Abstract
Dementia with Lewy bodies (DLB) is often associated with occipital hypometabolism or hypoperfusion, as well as deficits in cholinergic neurotransmission. In this study, 11 mild DLB, 16 mild AD and 16 age-matched controls underwent arterial spin-labeled perfusion MRI (ASL-pMRI) and neuropsychological testing. Patterns of cerebral blood flow (CBF) and cognitive performance were compared. In addition, combined ASL-pMRI and ChEI drug challenge (pharmacologic MRI) was tested as a probe of cholinergic function in 4 of the DLB participants. Frontal and parieto-occipital hypoperfusion was observed in both DLB and AD but was more pronounced in DLB. Following ChEI treatment, perfusion increased in temporal and parieto-occipital cortex, and cognitive performance improved on a verbal fluency task. If confirmed in a larger study, these results provide further evidence for brain cholinergic dysfunction in DLB pathophysiology, and use of pharmacologic MRI as an in vivo measure of cholinergic function.
Keywords: Arterial spin-labeled perfusion MRI, Pharmacologic MRI, Dementia with Lewy bodies, Cholinesterase inhibitors
Introduction
Decreased metabolism or cerebral blood flow (CBF) in the occipital cortex (Imamura et al. 1999; Ishii et al. 1998; Lobotesis et al. 2001; Pasquier et al. 2002; Varma et al. 1997) is a finding supportive of a diagnosis of dementia with Lewy Bodies (DLB) (McKeith 2006). However, this is not always present (Kemp et al. 2007), and other regions of hypometabolism or hypoperfusion have been reported in DLB, including parieto-occipital (Colloby et al. 2002; Lobotesis et al. 2001; Varma et al. 1997), frontal (Defebvre et al. 1999), or parietal, temporal, and occipital lobes (Ishii et al. 2007). The majority of these studies included patients with mild to moderate cognitive impairments (MMSE 13–20), while only a few studies included mild (MMSE 22–24) disease (Ishii et al. 2007; Whitwell et al. 2007). Therefore, differences in severity and stage of illness may contribute to the variable findings; other reasons may arise from the small numbers of participants in these studies, and differences in the imaging modality and analysis used.
In DLB there is a greater deficit in choline acetyltransferase (ChAT) activity in the temporoparietal cortex (Tiraboschi et al. 2002) and a greater loss of cholinergic projections from the striatum and pedunculopontine nucleus to the thalamus (Francis and Perry 2007) than in Alzheimer’s disease (AD). Recent PET imaging studies in DLB confirm widespread reductions in cholinergic activity throughout the cerebral cortex (Shimada et al. 2009) with reduced acetylcholinesterase binding in occipital, parietal, temporal, and frontal lobes (Klein et al. 2010). In contrast, the cholinergic deficit in early AD is minimized by compensatory up-regulation of ChAT activity in the hippocampus and the frontal cortex (DeKosky et al. 2002). These findings are supported by observations that the clinical response to cholinesterase inhibitor (ChEI) treatment in DLB is often dramatic (McKeith et al. 2000), in contrast to modest effects observed in AD (Aarsland et al. 2003).
For these reasons, we were motivated to further characterize the patterns of cerebral blood flow in patients with mild DLB, and to investigate the contributions of the cholinergic system to such changes in CBF. Arterial spin-labeled perfusion MRI (ASL-pMRI) (Detre and Alsop 1999) is a non-invasive technique that uses magnetic labeling of water in arterial blood as a diffusible tracer to generate quantitative measures of absolute CBF. ASL-pMRI has been applied to the study of AD, stroke, epilepsy, and normal brain function (Detre et al. 2009) and the findings in these studies are consistent with other established neuroimaging methods. In addition to use in differential diagnosis, ASL-pMRI is ideally suited for pharmacologic MRI (phMRI), a technique that measures the effects of drug challenges on neuronal activity and can provide an assessment of in vivo neurotransmitter function with high anatomic resolution. For example, phMRI can provide insight into the cholinergic deficits observed in dementia. Previous studies in AD have shown that a single dose of ChEI increases posterior parietotemporal and superior frontal perfusion (Ebmeier et al. 1992; Geaney et al. 1990); or, after 6 months of treatment with ChEI, AD patients who had improvement in clinical status, reasoning, working memory, and sustained attention had corresponding increases in CBF (Venneri et al. 2002). In DLB patients with hallucinations, treatment with ChEI results in a focal increase in CBF in the occipital cortex, and this correlated with a reduction in hallucinations (Mori et al. 2006).
In the present study, ASL-pMRI was used to examine CBF in patients with mild DLB compared to AD (comparison study), and in a small group of DLB participants before and after treatment with a ChEI (phMRI study). We propose that ASL-pMRI is capable of detecting hypoperfusion even in mild DLB compared to mild AD, and that combined ASL-pMRI and pharmacologic challenge (ChEI) can be used as a probe of cholinergic function.
Materials and methods
Participants
Two separate studies were performed, a cross-sectional comparison study, and a separate pilot pharmacologic study (phMRI study), with some participants completing both. Participants with probable DLB, probable AD, and normal age-matched controls were recruited for the studies. For the comparison study, 43 participants (11 probable DLB, 16 probable AD, and 16 normal age-matched controls) underwent ASL-pMRI and cognitive testing. Participants with dementia were limited to those with MMSE scores greater than 20. One DLB participant was excluded from the comparison study due to a frontal venous anomaly, to make the final N=10 in the comparison study. The AD and control participants were a subset of a larger cohort previously analyzed and published elsewhere (D. C. Alsop et al. 2008). A separate pilot study to test the utility of ASL-pMRI to measure perfusion changes in DLB following treatment with ChEI (phMRI study) was conducted, as described below. The phMRI study included 4 DLB participants, three of whom were in the comparison study, plus one additional DLB participant excluded from the comparison study (frontal venous anomaly). Diagnosis of AD or DLB was made by 2 experienced neurologists using the NINCDS/ADRDA criteria for AD (Tierney et al. 1988) or consensus criteria for “probable” DLB (McKeith 2006). Demographic information is summarized in Table 1. Eligible participants were recruited from the Behavioral Neurology Unit at Beth Israel Deaconess Medical Center, Boston, MA. Age- and education-matched controls were recruited from amongst spouses of participants in this and other research studies, to minimize recruitment biases. The Committee on Clinical Investigations of Beth Israel Deaconess Medical Center approved the study protocol. All participants gave written informed consent. Participants with dementia who demonstrated ability to make choices and who were capable of understanding associated risks and benefits to study participation were allowed to provide their own consent. Participants with limited cognitive abilities were required to provide assent for study participation, and a health care proxy, spouse, or close family member provided informed consent.
