Abstract
Cellular energy homeostasis is a crucial function of oxidative tissues and is altered in obesity, a continuously rising health problem. Lipid droplets are thought to play a central role in lipid homeostasis by mediating the transient storage of fatty acids in the form of triglyceride, while preventing high levels of toxic lipid intermediates or oxidized lipids that mediate cellular lipotoxicity. Members of the perilipin protein family coating lipid droplet surfaces have been found to serve important regulatory and structural functions critical to regulation of lipid stores. This review examines results of studies on one of the newest members of the perilipin family, perilipin 5, which has emerged as a putative key player in lipid droplet function in oxidative tissues.
Metabolic Syndrome: Prevalence of Lipid Droplets in Non-Adipose Tissue (Ectopic Fat)
The striking surge in obesity has serious health consequences that increase risk for hypertension, insulin resistance, diabetes and coronary heart disease [1]. An important clue to the pathogenesis of these metabolic diseases is the presence of ectopic fat in liver, heart and skeletal muscle of patients with “metabolic syndrome” [2]. Normally, excess energy from dietary consumption, in the form of non-esterified fatty acids (FA), is converted into triglyceride (TAG), i.e. fat, and stored in lipid droplets (LDs) within adipose tissue, a highly specialized and expandable organ. Poorly understood defects in adipose tissue fat storage lead to chronically elevated levels of circulating FA and are thought to mediate accumulation of ectopic fat, triglyceride accumulation in non-adipose organs, most prominently liver, muscle and heart [3].
Ectopic fat correlates with insulin resistance in skeletal muscle, hepatic steatosis, dysregulated insulin secretion and heart failure [3–4]. However, despite a well-established connection with these diseases, studies of endurance trained athletes found that increased intramyocellular lipid (IMCL) correlates with insulin sensitivity [5–6]. Transgenic mice with skeletal muscle over-expression of diacylglycerol acyltransferase-1 (DGAT-1), an enzyme that catalyzes the last step in TAG synthesis, replicate the “athlete paradox”. These mice have comparable levels of intramuscular LDs as mouse models with fat-induced insulin resistance; however, DGAT-1 mice are insulin sensitive, have reduced levels of diacylglycerols and ceramides and increased levels of fatty acid oxidation efficiency [7]. Inhibiting hydrolysis of LD triglyceride also appears to improve systemic insulin sensitivity, because mice lacking ATGL, a key ubiquitous lipolytic enzyme, are insulin sensitive although their phenotype is also characterized by the presence of ectopic fat [8].
Strikingly, triglyceride accumulation is most pronounced in hearts of ATGL KO heart, causing severe cardiomyopathy and premature death [9]. It remains to be determined if this heart failure is due solely to physical damage from a large amount of fat accumulated within cardiomyocytes or from an underestimated but critical contribution of cardiac LD hydrolysis to supply energy or lipid intermediates to cardiac muscle. Patients with defective ATGL function also suffer cardiomyopathy, illustrating the importance for proper LD hydrolysis regulation in the mammalian heart [10]. The complexity of the role of the lipid droplet in cardiac lipid homeostasis is further evidenced by the phenotype of mice over-expressing DGAT-1 specifically in heart. These mice have similar levels of cardiac lipid content to established mice models of cardiac lipotoxicity via over-expression of long chain acyl CoA synthetase, fatty acid transport protein 1, LPL and PPARγ [11–14], but DGAT-1 mice are free of cardiac lipotoxicity and dysfunction [14].
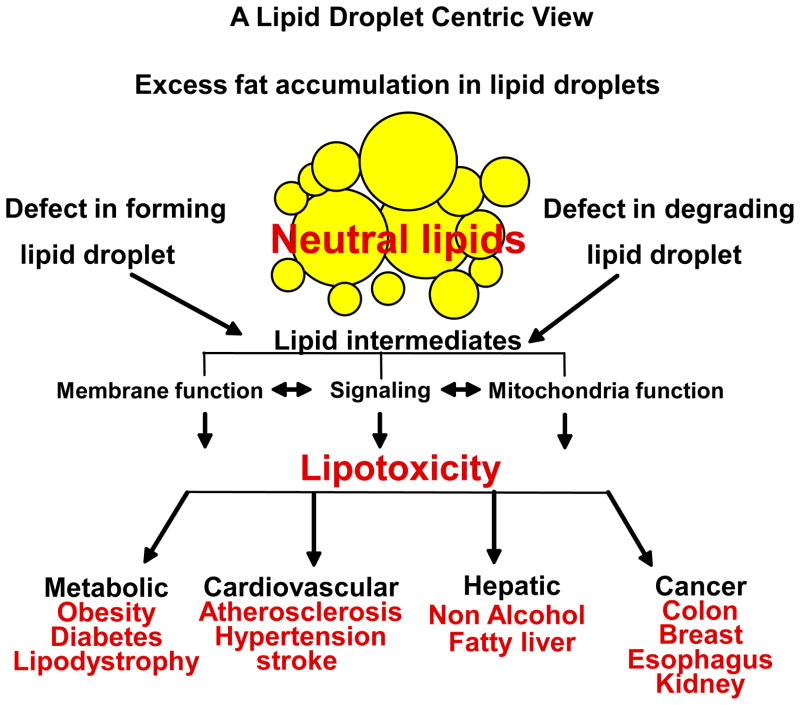
Overall, it is clear that development of tissue lipotoxicity and dysfunction is not simply due to presence of fat in non-adipose tissues but is also due to alterations in LD function. Whether LD biogenesis alone or regulation of LD TAG hydrolysis, or perhaps both, are altered in conditions of excess cellular supply remains to be explored, as both pathways release lipid intermediates with subsequent metabolic and signaling capabilities (Figure 1). Thus, identifying key components regulating non-adipose tissue LD function will be essential to prevent cellular dysfunction induced by lipotoxicity and reduce the adverse impact of metabolic diseases. Here, we discuss the role of perilipin 5, a lipid droplet surface scaffolding protein and a potential prime candidate to regulate LD hydrolysis function in oxidative tissues.
