based on the facts that either ethylene or auxin inhibitors blocked the enhanced ferric reductase activity and the acidification in Fe-deficient plants.7,16 On the other hand, there were results showing that the ethylene precursor ACC enhanced ferric reductase activity in plants grown with low levels of Fe.7,16 In relation to auxin, there were some results showing that 2,4-D (synthetic auxin) did not induce either enhanced ferric reductase activity or acidification when applied to Fe-sufficient plants.7 These results, along with results showing that ethylene insensitive mutants, like the Arabidopsis ein2, could enhance ferric reductase activity when grown under Fe deficiency, led to the conclusion that hormones were not involved in the regulation of physiological responses to Fe deficiency.31
After the work of Lucena et al.9 showing that ACC can upregulate several Fe acquisition genes, such as AtFIT, AtFRO2, AtIRT1, SlFER, SlFRO1 and SlIRT1, in Arabidopsis and in tomato plants grown with low levels of Fe, it is clear that the above conclusion can not be longer accepted. Furthemore, Graziano and Lamattina also found that NO can upregulate SlFER, SlFRO1 and SlIRT1 in tomato plants grown without Fe.11 In addition, García et al. have recently found that ethylene and NO are involved in the upregulation of many Fe acquisition genes in Arabidopsis plants grown with low levels of Fe.17 In that work, it was also found that there was enhanced expression of Fe acquisition genes upon ethylene treatment in the Arabidopsis ethylene insensitive mutant ein2.17 This suggests that this mutant is not totally insensitive to ethylene and agrees with recent results showing that ethylene signalling is far more complex than previously thought.34 Finally, Chen et al. have also shown that auxin can upregulate AtFIT and AtFRO2 in Arabidopsis plants grown without Fe.13 All these results clearly show that auxin, ethylene and NO can upregulate the expression of several Fe acquisition genes, responsible for the physiological responses to Fe deficiency. It should be mentioned that the above-cited researchers have shown that auxin, ethylene and NO can upregulate the expression of Fe acquisition genes in plants grown with low levels of Fe (or without Fe) but have almost no effect in plants grown with high levels of Fe.9,11,13 This suggests that the upregulation of Fe acquisition genes does not depend only on hormones (auxin, ethylene and NO), that act as activators, but also on Fe, that acts as inhibitor. From this suggestion, two questions arise: (1) Which hormone acts as the last activator of Fe acquisition genes? (2) Which form of Fe acts as an inhibitor?
The answer to the first question is not yet clear, but different results suggest that NO and ethylene act downstream of auxin. First, auxin did not induce either subapical root hairs or enhanced ferric reductase activity in plants simultaneously treated with ethylene inhibitors.7 Second, Chen et al. have presented evidence showing that NO acts downstream of auxin in the regulation of ferric reductase activity by Fe-deficient Arabidopsis plants.13 The question of whether ethylene acts downstream of NO or NO downstream of ethylene is less clear. In a recent work, García et al. have presented evidence that NO enhances ethylene production by upregulating several ethylene synthesis genes and also that ethylene enhances NO production in the subapical regions of the roots (Fig. 2).25 We have suggested that this mutual influence could contribute to the amplification of an activating signal, that would trigger the activation of Fe acquisition genes (Fig. 1), such as AtFIT, AtFbHLH38, AtbHLH39 and SlFER. But, is this activating signal more directly related to ethylene or to NO? Presently, this is unknown, but recent results by Bauer's group suggest that it could be more directly related to ethylene.35 These authors have found, by using yeast-two hybrid screening, that the transcription factor FIT (that activates FRO2 and IRT1) interacts directly with EIN3/EIL1, proteins related to ethylene signaling.35,36
As to the second question, Lucena et al. proposed a model to explain the regulation of Fe acquisition genes in Strategy I plants, according to which ethylene acts as an activator of SlFER (or AtFIT) expression, while phloem Fe acts as inhibitor of their expression.9 Lucena et al. proposed phloem-Fe (or some signal derived from it) as an inhibitor, based on the fact that some mutants, like the Arabidopsis frd3, that accumulate high levels of Fe in their roots, however present a constitutive activation of Fe acquisition genes.9 This clearly indicates that total Fe in the roots is not responsible for inhibiting the expression of Fe acquisition genes. Furthermore, the constitutively-expressed Fe acquisition genes of the frd3 mutant are downregulated when its leaves are sprayed with Fe thus suggesting an inhibiting role of the phloem transported Fe.9
Conclusions and Perspective
The results show that auxin, ethylene and NO increase under Fe deficiency, which could be necessary for the upregulation of Fe acquisition genes in Strategy I plants (Fig. 1). Each one influences the production of the other two (Fig. 1), and all of them require low Fe (probably low phloem Fe) to be effective. This low Fe requirement would give specificity to the response, with phloem Fe (or some signal derived from it) acting as a lock to prevent the induction of Fe responses under other stresses that also enhance auxin, ethylene and/or NO synthesis. In addition, the involvement of phloem Fe on Fe responses would allow the plant to establish a feedback loop between the aerial parts and the root system. The collected body of research suggests that auxin acts upstream of ethylene and NO, but it is unknown which of them, ethylene or NO, is the last activator of Fe acquisition genes. Some preliminary results suggest that, perhaps, it is ethylene, but this question deserves future research.