Table 1.
Demographic characteristics of study groups. The total DLB cohort (n=11) is described separately by participant inclusion in comparison study (n =10) or the treatment study (n=4). Of the total DLB cohort, one participant was excluded from the comparison study because of a small venous angioma (right frontal lobe), but kept in the treatment study; three participants were included in both the treatment and comparison group
| Characteristic | DLB (n=10) comparison | DLB (n=4) treatment | AD (n=16) | Control (n=16) | Pa |
|---|---|---|---|---|---|
| Age, years (SD) | 71.1 (10.2) | 62.3 (10.2) | 78.0 (8.6) | 71.5 (8.9) | 0.08 |
| Sex (F:M) | 1:9 | 1:3 | 10:6 | 9:7 | – |
| Education, years (SD) | 17.1 (2.8) | 15.3 (3.0) | 15.8 (2.8) | 14.3 (3.8) | 0.10 |
| MMSE (SD) | 28.1 (1.7) | 29.5 (0.6) | 25.3 (2.6) | 28.1 (2.7) | 0.003 |
| ChEI treatment (%) | 8 (80%) | – | 11 (69%) | 0 | – |
One-way ANOVA was used to compare continuous variables between DLB Comparison, AD, and Control groups. Subgroup comparisons two-tailed t-test for MMSE was p<0.05 for AD vs. controls, and AD vs. DLB Comparison
All potential participants underwent detailed history and physical examinations, routine laboratory testing and imaging as part of a diagnostic clinical evaluation. Participants with clinical depression, history of brain trauma, or epilepsy were excluded to eliminate any potentially confounding effects from such conditions. Participants with significant cerebrovascular disease, either by clinical history or by neuroimaging (CT or MRI showing territorial or lacunar infarcts, or extensive white matter changes) were also excluded, to minimize the co-occurrence of vascular dementia. All participants were proficient in the English language.
Cognitive assessment and clinical severity rating
Neuropsychological testing included the MMSE (Folstein et al. 1975), the Consortium to Establish a Registry for Alzheimer’s Disease neuropsychological battery (CERAD), controlled oral word association test (COWAT), Boston Naming Test (BNT), and digit span. For the phMRI study, participants were given a modified battery testing similar domains, including the Rey auditory verbal learning task (RAVLT) instead of the CERAD to assess memory. All but 3 of the 43 enrolled participants were tested on the day of the MRI scan. Due to unavoidable logistical constraints, two AD participants were tested 2 weeks after their scan and one control participant was tested 5 months after the MRI. Some cognitive data were missing due to participant fatigue.
Image acquisition
Brain MRI scans were acquired using one of two 3 Tesla whole body scanners (GE Healthcare Technologies, Milwaukee WI). Subjects participating only in the comparison study, which preceded the phMRI study, were studied on a 3T VH/I scanner using the transmit receive head coil; for the phMRI study, participants were scanned using a 3T HD scanner and the 8 channel array receive coil. ASL-pMRI (Williams et al. 1992) was performed in the entire supratentorial brain with 3D imaging sequences. The comparison study subjects were imaged identically to the methods described in Alsop et al (D. C. Alsop et al. 2008). Briefly, continuous ASL was achieved using flow driven adiabatic inversion and an improved version of a previously published method for subtracting off-resonance saturation effect (D. C. Alsop and Detre 1998). A background suppressed 3D Fast Spin Echo sequence with cubic resolution of 3.8 mm was employed. Acquisition of the perfusion image and the reference images needed for perfusion quantification required approximately 30 min. For the phMRI study, a faster 3D Spiral FSE sequence was employed using pulsed continuous labeling (Dai et al. 2008). The sequence had nominal spatial resolution of 3.8×3.8×4 mm, however the images appear slightly blurrier due to T2 decay during the longer FSE readout. Quantification of both ASL sequences was identical to that previously described (D. C. Alsop et al. 2008). Foam padding inserted around the participant’s head was used to minimize motion. All patients tolerated the 3T MRI without adverse effects.
Pilot pharmacological MRI study
Four DLB participants took part in a randomized double-blinded, placebo-controlled pilot study (phMRI study) where CBF was measured after a single dose of donepezil or placebo. Each participant was studied in two separate sessions held 2 weeks apart. Half of the participants were randomized to receive placebo at the first visit, followed by donepezil 5 mg at the second visit; the other half were treated in reverse order. MRI scans were obtained 3 h following oral administration of donepezil or placebo, at the time of peak plasma concentration. Following the MRI scan, participants underwent a battery of cognitive testing. None of the participants experienced adverse effects during the study. Investigators and participants were blinded to the treatment groups.