Figure 1. Importance of the role of LDs in cellular LD homeostasis.
In physiological conditions, the main role of the lipid droplet (LD) is to store excess intracellular fatty acid (FA) as neutral lipid, triglyceride (TAG). In chronic excess of cellular FA (for example, with obesity), presence of ectopic fat is found highly associated to disease states. A growing body of evidence supports the concept that defects in LD biogenesis, hydrolysis and/or turnover lead to an excessive flow of lipid intermediates and/or oxidized lipids into other metabolic pathways, perturbing ER and other membrane function, altering signaling by stimulating PKCs and/or by upregulating PPARs and affecting mitochondria function by increasing ROS and oxidized lipid formation.
LD Storage and Release of Lipid Intermediates – Cell Specific Energy Homeostasis
The LD compartment is recognized as a storage organelle, but its role in maintaining lipid homeostasis and metabolism is now also being realized. LD biogenesis is a fundamental cellular function; most if not all mammalian and non-mammalian cells convert excess FA into triglyceride for storage within LDs [15]. LD accumulation in “normal” cells maintains low but sufficient intracellular FA levels for essential activities, e.g. β-oxidation, membrane phospholipid synthesis and steroid production, depending on cell type and developmental state. More importantly, increasing evidence indicates that LDs play an important role in releasing endogenous ligands necessary to maintain activities of transcription factors, such as PPARs, involved in regulating lipid metabolism [16–19].
Cell-specific FA needs dictate a highly dynamic LD compartment, to store and release FA tailored to accommodate the cell’s requirements and function, ranging from highly efficient FA storage/release from or into blood (white adipocytes, [20]), mitochondrial FA oxidation for thermal regulation (brown adipocytes, [21]), FA oxidation for long term mobility demands (skeletal slow-twitched fiber muscles, [22]), lipidation of very low density lipoprotein production and mitochondria β-oxidation (liver, [23]), milk production (mammary epithelial cells, [24]) and surfactant production (specialized lung cells [25]).
Physiological evidence supports an essential role of LD in cellular lipid homeostasis. During fasting, adipose tissue lipolysis provides a convenient source of FA fuel for energy demands in non-adipose tissues. Intriguingly, concurrent with increasing fatty acid uptake and use, oxidative tissues such as liver and heart also increase their LD content [26–27]. Progress in imaging has confirmed in humans what had been observed in rodent models: an inherent dynamic role of myocardial LDs, increasing 3-fold following a 48hr fast in healthy individuals [28].
A growing body of evidence reveals the important role of LDs to store excess FA in the form of TAG, in fact acting as a temporary local warehouse for subsequent cellular demands. Evidence also indicates that LDs protect cells against lipotoxicity by efficiently storing bioactive lipids and toxic lipid species when FA supply exceeds demand [29]. However, the mechanisms underlying LD biogenesis remain to be fully understood. Nonetheless, progress in understanding LD TAG hydrolysis regulation has been boosted considerably by the discovery of a family of LD surface-associated proteins, the perilipins, which provide convenient tools to explore the role of LD in cellular lipid homeostasis.
The Perilipin Family: LD Surface Scaffolding and Regulators
Results from proteomic studies have highlighted the importance of LD surface-associated proteins as a critical interface for lipid metabolism [30]. Even more importantly, these studies identified a proteome “signature” for LDs in most eukaryotic organisms (except yeast) that consistently include at least one member of the perilipin protein family, originally named PAT proteins for Perilipin, ADRP and TIP47. The perilipin family currently includes five members; here we will use the recently adopted nomenclature [31]: perilipin 1 (Perilipin), perilipin 2 (ADRP), perilipin 3 (TIP 47), perilipin 4 (S3–12) and perilipin 5 (MLDP, OXPAT, LDSP5). The perilipins are defined by a conserved primary sequence homology across species [32–33]. A comprehensive overview of the perilipin family has been recently published elsewhere [34], and some of the relationships between perilipin family members are emerging.
The signature feature in all perilipin proteins is that they bind the LD surface in various cell types cultured in the presence of FAs. Perilipins can be classified into two groups according to their protein stability in the absence of LDs. Perilipin 1 and 2 are termed non-exchangeable perilipin proteins and are always found in an LD-bound state; in absence of LDs, perilipin 1 and 2 are rapidly degraded by lysosomal or proteosomal degradation, respectively [35–37]. On the other hand, perilipin 3, 4 and 5 are termed exchangeable perilipin proteins and exist either in a LD-bound state or in the cytosol [38]. Based on these differences in protein stability, it was proposed that perilipin 3, 4 and 5 bind to more transient pools of LDs, while perilipin 1 and 2 associate with more constitutive pools of LDs [38]. This lays the foundation for an interesting concept of specialized pools of LDs, even within the same cell, possibly reflecting different LD utilization.