Acknowledgements
This work was supported by the Ministerio de Educación y Ciencia (Project AGL2007-64372) and the Junta de Andalucía (Research Groups AGR115 and BIO-159 and Project AGR-3849). The authors want to thank Jon Shaff, from the Robert Holley Center for Agriculture and Health (Ithaca, New York USA), for the English correction of the manuscript and Inma Montilla and Pilar García for their technical support during the last years.
Abbreviations
- ACC
1-aminocyclopropane-1-carboxylic acid
- DAF-2 DA
4,5-diaminofluorescein diacetate solution
- GSNO
S-nitrosoglutathione
References
- 1.Walker EL, Connolly EL. Time to pump iron: iron-deficiency-signaling mechanisms of higher plants. Curr Opin Plant Biol. 2008;11:530–535. doi: 10.1016/j.pbi.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Yuan YX, Wu HL, Wang N, Li J, Zhao WN, Du J, et al. FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Res. 2008;18:385–397. doi: 10.1038/cr.2008.26. [DOI] [PubMed] [Google Scholar]
- 3.Brumbarova T, Bauer P. Iron-mediated control of the basic helix-loop-helix protein FER, a regulator of iron uptake in tomato. Plant Physiol. 2005;137:1018–1026. doi: 10.1104/pp.104.054270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santi S, Cesco S, Varanini Z, Pinton R. Two plasma membrane H+-ATPase genes are differentially expressed in iron-deficient cucumber plants. Plant Physiol Bioch. 2005;43:287–292. doi: 10.1016/j.plaphy.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Klatte M, Schuler M, Wirtz M, Fink-Straube C, Hell R, Bauer P. The analysis of Arabidopsis nicotianamine synthase mutants reveals functions for nicotianamine in seed iron loading and iron deficiency responses. Plant Physiol. 2009;150:257–271. doi: 10.1104/pp.109.136374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durrett TP, Gassmann W, Rogers EE. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol. 2007;144:197–205. doi: 10.1104/pp.107.097162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romera FJ, Lucena C, Alcántara E. Plant hormones influencing iron uptake in plants. In: Barton LL, Abadía J, editors. Iron Nutrition in Plants and Rhizospheric Microorganisms. Dordrecht, The Netherlands: Springer; 2007. pp. 251–278. [Google Scholar]
- 8.Landsberg EC. Fe stress induced transfer cell formation—Regulated by auxin? Plant Physiol. 1981;67:563. [Google Scholar]
- 9.Lucena C, Waters BM, Romera FJ, García MJ, Morales M, Alcántara E, et al. Ethylene could influence ferric reductase, iron transporter and H+-ATPase gene expression by affecting FER (or FER-like) gene activity. J Exp Bot. 2006;57:4145–4154. doi: 10.1093/jxb/erl189. [DOI] [PubMed] [Google Scholar]
- 10.Waters BM, Lucena C, Romera FJ, Jester GG, Wynn AN, Rojas CL, et al. Ethylene involvement in the regulation of the H+-ATPase CsHA1 gene and of the new isolated ferric reductase CsFRO1 and iron transporter CsIRT1 genes in cucumber plants. Plant Physiol Bioch. 2007;45:293–301. doi: 10.1016/j.plaphy.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Graziano M, Lamattina L. Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J. 2007;52:949–960. doi: 10.1111/j.1365-313X.2007.03283.x. [DOI] [PubMed] [Google Scholar]
- 12.Graziano M, Beligni MV, Lamattina L. Nitric oxide improves internal iron availability in plants. Plant Physiol. 2002;130:1852–1859. doi: 10.1104/pp.009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen WW, Yang JL, Qin C, Jin CW, Mo JH, Ye T, et al. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis thaliana. Plant Physiol. 2010;154:810–819. doi: 10.1104/pp.110.161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Römheld V, Marschner H. Mobilization of iron in the rhizosphere of different plant species. Adv Plant Nutr. 1986;2:155–204. [Google Scholar]
- 15.Bacaicoa E, Zamarreño AM, Leménager D, Baigorri R, García-Mina JM. Relationship between the hormonal balance and the regulation of iron deficiency stress responses in cucumber. J Amer Soc Hort Sci. 2009;134:589–601. [Google Scholar]
- 16.Romera FJ, Alcántara E. Ethylene involvement in the regulation of Fe-deficiency stress responses by Strategy I plants. Funct Plant Biol. 2004;31:315–328. doi: 10.1071/FP03165. [DOI] [PubMed] [Google Scholar]