Statistical analysis
Perfusion images were transformed to a standard atlas based on the intensities in the anatomical reference images. The voxel size in the transformed images was 2 * 2 * 2 mm3. For the comparison study, voxelwise statistical comparison was performed using the SPM2 (Wellcome Department of Cognitive Neurology) compare groups function after global normalization and smoothing (using Gaussian kernel of full width half maximum (FWHM) 8 mm) to test for group differences among the control group, the DLB group, and the AD group. Gender was included as a co-variate in a two-way analysis of covariance (ANCOVA) model. Clusters were analyzed on maps thresholded at p<0.05 and only clusters of at least 1600 voxels corresponding to a corrected p<0.05 were retained. A toolbox for SPM that calculates the percentage of a cluster in each of a number of predefined regions within the atlas (Tzourio-Mazoyer et al. 2002) was used to systematically identify the anatomical location of clusters. All regions containing at least 2% of each cluster were recorded. One-way ANOVA was used for comparison of cognitive performance. For participants in both the comparison and phMRI study, the imaging and cognitive data obtained on the day of donepezil treatment were used in the DLB group comparisons.
Analysis of the pilot phMRI study was performed using Statistical nonParametric Mapping (SnPM, http://www.sph.umich.edu/ni-stat/SnPM/). A non-parametric approach was chosen because the small sample size could not be assumed to have a normal distribution and the sample has a low degree of freedom. A voxel-level pseudo-t threshold of 2.6 (t-value of 2.35 corresponding to a voxel-level p-value of 0.05) was chosen to define clusters in each permutation. The largest cluster size in each permutation was then used to calculate the empirical distribution in order to correct for multiple comparisons among clusters. Only the clusters with corrected p-values less than 0.05 were reported.
Results
Demographic characteristics
Participant characteristics are shown in Table 1. Across groups there were no differences in age or education, although the AD group was slightly older than control and DLB groups. For both control and AD groups, slightly more participants were female whereas for the DLB group there was only one female participant. This is consistent with epidemiologic studies showing DLB more often affects males, and persons with AD presenting over the age of 70 more likely to be female (Barker et al. 2002). A majority of the DLB and AD participants were treated with a ChEI at the time of MRI scanning. All participants were clinically rated as “mild” based on MMSE (score >20), however performance was significantly more impaired for the AD group compared to both the control and DLB groups (p<0.05) (Table 1).
Neuropsychological testing
Performance on testing was consistent with cognitive profiles described for DLB and AD. The DLB group had significantly more difficulty with delayed recall compared with controls, but did better on both delayed recall and recognition testing than the AD group, as expected in early DLB where the memory difficulty is usually an encoding and retrieval deficit (Salmon et al. 1996). The DLB group also showed relative preservation of performance on confrontation naming (BNT) and semantic fluency, in contrast to AD (Table 2).
Table 2.
Neuropsychological test results across study groups
| Domain/Test | DLB Comparison (n=10) | AD (n=16) | Control (n=16) | Pa |
|---|---|---|---|---|
| Memoryb | ||||
| CERAD- delayed recall (SD)c, d | 2.9 (2.5) | 0.9 (1.5) | 6.7 (2.8) | <0.0001 |
| CERAD – recognition (SD)c | 6.9 (3.4) | 4.6 (3.2) | 8.9 (2.5) | 0.002 |
| RAVLT – total recall (SD) | 26.3 (9.5) | |||
| RAVLT – delayed recall (SD) | 4.3 (2.3) | |||
| RAVLT – recognition (SD) | 11.3 (2.9) | |||
| Working Memory | ||||
| Semantic fluency (SD)c | 14.3 (5.5) | 9.9 (4.0) | 19.7 (7.7) | 0.0002 |
| Digit Span – forward (SD) | 8.2 (1.9) | 9.3 (1.8) | 9.8 (3.1) | 0.28 |
| Digit Span – back (SD) | 5.4 (2.2) | 6.7 (2.2) | 7.1 (2.7) | 0.24 |
| Language | ||||
| BNT (SD) | 13.6 (1.0) | 10.8 (2.8) | 13.1 (3.8) | 0.04 |
| FAS (SD) | 38.4 (13.8) | 37.0 (17.6) | 42.6 (21.1) | 0.67 |
Data are missing from one control participant for all tests except FAS; from one AD participant for digit span; and for two AD participants on CERAD. For the DLB comparison group, 3 subjects did not undergo the CERAD and instead were tested on the RAVLT) and showed similar patterns of performance, with impairments in verbal recall and relatively better performance on recognition testing
BNT Boston Naming Test (score 0–15, higher score=better performance), CERAD Consortium to Establish a Registry for Alzheimer’s Disease (scores, delayed recall 0–10; recognition 0–10; higher score=better performance), RAVLT Rey Auditory Verbal Learning Test (scores, total recall 0–75; delayed recall 0–15; recognition 0–15; higher score=better performance), FAS phonemic fluency test (higher score=better performance)
One-way ANOVA was used to compare continuous variables between groups
For DLB participants on memory tests, n=7 for CERAD; n=3 for RAVLT
Post hoc Tukey, p<0.01 for Controls vs. AD
Post hoc Tukey, p<0.01 for Controls vs. DLB
In the phMRI study, all four DLB participants had similar performance on MMSE at baseline (29.5±0.3). Following a single dose of donepezil, significant improvement was found on performance on COWAT, a measure of sustained attention, working memory, and language, across all four participants (Fig. 1). Participants 1 and 4, who had the greatest improvement in COWAT performance also had the greatest fractional increase in CBF, 24.3% and 19.8%, respectively, while Participants 2 and 3 with only minimal improvement on COWAT had no significant fractional increases in CBF. The improvement on COWAT performance did not appear to be related to practice effect, as participants 1 and 3 were given placebo at visit 1 and donepezil at visit 2, and for participants 2 and 4 the order of drug administration was reversed.
Fig. 1.