Perilipin distribution is also clearly tissue- and utilization-dependent. While perilipin 1 and 4 are limited to adipose tissue, perilipin 2 and 3 are ubiquitous with perilipin 2 highly abundant in liver; interestingly, perilipin 5 is found primarily in oxidative tissues [34]. When LDs from human and rat skeletal muscles are immunostained with specific antibodies against perilipin 2 and 5, it was shown that both perilipin proteins can either partially co-localize or be segregated to individual droplets [39]. This clearly illustrates the dynamic nature of the perilipins at the surface of the LD in oxidative tissues. Whether these differences in perilipin protein composition of LD surface in oxidative tissues are indicative of specific changes in LD metabolism remain to be determined.
Among the perilipin proteins, perilipin 1 is the best studied and to date remains the only perilipin in which a key role regulating adipocyte lipolysis has been well established [40]. On the basis of the work performed by several groups, it is now accepted that perilipin 1, a multi-phosphorylated protein, acts as a scaffolding protein for key lipolytic proteins. Perilipin 1 regulates lipolysis by phosphorylation/dephosphorylation events that induce changes in protein/protein interactions at the LD surface [41–46]. The detailed molecular mechanisms by which perilipin 1 facilitates LD TAG hydrolysis are still being investigated, but the general working model is that perilipin 1 phosphorylation simultaneously triggers 1) release of CGI-58, a co-lipase essential for ATGL activation; 2) ATGL activation including access to its substrate triglyceride; and 3) binding of hormone sensitive lipase (HSL), acting mainly as a diacylglycerol lipase for the second step in the lipolytic pathway [46–48]. In addition, perilipin 1 phosphorylation is responsible for important structural modifications at the LD surface, inducing fragmentation of large LDs into micro-LDs. This structural change is proposed to promote rapid surface expansion of the LD surface, increase access to ATGL and therefore increase the lipolytic rate [49].
Perilipin 5 is the most recently established family member, highly expressed in oxidative tissues, but its function remains poorly understood. Here we review the recent published evidence that perilipin 5, like perilipin 1, is a scaffolding protein for key lipolytic players and also a multi-phosphorylated protein. Therefore, perilipin 5 may play an important role in regulating LD TAG hydrolysis in oxidative mammalian tissues and be a convenient tool to investigate oxidative tissue lipid homeostasis
Perilipin 5: a putative regulator of oxidative tissue LDs
The perilipin 5 gene, Plin5, is only found in mammalian species, mapped to mouse chromosome 17D and human chromosome 19p13.3. Interestingly, in both species, it is located adjacent to Plin4 and is in close proximity to Plin3 [50]. Evolution driven gene duplication is a potential mechanism by which Plin3 is most likely the primordial gene and plin4 and 5 ortholog genes, sharing related structures but more specialized functions [50]. A comparison of murine perilipin 5 sequence with other perilipins reveals that its highest sequence similarity is to perilipin 2 and 3 [50]. Homology to perilipin 1 is only in the amino terminal “PAT-1” domain and in the 11-mer repeat region sometimes referred as the PAT-2 domain [50]. The conserved homologous sequences and variances found in different perilipins offer strategic points to further investigate perilipin function and by extension, LD function.
In mice, Plin5 mRNA expression is highly restricted to oxidative tissues: heart, slow-twitch fibers of skeletal muscle, brown adipose tissue and liver. Plin5 is also induced by fasting [50–52]. This tissue-selective protein expression pattern and regulation are found to be highly similar to the tissue distribution of the fasting-induced nuclear receptor PPARα, suggesting similar roles for perilipin 5 in fatty acid utilization and lipid metabolism [50–52]. Intriguingly, although perilipin 5 appears to be regulated by PPARα, the murine perilipin 5 promoter does not contain a DR-1 type response element, and does not respond to PPARα activation based on in vitro transfection studies [50]. In addition, in mouse and human subcutaneous adipose tissues, transcriptional regulation of Plin5 seems to be instead regulated by PPARγ [52]. Still, treatments with rosiglitazone or pioglitazone do not affect Plin5 expression in skeletal muscle of glucose intolerant patients (rosiglitazone) [39] or of diabetic type 2 patients (pioglitazone) [39]. Due to the close proximity of the perilipin 4 and 5 genes, the promoter region for perilipin 5 has not yet been clearly defined, and additional work is necessary to fully comprehend the regulation of perilipin 5 by the PPARs.
Relying on reconstituted cell culture models using perilipin 5 over-expression systems, the current general consensus is that perilipin 5 plays an important role in LD accumulation [50–52]. Different underlying mechanisms have been proposed so far for this role, either via increased fatty acid uptake [52] or decreased lipolysis [50]. Intriguingly, perilipin 5, despite its role in increasing TAG accumulation, paradoxically also increases fatty acid oxidation [52]. Concomitant coupling of TAG synthesis and fatty acid oxidation has also been observed in skeletal muscle over-expressing DGAT-1 [14]. This suggests that perilipin 5, like DGAT-1, acts by keeping the intracellular levels of FA metabolites below their lipotoxic level by channeling lipid intermediates efficiently both into storage and utilization. Future work is needed to better characterize perilipin 5’s role in cellular lipid metabolism and define its potential effects on insulin sensitivity.