- 17.García MJ, Lucena C, Romera FJ, Alcántara E, Pérez-Vicente R. Ethylene and nitric oxide involvement in the upregulation of key genes related to iron acquisition and homeostasis in Arabidopsis. J Exp Bot. 2010;61:3885–3899. doi: 10.1093/jxb/erq203. [DOI] [PubMed] [Google Scholar]
- 18.Gazzarrini S, McCourt P. Cross-talk in plant hormone signalling: what Arabidopsis mutants are telling us. Ann Bot. 2003;91:605–612. doi: 10.1093/aob/mcg064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen H, Grossmann K. Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol. 2000;124:1437–1448. doi: 10.1104/pp.124.3.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swarup R, Parry G, Graham N, Allen T, Bennett M. Auxin cross-talk: integration of signalling pathways to control plant development. Plant Mol Biol. 2002;49:411–426. doi: 10.1007/978-94-010-0377-3_12. [DOI] [PubMed] [Google Scholar]
- 21.Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Ann Rev Plant Physiol Plant Mol Biol. 1984;35:155–189. [Google Scholar]
- 22.Leshem YY, editor. Nitric Oxide in Plants. The Netherlands: Kluwer Academic Publishers; 2000. [Google Scholar]
- 23.Mur LAJ, Laarhoven LJJ, Harren FJM, Hall MA, Smith AR. Nitric oxide interacts with salicylate to regulate biphasic ethylene production during the hypersensitive response. Plant Physiol. 2008;148:1537–1546. doi: 10.1104/pp.108.124404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang H, Liang X, Wan Q, Wang X, Bi Y. Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress. Planta. 2009;230:293–307. doi: 10.1007/s00425-009-0946-y. [DOI] [PubMed] [Google Scholar]
- 25.García MJ, Suárez V, Romera FJ, Alcántara E, Pérez-Vicente R. A new model involving ethylene, nitric oxide and Fe to explain the regulation of Fe acquisition genes in Strategy I plants. Plant Physiol Bioch. 2011 doi: 10.1016/j.plaphy.2011.01.019. In press. [DOI] [PubMed] [Google Scholar]
- 26.Kolbert Z, Bartha B, Erdei L. Exogenous auxin-induced NO synthesis is nitrate reductase-associated in Arabidopsis thaliana root primordial. J Plant Physiol. 2008;165:967–975. doi: 10.1016/j.jplph.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Wang W, Yin H, Liu X, Sun H, Mi Q. Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant Soil. 2010;326:321–330. [Google Scholar]
- 28.Landsberg EC. Hormonal regulation of iron-stress response in sunflower roots: a morphological and cytological investigation. Protoplasma. 1996;194:69–80. [Google Scholar]
- 29.Schmidt W, Bartels M. Formation of root epidermal transfer cells in Plantago. Plant Physiol. 1996;110:217–225. doi: 10.1104/pp.110.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi H, Kawahara A, Inoue Y. Ethylene promotes the induction by auxin of the cortical microtubule randomization required for low-pH-induced root hair initiation in lettuce (Lactuca sativa L.) seedlings. Plant Cell Physiol. 2003;44:932–940. doi: 10.1093/pcp/pcg119. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt W, Tittel J, Schikora A. Role of hormones in the induction of iron deficiency responses in Arabidopsis roots. Plant Physiol. 2000;122:1109–1118. doi: 10.1104/pp.122.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schikora A, Schmidt W. Iron stress-induced changes in root epidermal cell fate are regulated independently from physiological responses to low iron availability. Plant Physiol. 2001;125:1679–1687. doi: 10.1104/pp.125.4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Li C. Is ethylene involved in regulation of root ferric reductase activity of dicotyledonous species under iron deficiency? Plant Soil. 2004;261:147–153. [Google Scholar]
- 34.Cho YH, Yoo SD. Emerging complexity of ethylene signal transduction. J Plant Biol. 2009;52:283–288. [Google Scholar]
- 35.Fleischer J, Bauer P. Hormonal influence on irondeficiency responses mediated by FIT protein interaction. 15th International Symposium on Iron Nutrition and Interactions in Plants. 2010;9 [Google Scholar]
- 36.Chang C, Stadler R. Ethylene hormone receptor action in Arabidopsis. BioEssays. 2001;23:619–627. doi: 10.1002/bies.1087. [DOI] [PubMed] [Google Scholar]