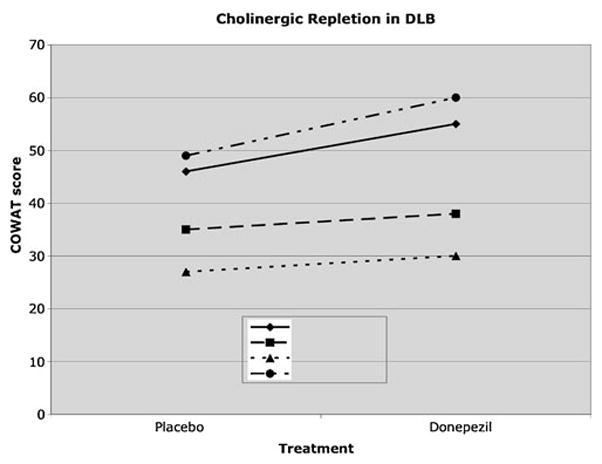
Cognitive performance in participants with DLB tested after a single dose of donepezil on a test of verbal fluency; p<0.05, paired t-test. COWAT= Controlled Oral Word Association Task. Participants 1 and 4 with the largest change in COWAT score also had the largest percent increase in perfusion. Half of the participants were tested on placebo first (participants 1 and 3) and half tested on donepezil first (participants 2 and 4)
Regional cerebral blood flow
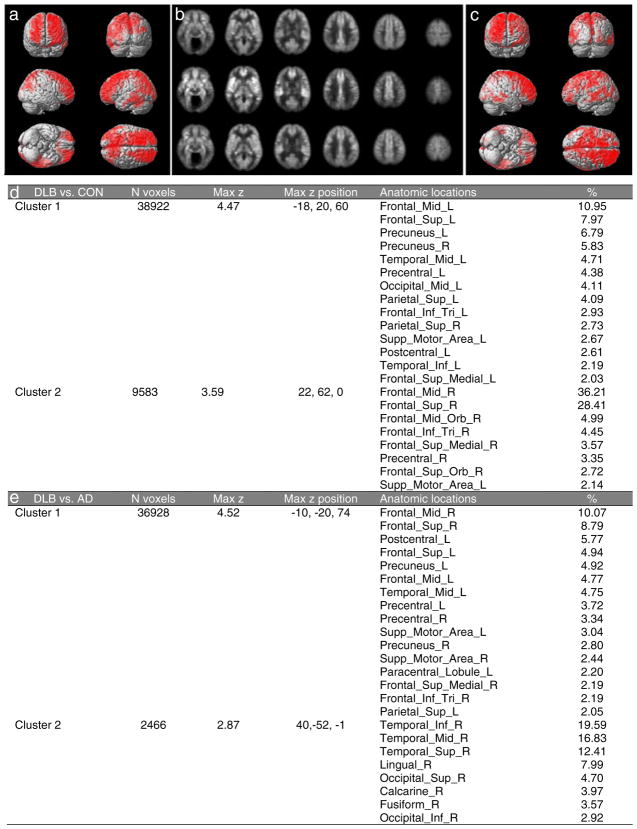
Images of high quality were obtained in all participants. Reductions in CBF were found in DLB, most notably in the frontal, precuneus, parietal, and occipital lobes bilaterally, compared to controls (Fig. 2a and b) by 10 to 20% (Table 3). For the contrast of DLB CBF greater than control, no significant regions were identified. When compared to AD, CBF was decreased in the DLB group (Fig. 2b and c) in bilateral frontal, precuneus, and right temporal and occipital lobes by 10–12% (Table 3). There were no significant regions found for the contrast of DLB CBF greater than AD. Cluster statistics for reduced perfusion for both DLB compared to control and DLB compared to AD is shown in Fig. 2d and e with anatomical regions containing more than 2% of the cluster size listed. A technical limitation of note is that images for 3 of the DLB participants in the comparison study were obtained using a different scanner and sequence. However, a separate analysis that excluded these 3 participants yielded qualitatively similar results (data not shown).
Fig. 2.
Decreased perfusion in DLB measured using ASL-perfusion MRI. a Statistical parametric maps showing areas (red) with decreased perfusion in DLB (n=10) relative to controls (n=16). b Average axial blood flow images in controls, n=16 (top), AD, n=16 (middle), and DLB, n=10 (bottom). c Statistical parametric maps showing areas (red) with decreased perfusion in DLB (n=10) relative to AD (n=16). d Clusters with significant decreases in globally normalized CBF in DLB relative to age-matched controls or e AD. % indicates the percentage of each cluster that falls within the defined region. For (a) and (c), the corrected p-value <0.05 corresponds to clusters of at least 1600 voxels
Table 3.
Cerebral blood flow (ml/100 g tissue/minute) in clusters of significantly decreased globally normalized blood flow in DLB compared to (a) control or (b) AD. Clusters 1 and 2 in (a) and (b) refers to Clusters 1 and 2 in Fig. 2d and e, respectively
| Cluster 1 | Cluster 2 | |
|---|---|---|
| a DLB vs. CON | ||
| Mean DLB (SD) | 52.4 (7.2) | 55.1 (6.0) |
| Mean Control (SD) | 64.2 (5.9) | 64.1 (5.9) |
| b DLB vs. AD | ||
| Mean DLB (SD) | 56.9 (4.5) | 44.2 (7.0) |
| Mean AD (SD) | 66.3 (5.3) | 52.2 (8.5) |
For the phMRI study, global perfusion after donepezil fractionally increased across the group by 14.5%±5.3% (p<0.05, one-tailed t-test), whereas there was no decrease in CBF following ChEI compared to placebo. SnPM analysis revealed significant regions of increased perfusion bilaterally in temporal, parietal, and occipital regions (Fig. 3a). Perfusion maps from a single subject in the phMRI group is shown (Fig. 3b). Cluster statistics are reported in Fig. 3c, with anatomical regions containing more than 2% of the cluster size listed. The cluster had a corrected p-value of 0.03.
Fig. 3.