At the molecular level, independent work from two groups strongly supports a role for perilipin 5 in LD TAG hydrolysis with a scaffolding role for three major key lipolytic players: CGI-58, ATGL and HSL [53–55). It was first reported that perilipin 5 interacts with CGI-58, the co-lipase for a key enzyme in LD TAG hydrolysis [54]. In experiments using Fluorescent Resonance Energy Transfer (FRET) protein complementation technology, CGI-58 protein was found to bind murine perilipin 5 with even greater affinity than to perilipin 1. A single-point mutation in mouse CGI-58 (E262K) greatly disrupts its interaction with perilipin 5, and a homologous mutation in human CGI-58 (E260K) inhibits CGI-58 binding to perilipin 1 [54]. This initial work was confirmed by co-immunoprecipitation studies [56]. Interestingly, CGI-58 interaction with perilipin 2 was found in a yeast two-hybrid screen [56], but exhibits weak interaction by FRET [54]. These perilipin-driven differences in CGI-58 recruitment may play a role in cell specific LD metabolism. Interaction between perilipin1 and CGI-58 has been proposed to be an important regulatory step of adipose tissue hydrolysis, and subsequent studies indicate that it may also be an important step regulating perilipin 5-coated LD in oxidative tissues [54,55].
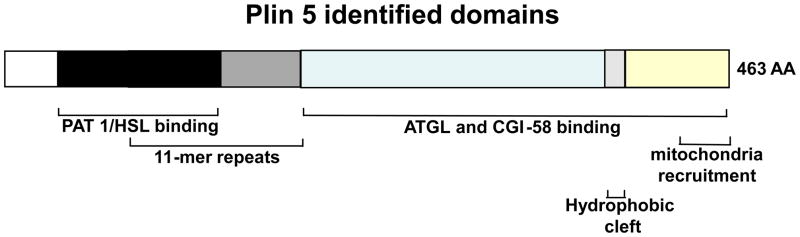
Murine perilipin 5 also interacts with ATGL [53,55]. Murine perilipin 5’s ATGL binding domain has been determined to be between amino acids 200 and 463 [53,55]. Interestingly, this region also appears to be responsible for binding CGI-58, since ATGL and CGI-58 binding are mutually exclusive [53]. Figure 2 illustrates structural features and domains of perilipin 5. Experiments using FRET protein-complementation indicate that perilipin 5, like other perilipins, self-assembles into oligomers. It has been proposed that perilipin 5 oligomerization at the LD surface facilitates recruitment of ATGL and CGI-58 to elicit efficient oxidative tissue lipolysis [53]. Alternatively, based on measurements of lipolysis in reconstituted cellular systems and the novel finding that perilipin 5 is a multi-phosphorylated protein, it can be proposed that perilipin 5 protects TAG in LD against lipolysis by sequestering ATGL and limiting interactions between ATGL and CGI-58, with maximum lipolysis elicited through changes in perilipin 5 phosphorylation [55].
Figure 2. Schematic diagram of the structural features of murine perilipin 5.
Structural domains that are shared by some of the perilipin family of proteins are depicted in shades of black and grey. The N terminus of perilipin 5 contains 100 amino acid (AA) sequences that are highly conserved between perilipin 1, 2, 3 but not 4 (PAT-1 domain, (black)). Overlapping with these sequences are stretches of amino acids containing 11-mer repeat sequences, a common feature for all perilipin proteins (PAT-2 domain, (medium grey)). The C-terminus of perilipin 5 contains a highly conserved sequence of 14 amino acids that folds into a hydrophobic cleft in perilipin 2, 3 and 4 but not in perilipin 1 (light grey). Unique to perilipin 5 are the ATGL and CGI-58 binding domain (blue) between 189 amino acids (AA) and 391 AA as well as a mitochondria-binding domain between 443 AA and 463 AA (yellow).
Perilipin 1 and 5 appear to share a mechanism for posttranslational regulation of LD TAG hydrolysis. Both may modulate ATGL/CGI-58 protein/protein interactions via phosphorylation, but perilipin 5 phosphorylation sites and regulation have yet to be identified. Perilipin 5 is reported to bind Hormone Sensitive Lipase (HSL) via its N-terminal domain or PAT-1 domain, a property shared with perilipin 1, 2 and 3 [46]. However, at least one marked difference is that perilipin 5 is the only perilipin able to bind ATGL. Table 1 recapitulates the similarity and differences between perilipin 1 and perilipin 5. These variations may reveal important tissue differences in regulation of LD TAG hydrolysis in adipocytes versus oxidative tissues.
Table 1.
Shared and different properties between murine perilipin 1 and 5
| perilipin proteins | Perilipin 1 | references | Perilipin 5 | references |
|---|---|---|---|---|
| Tissue distribution | Specific
|
[68–69] | Specific
|
[50–52] |
| Tissue energetic function | Storage | Utilization | [50–52] | |
| Cell localization | Lipid droplet surface | Cytosol/lipid droplet surface | ||
| Metabolic pathway | Lipid droplet hydrolysis | [70] | Lipid droplet hydrolysis | [50] |
| Shared structural features with perilipin protein family |
|
[32] |
|
[50] |
| Unique sequence features | Hydrophobic sequences | [71] | Binding domains for mitochondria recruitment | [66] |
| Scaffolding properties |
|
[44-46,48] |
|
[46,53–55] |
| Regulation |
|
[41–42] [72] |
|
[55] [50–52] |
Perilipin 5 provides a physical link between LDs and mitochondria
For thermoregulation and other cellular energy functions, LDs and mitochondria are intimately and functionally related. Mitochondrial dysfunction results in lipid accumulation and cardiomyopathy in both mice and humans [57]. Likewise, lipid storage is increased after treatment with RNAi targeting genes encoding mitochondrial FA β-oxidation proteins [58]. Conversely, lack of some LD-associated proteins such as perilipin 1, Cidec, are associated with increased mitochondrial FA β-oxidation in adipose cells [59,60]. Spatial interaction between LDs and mitochondria has been anecdotally reported in electron microscopic studies of adipocytes, heart and liver [61–63]. In skeletal muscle cells where lipids are utilized for energy production, interaction between LDs and mitochondria is enhanced by exercise [63]. Proteins usually associated with mitochondria are also found in LDs via proteomic studies [64]. While there is always the possibility that these are contaminants, interaction between these two organelles is expected because in mammalian cells, FA oxidation occurs in mitochondria. Both LDs and mitochondria have been shown to be dynamic organelles, and their movement relies essentially on the microtubule machinery, but the mechanisms underlying mitochondria recruitment at the LD surface have not been clarified. Recently, ex vivo experiments have revealed one SNARE protein, SNAP23, to be important in potentially recruiting mitochondria at the LD surface [65]. It also has been reported that perilipin 5 at the LD surface recruits mitochondria and that the physical interaction is dependent on its C-terminal domain [66]. Furthermore, the ability to constitutively recruit mitochondria to the LD appears to be unique to perilipin 5. Perilipin 3 was recently reported to associate to the mitochondria fraction, but this association happened only under specific stress conditions, in cell culture conditions and also required an intact perilipin 3 N-terminal domain [67].
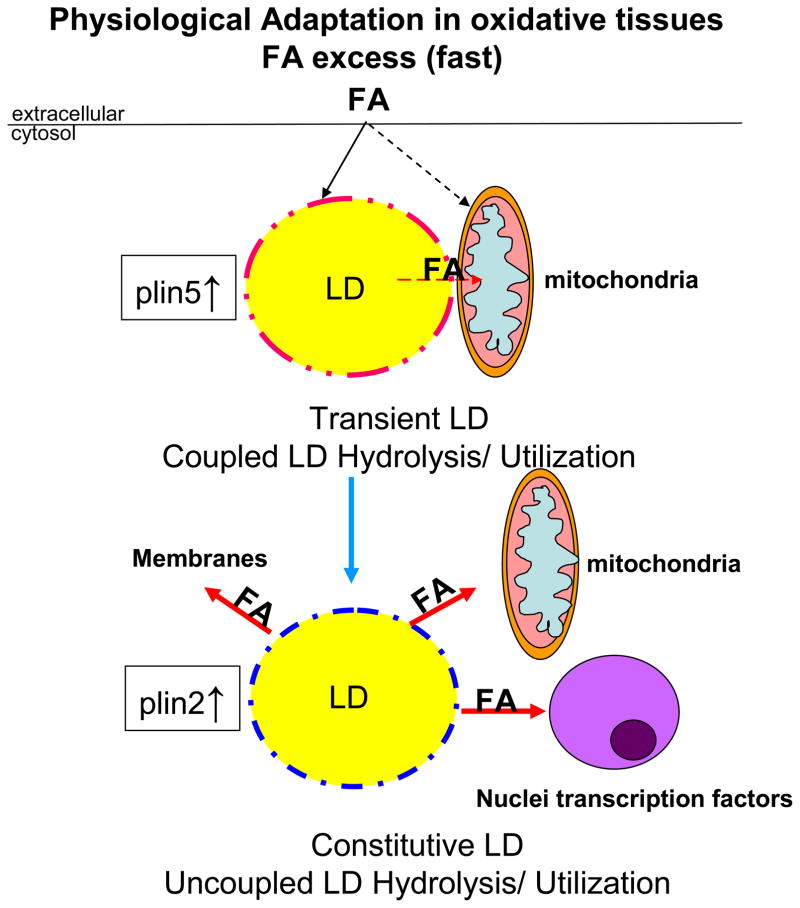
Based on current knowledge, and by analogy to perilipin 1’s established role in regulating adipocyte LD TAG hydrolysis in a PKA dependent manner, it can be hypothesized that perilipin 5 provides a similar crucial function in oxidative tissues. Perilipin 5 may play a part in protection against cellular lipotoxicity by transiently entrapping bioactive lipids in LDs in close proximity of mitochondria at times of increased cellular FA influx, and by regulating FA flux from LD to mitochondria (Figure 3). Further studies are necessary to fully investigate the function of perilipins, specifically perilipin 5, in oxidative tissues; such studies may provide insights into how energy metabolism is coupled to physical structures and how such connectivity can be altered in a lipotoxic environment.
Figure 3. Hypothetical model of the role of perilipin 5 in cellular lipid homeostasis in adaptive response to excess FA flux in oxidative cells under physiological conditions.
During fasting conditions, adipose tissue lipolysis provides FA fuel to other organs. Excess FA supply to liver or heart leads to a rapid increase in LD biogenesis. Based on present studies, it can be hypothesized that perilipin 5, already present in the cytosol, is rapidly recruited to the surface of a nascent pool of LDs and regulates LD TAG hydrolysis, being a novel protein kinase A (PKA) downstream target, by modifications in its scaffolding properties for ATGL and CGI-58, controlling FA utilization through recruitment of mitochondria to LDs by its C-terminal domain and finally protecting mitochondria against lipotoxicity. Perilipin 2 coats a pool of constitutive droplets which are not coupled to specific use.