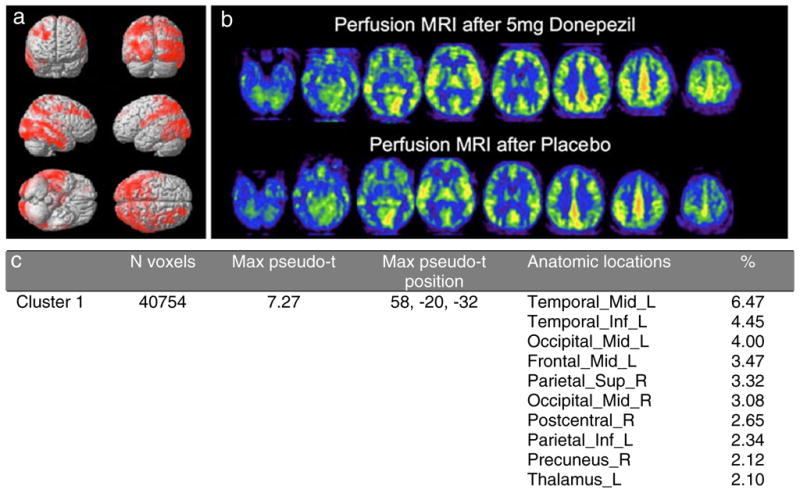
a Statistical non-parametric maps showing areas (red) of increased perfusion in 4 participants with DLB treated with a single dose of donepezil. Significant regions of increased perfusion were detected bilaterally in temporal, parietal, and occipital regions. P-value corrected for the cluster was 0.03. b Perfusion maps of an individual DLB participant following single dose donepezil or placebo. The right side of the image represents the right side of the brain, neurological convention. Red > yellow are the regions of highest flow. c Clusters with significant increases in globally normalized CBF in DLB following treatment with the ChEI donepezil. % indicates the percentage of each cluster that falls within the defined region
Discussion
In this study, we identified significant hypoperfusion in early stages of DLB. The most notable difference between DLB and AD was not only the regional pattern of hypoperfusion, but also the relative amount of CBF reduction among clinically defined mild DLB and AD participants. With a greater magnitude of hypoperfusion, DLB participants showed markedly different patterns of performance on cognitive testing. In our pilot phMRI study, ASL-pMRI detected an improvement in association cortex hypoperfusion following ChEI treatment, and this increase in CBF was associated with improvement in cognitive performance.
Regional changes in perfusion
Previous studies identify occipital hypoperfusion in DLB. This pattern is a supporting feature in the diagnosis of DLB (McKeith 2006), and may serve as a basis for the visuospatial deficits that frequently occur in this disease. However, a decrease in perfusion has also been observed in other regions (Colloby et al. 2002; Ishii et al. 2007), including the inferior frontal, temporal, and parietal cortex of participants with DLB compared to normal controls. Our findings looking at clusters of decreased perfusion are consistent with these previously reported patterns, and correlate with the attentional, executive function, episodic memory, and visuospatial dysfunction commonly observed. Furthermore, there is greater reduction in CBF in DLB than in AD.
Cholinergic neurons in the basal forebrain may be one of the earliest brain regions affected in DLB with moderate neuronal loss and gliosis in the nucleus basalis of Meynert (NBM) (Albin et al. 1996; Braak et al. 2003). Recent studies in DLB patients with mild disease (MMSE 22–24) find distinct patterns of atrophy in the dorsal midbrain, hypothalamus, and substantia inominata with sparing of cortical involvement (Whitwell et al. 2007). Others describe striatal atrophy with more intense hypometabolism in frontal, temporoparietal, and occipital association cortex (Ishii et al. 2007). A loss of widespread projections to the cortex is a likely explanation for these findings, and a recent study showing reduced cortical acetylcholinesterase binding and glucose metabolism in PET studies (Klein et al. 2010) supports this notion. In addition to cortical projections, cholinergic neurons from the NBM project to cerebral blood vessels, particularly in frontoparietal cortex (Sato et al. 2002). It has been suggested that the physiologic role of this cholinergic vasodilator system is in preparation for increasing cerebral neuronal activity (Sato and Sato 1992). Thus, regional hypoperfusion in DLB may reflect reduced neuronal activity from both the loss of cholinergic projections from subcortical neurons and dynamic vascular effects, and may contribute to the greater hypoperfusion noted in our DLB compared to AD group.
Cognitive performance and perfusion
Regional reductions in CBF might explain some of the early differences in cognitive impairment in DLB compared to AD. For example, frontal hypoperfusion may be related to symptoms in DLB such as inattention and difficulties on tests of sustained attention, whereas decreased perfusion in the precuneus may play an important role in the impairments in visuospatial function and episodic memory retrieval (Cavanna and Trimble 2006) commonly seen in DLB. In this study, despite a 10–12% decrease in CBF in mild DLB compared to mild AD, participants with DLB had relatively intact performance on the MMSE. A similar finding of disproportionate widespread cortical hypometabolism in DLB relative to AD patients with comparable performance on cognitive testing has been described in a previous study in patients with an average MMSE of 24 (Ishii et al. 2007). Ishii et al. speculated that this finding may be a result of better “cognitive reserve” in the DLB subjects or that the MMSE is not sensitive enough to detect the degree of neurodegeneration in DLB. Given that ChAT activity is more reduced in DLB relative to AD (Tiraboschi et al. 2002), it is possible that the hypoperfusion observed may in part be related to cholinergic dysfunction.