Concluding Remarks
The LD is an exquisite barometer of cellular lipid homeostasis in oxidative tissues, and its presence frequently represents a mismatch between supply and use, for instance in mitochondrial β-oxidation. In adaptive responses to fasting or moderate exercise training, excess FAs are transiently packed into LD in the form of TAG, with an effective tight coupling between TAG hydrolysis and FA utilization. However, in conditions of chronic excess FA, the LD ceases to be an effective buffer for lipid supply and utilization. Identifying key genes in the pathways that efficiently couple the LD storage compartment to the mitochondria FA utilization compartment will help unravel the underpinnings of cellular lipid homeostasis.
Perilipin 5 is a promising candidate to investigate LD function in oxidative tissues, just as studies of perilipin 1 helped reveal the role and regulation of adipocyte LDs. So far, our knowledge has relied on in vitro experiments and perilipin 5 over-expressing model systems. Lack of endogenous expression of perilipin 5 in established cell lines and difficulties in culturing primary culture of oxidative cells limit our capacity to undertake studies of the endogenous function of perilipin 5. For example, perilipin 5 endogenous protein expression in oxidative cell line models is undetectable, probably due to experimental conditions inducing a cellular energy shift from an oxidative to a glycolytic state. In addition, the limited amount of LDs present in oxidative tissues requires large amounts of tissue. Much can be learned by generating tissue specific transgenic mice with down- or up-regulated perilipin 5. It is likely that multiple transgenic models may have to be generated to fully comprehend perilipin 5 function, because of the presence of other perilipins and the extremely dynamic nature of LDs.
Acknowledgments
We thank Dr Martin Woodle and Mrs Darcy Tell for careful review and helpful editing of the manuscript. We thank Dr Londos for having pioneered this field of investigation, for having trained many of us and for being an inspiration to our continuously expanding group of “ lipid droplet” investigators. This work was supported by a career development award 1-05-CD-17 from the American Diabetes Association (to C.S.), a grant from NIH 1RO1 DK 075017(to C.S.), the Geriatric Research, Education and Clinical Center, Baltimore Veterans Affairs Health Care Center, the Clinical Nutrition Research Unit of Maryland (DK072488), and Intramural Research Programs of NIDDK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meigs JB. Epidemiology of type 2 diabetes and cardiovascular disease: translation from population to prevention: the Kelly West award lecture. Diabetes Care, 1865–1871. 2010 doi: 10.2337/dc10-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckel RH, et al. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 3.Unger RH, et al. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;180:209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Szendroedi J, Roden M. Ectopic lipids and organ function. Curr Opin Lipidol. 2009;20:50–56. doi: 10.1097/mol.0b013e328321b3a8. [DOI] [PubMed] [Google Scholar]
- 5.Machann J, et al. Intramyocellular lipids and insulin resistance. Diabetes Obes Metab. 2004;6:239–248. doi: 10.1111/j.1462-8902.2004.00339.x. [DOI] [PubMed] [Google Scholar]
- 6.Russell AP. Lipotoxicity: the obese and endurance-trained paradox. Int J Obes Relat Metab Disord. 2004;28:S66–71. doi: 10.1038/sj.ijo.0802859. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, et al. Upregulation of myocellular DGAT1 augments triglyceride synthesis in skeletal muscle and protects against fat induced insulin resistance. J Clin Invest. 2007;117:1679–1689. doi: 10.1172/JCI30565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoy AJ, et al. Adipose triglyceride lipase-null mice are resistant to high-fat diet-induced insulin resistance despite reduced energy expenditure and ectopic lipid accumulation. Endocrinology. 2011;152:48–58. doi: 10.1210/en.2010-0661. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann R, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 10.Schweiger M, et al. Neutral lipid storage disease: genetic disorders caused by mutations in ATGL/PNPLA2 or CGI-58/ABHD5. Am J Physiol Endocrinol Metab. 2009;297:E289–E296. doi: 10.1152/ajpendo.00099.2009. [DOI] [PubMed] [Google Scholar]
- 11.Chiu HC, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu HC, et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96:225–233. doi: 10.1161/01.RES.0000154079.20681.B9. [DOI] [PubMed] [Google Scholar]
- 13.Yagyu H, et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111:419–426. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Son NH, et al. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest. 2007;117:2791–2801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi X, et al. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284:36312–36323. doi: 10.1074/jbc.M109.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beller M, et al. Lipid droplets: a dynamic organelle moves into focus. FEBS Lett. 2010;584:2176–2182. doi: 10.1016/j.febslet.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Aishan H, et al. Physiologic roles of hepatic lipid droplets and involvement of peroxisome proliferator-activated receptor alpha in their dynamism. Biol Pharm Bull. 2010;33:351–354. doi: 10.1248/bpb.33.351. [DOI] [PubMed] [Google Scholar]
- 18.Ong KT, et al. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatology. 2010;53:116–126. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen WJ, et al. Hormone-sensitive lipase modulates adipose metabolism through PPARγ. Biochim Biophys Acta. 2011;1811:9–16. doi: 10.1016/j.bbalip.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu L, et al. Diacylglycerol Acyl transferase 1 deficiency decreases PPAR expression and does not lead lipotoxicity in cardiac and skeletal muscle. J Lipid Res. 2011 doi: 10.1194/jlr.M011395. ( http://jlr.org/) [DOI] [PMC free article] [PubMed]
- 21.Ahmadian M, et al. Lipolysis in adipocytes. Int J Biochem Cell Biol. 2010;42:555–559. doi: 10.1016/j.biocel.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricquier D. Biology of brown adipose tissue: view from the chair. Int J Obes (Lond) 2010;34:S3–6. doi: 10.1038/ijo.2010.176. [DOI] [PubMed] [Google Scholar]
- 23.Moro C, et al. Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am J Physiol Endocrinol Metab. 2008;294:E203–213. doi: 10.1152/ajpendo.00624.2007. [DOI] [PubMed] [Google Scholar]
- 24.Olofsson SO, et al. Lipid droplets as dynamic organelles connecting storage and efflux of lipids. J Biochim Biophys Acta. 2009;1791:448–458. doi: 10.1016/j.bbalip.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Bauman DE, et al. Major advances associated with the biosynthesis of milk. J Dairy Sci. 2006;89:1235–1243. doi: 10.3168/jds.S0022-0302(06)72192-0. [DOI] [PubMed] [Google Scholar]
- 26.Magra AL, et al. Role of adipose differentiation-related protein in lung surfactant production: a reassessment. J Lipid Res. 2006;47:2367–2373. doi: 10.1194/jlr.M600157-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Jacob S, et al. Lipid droplet accumulation in the heart during fasting. Acta Histochem. 1987;82:149–152. doi: 10.1016/S0065-1281(87)80020-X. [DOI] [PubMed] [Google Scholar]
- 28.Kersten S, et al. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reingold JS, et al. Determination of triglyceride in the human myocardium by magnetic resonance spectroscopy: reproducibility and sensitivity of the method. Am J Physiol Endocrinol Metab. 2005;289:E935–E939. doi: 10.1152/ajpendo.00095.2005. [DOI] [PubMed] [Google Scholar]
- 30.Muoio DM. Intramuscular triacylglycerol and insulin resistance: guilty as charged or wrongly accused? Biochim. Biophys Acta. 2010;1801:281–288. doi: 10.1016/j.bbalip.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hodges BD, Wu CC. Proteomic insights into an expanded cellular role for cytoplasmic lipid droplets. J Lipid Res. 2010;51:262–273. doi: 10.1194/jlr.R003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimmel AR, et al. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res. 2010;51:468–471. doi: 10.1194/jlr.R000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miura S, et al. Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J Biol Chem. 2002;277:32253–32257. doi: 10.1074/jbc.M204410200. [DOI] [PubMed] [Google Scholar]
- 34.Lu X, et al. The murine perilipin gene: the lipid droplet-associated perilipins derive from tissue-specific, mRNA splice variants and define a gene family of ancient origin. Mamm Genome. 2001;9:741–749. doi: 10.1007/s00335-01-2055-5. [DOI] [PubMed] [Google Scholar]
- 35.Bickel PE, et al. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta. 2009;1791:419–440. doi: 10.1016/j.bbalip.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu G, et al. Degradation of perilipin is mediated through ubiquitination-proteasome pathway. Biochim Biophys Acta. 2006;1761:83–90. doi: 10.1016/j.bbalip.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Xu G, et al. Post-translational regulation of adipose differentiation-related protein by the ubiquitin/proteasome pathway. J Biol Chem. 2005;280:42841–42847. doi: 10.1074/jbc.M506569200. [DOI] [PubMed] [Google Scholar]
- 38.Kovsan J, et al. Regulation of adipocyte lipolysis by degradation of the perilipin protein: nelfinavir enhances lysosome-mediated perilipin proteolysis. J Biol Chem. 2007;282:21704–21711. doi: 10.1074/jbc.M702223200. [DOI] [PubMed] [Google Scholar]
- 39.Wolins NE, et al. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–5491. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 40.Minnaard R, et al. Adipocyte differentiation-related protein and OXPAT in rat and human skeletal muscle: involvement in lipid accumulation and type 2 diabetes mellitus. J Clin Endocrinol Metab. 2009;94:4077–4085. doi: 10.1210/jc.2009-0352. [DOI] [PubMed] [Google Scholar]
- 41.Tansey JT, et al. The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life. 2004;56:379–385. doi: 10.1080/15216540400009968. [DOI] [PubMed] [Google Scholar]
- 42.Zhang HH, et al. Lipase-selective functional domains of perilipin A differentially regulate constitutive and protein kinase A-stimulated lipolysis. J Biol Chem. 2003;278:51535–5142. doi: 10.1074/jbc.M309591200. [DOI] [PubMed] [Google Scholar]
- 43.Tansey JT, et al. Functional studies on native and mutated forms of perilipins. A role in protein kinase A-mediated lipolysis of triacylglycerols. J Biol Chem. 2003;278:8401–8406. doi: 10.1074/jbc.M211005200. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi T, et al. CGI-58 interacts with perilipin and is localized to lipid droplets. Possible involvement of CGI-58 mislocalization in Chanarin-Dorfman syndrome. J Biol Chem. 2004;279:30490–30497. doi: 10.1074/jbc.M403920200. [DOI] [PubMed] [Google Scholar]
- 45.Subramanian V, et al. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3-L1 adipocytes. J Biol Chem. 2004;279:42062–42071. doi: 10.1074/jbc.M407462200. [DOI] [PubMed] [Google Scholar]