Response to cholinesterase inhibitors
The clinical response to ChEI treatment in DLB is well described and often dramatic (McKeith et al. 2000). For this reason, treatment of DLB with ChEI is an ideal model for testing the utility of combined ASL-pMRI and drug challenge as an in vivo probe of cholinergic function. Our pilot phMRI study demonstrates that ASL-pMRI can detect changes in perfusion following a single oral dose of donepezil in DLB. The test-retest reliability of ASL-pMRI for measuring blood flow in young healthy subjects demonstrated a 5.8% standard deviation in whole brain blood flow values when measured one hour apart, and a 13% standard deviation when measured 1 week apart. For regional values the standard deviations were 13% and 14% for one hour and 1 week apart, respectively (Floyd et al. 2003). Others have confirmed the reproducibility of repeated ASL-pMRI scans, with a 95% confidence limit of 12% for whole brain perfusion and 11% for hemispheric perfusion (Parkes et al. 2004). While the small number of participants limits this study, it is interesting to note the regions of increased perfusion in DLB following ChEI treatment correspond to regions of hypoperfusion observed at baseline. Furthermore, we observed both improved CBF and performance on cognitive tests, specifically, tests of verbal fluency. This is consistent with the notion that frontosubcortical dysfunction may be more directly related to cholinergic functioning (Bohnen et al. 2005), and observations that symptoms of DLB have a greater response to ChEI treatment than the cognitive symptoms in AD (McKeith et al. 2000). Further, PET studies comparing DLB with Parkinson’s disease without dementia suggest that a cholinergic deficit is necessary for the development of cognitive symptoms (Klein et al. 2010). The findings in this study, while descriptive, will need to be confirmed in a larger number of participants, and should serve only as a basis for additional future studies. It also must be noted that the participant excluded from the comparison study because of a venous anomaly was kept in the phMRI study, as we did not believe this would affect the CBF response to treatment, except in the nearby frontal lobe.
Limitations
One limitation of this study is that the DLB and AD groups differed in MMSE scores. While the MMSE is often used as a measure of disease severity in AD, it has not been validated in DLB. In addition, matching based on MMSE score is likely flawed due to differences in cognitive domains affected in DLB and AD, as methods which place more weight on orientation, attention, and construction aspects from the MMSE may be more sensitive for differentiating DLB from AD (Ala et al. 2002; Palmqvist et al. 2009). However, even without accurate matching on disease severity, the finding of greater hypoperfusion in DLB compared to AD despite a “normal” performance on MMSE remains a clinically important finding.
Second, none of the cases were pathologically confirmed. Without autopsy-confirmed diagnosis, it is possible that participants may have had varying degrees of co-morbid AD pathology, which might produce variability in outcome. However, the DLB consensus criteria have shown high specificity (0.91) and sensitivity (0.83) in a previous prospective study (McKeith 2002). In our cohort with preserved MMSE and naming performance, concomitant AD pathology seems less likely. Assuming any cross-contamination in the diagnosis, the outcome would be a reduction in power to detect differences and would bias against a positive finding. Thus, our findings of significance differences, despite a potentially conservative bias, provide greater evidence for the robustness of our results.
A key difference between this study and previous CBF studies of DLB patients is the severity of cognitive impairment, and presumably, the stage of illness. Prior studies (Colloby et al. 2002; Lobotesis et al. 2001) have included more “advanced” DLB participants with an average MMSE of <18. Participants in the current study were clinically very mild and younger than the AD participants. Changes in CBF over the disease course are not known, but presumably, hypoperfusion reflecting neuronal dysfunction or dysregulation of cholinergic microvascular tone would remain stable or worsen over time, rather than improve. Evidence from a single longitudinal study of CBF in DLB and Parkinson’s disease dementia showed no change in cortical perfusion, although putaminal perfusion increased over 1 year (Firbank et al. 2005). Many prior studies do not specifically comment on ChEI usage at the time of neuroimaging, and it is possible that such studies may have confounded and underestimated the degree of hypoperfusion in the DLB group. In our own comparison study, 80% of the DLB and 69% of the AD participants were being treated with ChEI at the time of the scan, which raises the possibility that the perfusion difference measured here underestimates the true pathophysiologic state. Further, PET studies using fixed doses of donepezil in patients with AD do show variation in the extent of AChE inhibition (Bohnen et al. 2005), and it is possible that there is significant variability in AChE inhibition in the DLB compared to AD groups due to differences in underlying pathophysiology.
Finally, due to the small sample size of this study, covariates, including the effect of severity of parkinsonism could not be fully addressed.
Conclusions
In conclusion, this study demonstrates that even in mild DLB ASL-pMRI can detect profound decreases in CBF. Following a single dose of ChEI, improvements in both hypoperfusion and cognitive impairments were found. If confirmed in a larger number of participants, these results suggest that the hypoperfusion in DLB may be due to a cholinergic deficiency and that phMRI is a useful tool for the in vivo study of cholinergic function.
Further studies looking at response to ChEI visualized using phMRI are needed to better understand how the response to ChEI changes over time in DLB as well as in normal aging, and in comparison with other dementias such as AD. Ultimately, phMRI may become an important tool to study the pathophysiology of dementia and to advance the development and testing of new and more effective treatment strategies for these conditions.
Acknowledgments
Supported in part by F32NS047431(Fong) from the National Institute of Neurological Disorders and Stroke, K23AG031320 (Fong), K24AG000949 (Inouye) and RO1AG19599 (Alsop) from the National Institute on Aging, IIRG-08-88737 (Inouye) from the Alzheimer’s Association, and the Aging Brain Center, Institute for Aging Research, Hebrew SeniorLife. Support provided to Dr. Fong, Department of Neurology, Beth Israel Deaconess Medical Center and the Clinical Investigator Training Program: Beth Israel Deaconess Medical Center-Harvard/MIT Health Sciences and Technology, in collaboration with Pfizer Inc. and Merck & Co., and in part by the John A. Hartford Harvard Center of Excellence. Dr. Inouye holds the Milton and Shirley F. Levy Family Chair. Study medications (donepezil and placebo) were provided by a grant from Pfizer/Eisai, Inc.
Footnotes
Disclosure The authors report no conflicts of interest.
Contributor Information
Tamara G. Fong, Email: tfong@bidmc.harvard.edu, Aging Brain Center, Institute for Aging Research, Hebrew SeniorLife, 1200 Centre Street, Boston, MA 02131, USA. Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
Sharon K. Inouye, Aging Brain Center, Institute for Aging Research, Hebrew SeniorLife, 1200 Centre Street, Boston, MA 02131, USA. Department of Medicine, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
Weiying Dai, Department of Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA.