- 46.Granneman JG, et al. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) 2009;284:34538–34544. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, et al. Activation of hormone-sensitive lipase requires two steps, protein phosphorylation and binding to the PAT-1 domain of lipid droplet coat proteins. J Biol Chem. 2009;284:32116–32125. doi: 10.1074/jbc.M109.006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Granneman JG, et al. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem. 2007;282:5726–5735. doi: 10.1074/jbc.M610580200. [DOI] [PubMed] [Google Scholar]
- 49.Shen WJ, et al. Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. J Lipid Res. 2009;50:2306–2313. doi: 10.1194/jlr.M900176-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Dalen KT, et al. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim Biophys Acta. 2007;1771:210–212. doi: 10.1016/j.bbalip.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi T, et al. MLDP, a novel PAT family protein localized to lipid droplets and enriched in the heart, is regulated by peroxisome proliferator-activated receptor alpha. J Biol Chem. 2006;281:14232–14240. doi: 10.1074/jbc.M601682200. [DOI] [PubMed] [Google Scholar]
- 53.Wolins NE, et al. OXPAT/PAT-1 is aPPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 2006;55:3418–3428. doi: 10.2337/db06-0399. [DOI] [PubMed] [Google Scholar]
- 54.Granneman JG, et al. Interactions of perilipin-5 (PLIN5) with adipose trigylceride lipase (ATGL) J Biol Chem. 2010 doi: 10.1074/jbc.M110.180711. ( http://www.jbc.org/cgi/) [DOI] [PMC free article] [PubMed]
- 55.Granneman JG, et al. Functional interactions between Mldp (LSDP5) and Abhd5 in the control of intracellular lipid accumulation. J Biol Chem. 2009 Jan;284:3049–3057. doi: 10.1074/jbc.M808251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang H, et al. Unique Regulation of Adipose Triglyceride Lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J Biol Chem. 2011 doi: 10.1074/jbc.M110.207779. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaguchi T, et al. Analysis of interaction partners for perilipin and ADRP on lipid droplets. Mol Cell Biochem. 2006;284:167–173. doi: 10.1007/s11010-005-9045-y. [DOI] [PubMed] [Google Scholar]
- 58.Marín-García J, Goldenthal MJ. Mitochondrial centrality in heart failure. Heart Fail Rev. 2008;13:137–150. doi: 10.1007/s10741-007-9079-1. [DOI] [PubMed] [Google Scholar]
- 59.Beller M, et al. COPI complex is a regulator of lipid homeostasis. PLoS Biol. 2008;6(11):e292. doi: 10.1371/journal.pbio.0060292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nishino N, et al. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Invest. 2008;118:2808–2821. doi: 10.1172/JCI34090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saha PK, et al. Metabolic adaptations in the absence of perilipin: increased beta-oxidation and decreased hepatic glucose production associated with peripheral insulin resistance but normal glucose tolerance in perilipin-null mice. J Biol Chem. 2004;279:35150–25158. doi: 10.1074/jbc.M405499200. [DOI] [PubMed] [Google Scholar]
- 62.Blanchette-Mackie EJ, Scow RO. Movement of lipolytic products to mitochondria in brown adipose tissue of young rats: an electron microscope study. J Lipid Res. 1983;24:229–244. [PubMed] [Google Scholar]
- 63.Kalashnikova MM, Fadeeva EO. Ultrastructural study of liver cells from rooks living in ecologically unfavorable areas. Biology Bulletin. 2006;33:99–106. [PubMed] [Google Scholar]
- 64.Shaw CS, et al. Network distribution of mitochondria and lipid droplets in human muscle fibres. Histochem Cell Biol. 2008;129:65–72. doi: 10.1007/s00418-007-0349-8. [DOI] [PubMed] [Google Scholar]
- 65.Liu P, et al. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem. 2004;279:3787–3792. doi: 10.1074/jbc.M311945200. [DOI] [PubMed] [Google Scholar]
- 66.Wickström Y, et al. Lipid droplets interact with mitochondria using SNAP23. Cell Biol Int. 2009;33:934–940. doi: 10.1016/j.cellbi.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 67.Wang H, et al. Perilipin 5 recruits mitochondria to the lipid droplet surface thru its C terminal domain. J Lipid Research. 2011 (Submitted) [Google Scholar]
- 68.Hocsak E, et al. TIP47 protects mitochondrial membrane integrity and inhibits oxidative-stress-induced cell death. FEBS Lett. 2010;584:2953–2960. doi: 10.1016/j.febslet.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 69.Blanchette-Mackie EJ, et al. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res. 1995;36:1211–1226. [PubMed] [Google Scholar]
- 70.Servetnick DA, et al. Perilipins are associated with cholesteryl ester droplets in steroidogenic adrenal cortical and Leydig cells. J Biol Chem. 1995;270:16970–16973. doi: 10.1074/jbc.270.28.16970. [DOI] [PubMed] [Google Scholar]
- 71.Brasaemle DL, et al. Post-translational regulation of perilipin expression. Stabilization by stored intracellular neutral lipids. J Biol Chem. 1997;272:9378–9387. doi: 10.1074/jbc.272.14.9378. [DOI] [PubMed] [Google Scholar]
- 72.Subramanian V, et al. Hydrophobic sequences target and anchor perilipin A to lipid droplets. J Lipid Res. 2004;45:1983–1991. doi: 10.1194/jlr.M400291-JLR200. [DOI] [PubMed] [Google Scholar]
- 73.Arimura N, et al. The peroxisome proliferator-activated receptor gamma regulates expression of the perilipin gene in adipocytes. J Biol Chem. 2004;279:10070–10076. doi: 10.1074/jbc.M308522200. [DOI] [PubMed] [Google Scholar]