Daniel Z. Press, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
David C. Alsop, Department of Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
References
- Aarsland D, Litvan I, Salmon DP, Galasko D, Wentzel-Larsen T, Larsen J. Performance on the dementia rating scale in Parkinson’s disease with dementia and dementia with Lewy bodies: comparison with progressive supranuclear palsy and Alzheimer’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 2003;74:1215–1220. doi: 10.1136/jnnp.74.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ala TA, Hughes LF, Kyrouac GA, Ghobrial MW, Elble RJ. The mini-mental state exam may help in the differentiation of dementia with Lewy bodies and Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2002;17(6):503–509. doi: 10.1002/gps.550. [DOI] [PubMed] [Google Scholar]
- Albin RL, Minoshima S, D’Amato CJ, Frey KA, Kuhl DA, Sima AA. Fluro-deoxyglucose positron emission tomography in diffuse Lewy body disease. Neurology. 1996;47:462–466. doi: 10.1212/wnl.47.2.462. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Detre JA. Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology. 1998;208:410–416. doi: 10.1148/radiology.208.2.9680569. [DOI] [PubMed] [Google Scholar]
- Alsop DC, Casement M, de Bazelaire C, Fong T, Press DZ. Hippocampal hyperperfusion in Alzheimer’s disease. Neuroimage. 2008;42(4):1267–1274. doi: 10.1016/j.neuroimage.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and Hippocampal sclerosis in the state of Florida brain bank. Alzheimer Disease and Associated Disorders. 2002;16(4):203–212. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti BJ, Koeppe RA, et al. Degree of inhibition of cortical acetylcholinesterase activity and cognitive effects by Donepezil treatment in Alzheimer’s disease. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76(3):315–319. doi: 10.1136/jnnp.2004.038729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Colloby SJ, Fenwick JD, Williams ED, Paling SM, Lobotesis K, Ballard C, et al. A comparison of (99M) TC-HMPAO SPET changes in dementia with Lewy bodies and Alzheimer’s disease using statistical parametric mapping. European Journal of Nuclear Medicine and Molecular Imaging. 2002;29(5):615–622. doi: 10.1007/s00259-002-0778-5. [DOI] [PubMed] [Google Scholar]
- Dai W, Garcia D, de Bazelaire C, Alsop DC. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magnetic Resonance in Medicine. 2008;60(6):1488–1497. doi: 10.1002/mrm.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defebvre LJ, Leduc V, Duhamel A, Lecouffe P, Pasquier F, Lamy-Lhullier C, et al. Technetium HMPAO spect study in dementia with Lewy bodies, Alzheimer’s disease and idiopathic Parkinson’s disease. Journal of Nuclear Medicine. 1999;40(6):956–962. [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, et al. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Annals of Neurology. 2002;51:145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- Detre JA, Alsop DC. Perfusion magnetic resonance imaging with continuous arterial spin labeling: methods and clinical applications in the central nervous system. European Journal of Radiology. 1999;30(2):115–124. doi: 10.1016/s0720-048x(99)00050-9. [DOI] [PubMed] [Google Scholar]
- Detre JA, Wang J, Wang Z, Rao H. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Current Opinion in Neurology. 2009;22(4):348–355. doi: 10.1097/WCO.0b013e32832d9505. [DOI] [PubMed] [Google Scholar]
- Ebmeier KP, Hunter R, Curran SM, Dougal NJ, Murray CL, Wyper DJ, et al. Effects of a single dose of the acetylcholinesterase inhibitor velnacrine on recognition memory and regional cerebral blood flow in Alzheimer’s disease. Psychopharmacology (Berl) 1992;108(1–2):103–109. doi: 10.1007/BF02245293. [DOI] [PubMed] [Google Scholar]
- Firbank MJ, Burn DJ, McKeith IG, O’Brien JT. Longitudinal study of cerebral blood flow spect in Parkinson’s disease with dementia, and dementia with Lewy bodies. International Journal of Geriatric Psychiatry. 2005;20(8):776–782. doi: 10.1002/gps.1359. [DOI] [PubMed] [Google Scholar]
- Floyd T, Ratcliffe S, Wang J, Resch B, Detre J. Precision of the casl-perfusion MRI technique for the measurement of cerebral blood flow in whole brain and vascular territories. Journal of Magnetic Resonance Imaging. 2003;18:649–655. doi: 10.1002/jmri.10416. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental-state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Francis PT, Perry EK. Cholinergic and other neurotransmitter mechanisms in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies. Movement Disorders. 2007;22(Suppl 17):S351–S357. doi: 10.1002/mds.21683. [DOI] [PubMed] [Google Scholar]
- Geaney DP, Soper N, Shepstone BJ, Cowen PJ. Effect of central cholinergic stimulation on regional cerebral blood flow in Alzheimer disease. Lancet. 1990;335(8704):1484–1487. doi: 10.1016/0140-6736(90)93028-n. [DOI] [PubMed] [Google Scholar]
- Imamura T, Ishii K, Hirono N, Al E. Visual hallucinations and regional cerebral metabolism in dementia with Lewy bodies (DLB) NeuroReport. 1999;10:1903–1907. doi: 10.1097/00001756-199906230-00020. [DOI] [PubMed] [Google Scholar]
- Ishii K, Imamura T, Sasaki M, Yamaji S, Sakamoto S, Kitagaki H, et al. Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer’s disease. Neurology. 1998;51(1):125–130. doi: 10.1212/wnl.51.1.125. [DOI] [PubMed] [Google Scholar]
- Ishii K, Soma T, Kono AK, Sofue K, Miyamoto N, Yoshikawa T, et al. Comparison of regional brain volume and glucose metabolism between patients with mild dementia with Lewy bodies and those with mild Alzheimer’s disease. Journal of Nuclear Medicine. 2007;48(5):704–711. doi: 10.2967/jnumed.106.035691. [DOI] [PubMed] [Google Scholar]
- Kemp PM, Hoffmann SA, Tossici-Bolt L, Fleming JS, Holmes C. Limitations of the HMPAO spect appearances of occipital lobe perfusion in the differential diagnosis of dementia with Lewy bodies. Nuclear Medicine Communications. 2007;28(6):451–456. doi: 10.1097/MNM.0b013e328155d143. [DOI] [PubMed] [Google Scholar]
- Klein JC, Eggers C, Kalbe E, Weisenbach S, Hohmann C, Vollmar S, et al. Neurotransmitter changes in dementia with Lewy bodies and Parkinson disease dementia in vivo. Neurology. 2010;74(11):885–892. doi: 10.1212/WNL.0b013e3181d55f61. [DOI] [PubMed] [Google Scholar]
- Lobotesis K, Fenwick JD, Phipps A, Ryman A, Swann A, Ballard C, et al. Occipital hypoperfusion on spect in dementia with Lewy bodies but not ad. Neurology. 2001;56(5):643–649. doi: 10.1212/wnl.56.5.643. [DOI] [PubMed] [Google Scholar]
- McKeith IG. Dementia with Lewy bodies. The British Journal of Psychiatry. 2002;180:144–147. doi: 10.1192/bjp.180.2.144. [DOI] [PubMed] [Google Scholar]
- McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Journal of Alzheimer’s Disease. 2006;9(3 Suppl):417–423. doi: 10.3233/jad-2006-9s347. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Grace JB, Walker Z, Byrne EJ, Wilkinson D, Stevens T, et al. Rivastigmine in the treatment of dementia with Lewy bodies: preliminary findings from an open trial. International Journal of Geriatric Psychiatry. 2000;15(5):387–392. doi: 10.1002/(sici)1099-1166(200005)15:5<387::aid-gps131>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Mori T, Ikeda M, Fukuhara R, Nestor PJ, Tanabe H. Correlation of visual hallucinations with occipital RCBF changes by Donepezil in DLB. Neurology. 2006;66(6):935–937. doi: 10.1212/01.wnl.0000203114.03976.b0. [DOI] [PubMed] [Google Scholar]
- Palmqvist S, Hansson O, Minthon L, Londos E. Practical suggestions on how to differentiate dementia with Lewy bodies from Alzheimer’s disease with common cognitive tests. International Journal Geriatric Psychiatry. 2009 doi: 10.1002/gps.2277. [DOI] [PubMed] [Google Scholar]
- Parkes LM, Rashid W, Chard DT, Tofts PS. Normal cerebral perfusion measurements using arterial spin labeling: reproducibility, stability, and age and gender effects. Magnetic Resonance in Medicine. 2004;51(4):736–743. doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- Pasquier J, Michel BF, Brenot-Rossi I, Hassan-Sebbag N, Sauvan R, Gastaut JL. Value of (99M)TC-ECD Spet for the diagnosis of dementia with Lewy bodies. European Journal of Nuclear Medicine and Molecular Imaging. 2002;29(10):1342–1348. doi: 10.1007/s00259-002-0919-x. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Galasko D, Hansen LA. Neuropsychological deficits associated with diffuse Lewy body disease. Brain and Cognition. 1996;31:148–165. doi: 10.1006/brcg.1996.0039. [DOI] [PubMed] [Google Scholar]
- Sato A, Sato Y. Regulation of regional cerebral blood flow by cholinergic fibers originating in the basal forebrain. Neuroscience Research. 1992;14(4):242–274. doi: 10.1016/0168-0102(92)90071-j. [DOI] [PubMed] [Google Scholar]
- Sato A, Sato Y, Uchida S. Regulation of cerebral cortical blood flow by the basal forebrain cholinergic fibers and aging. Autonomic Neuroscience. 2002;96(1):13–19. doi: 10.1016/s1566-0702(01)00367-8. [DOI] [PubMed] [Google Scholar]
- Shimada H, Hirano S, Shinotoh H, Aotsuka A, Sato K, Tanaka N, et al. Mapping of brain acetylcholinesterase alterations in Lewy body disease by pet. Neurology. 2009;73(4):273–278. doi: 10.1212/WNL.0b013e3181ab2b58. [DOI] [PubMed] [Google Scholar]
- Tierney MC, Fisher RH, Lewis AJ, Zorzitto ML, Snow WG, Reid DW, et al. The NINCDS-ADRDA work group criteria for the clinical diagnosis of probable Alzheimer’s disease: a clinicopathologic study of 57 cases. Neurology. 1988;38(3):359–364. doi: 10.1212/wnl.38.3.359. [DOI] [PubMed] [Google Scholar]
- Tiraboschi P, Hansen LA, Alford M, Merdes A, Masliah E, Thal LJ, et al. Early and widespread cholinergic losses differentiate dementia with Lewy bodies from Alzheimer disease. Archives of General Psychiatry. 2002;59(10):946–951. doi: 10.1001/archpsyc.59.10.946. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Varma AR, Talbot PR, Snowden JS, Lloyd JJ, Testa HJ, Neary D. A 99MTC-HMPAO single-photon emission computed tomography study of Lewy body disease. Journal of Neurology. 1997;244(6):349–359. doi: 10.1007/s004150050101. [DOI] [PubMed] [Google Scholar]
- Venneri A, Shanks MF, Staff RT, Pestell SJ, Forbes KE, Gemmell HG, et al. Cerebral blood flow and cognitive responses to rivastigmine treatment in Alzheimer’s disease. NeuroReport. 2002;13(1):83–87. doi: 10.1097/00001756-200201210-00020. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Weigand SD, Shiung MM, Boeve BF, Ferman TJ, Smith GE, et al. Focal atrophy in dementia with Lewy bodies on MRI: a distinct pattern from Alzheimer’s disease. Brain. 2007;130(Pt 3):708–719. doi: 10.1093/brain/awl388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(1):212–216. doi: 10.1073/pnas.89.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]