Abstract
Background
There is considerable controversy about the treatment of patients with malignant functional or nonfunctional pancreatic endocrine tumors (PETs). Aggressive surgery with dissection and/or reconstruction of major vascular structures is a potentially efficacious antitumor therapy, but is rarely performed, and considered a contraindication to surgery by many.
Hypothesis
Aggressive resection of locally advanced PETs in which preoperative studies suggest major vascular involvement can be performed with acceptable morbidity and mortality rates and may lead to extended survival.
Design
The combined databases of the prospective NIH study on PETs (gastrinomas) (from 1982) and Stanford (all PETs)(from 2004) were queried. All patients with possible involvement of major vascular structures were reviewed and preoperative studies, operative findings and surgical results/outcomes correlated.
Main Outcome Measures
Surgical procedure, pathologic characteristics, complications, mortality rates, and disease-free and overall survival rates.
Results
Of 273 patients with PETs, 46 (17%) had preoperative CT evidence of major vascular involvement. There were 21 men (45%). Mean age was 42 years (range 24-76). 32 (57%) had functional tumors with 30 gastrinomas and 2 glucagonomas; the remainder (n=14) had nonfunctional PETs. 12 patients (26%) had MEN-1. 44 of 46 underwent surgery. The mean size for the primary PET on preoperative CT was 5.8 cm. The involved major vessel was as follows: portal vein (n=20, 43%), SMV or SMA (n=16, 35%), IVC (n=4, 9%), splenic vein (n=4, 9%) and heart (n=2, 4%). 42 (91%) patients had PET removed: 12 (27%) primary only, 30 (68%) with lymph nodes, and 18 (41%) with liver metastases. PETs were removed by either enucleation (n=5, 12%) or resection (n=36, 86%). Resections included distal or subtotal pancreatectomy in 23 (55%), Whipple in 10 (23%) and total in 2 (5%). 19 (45%) patients had concomitant liver resection: 10 (23%) wedge resection and 9 (21%) anatomic resections (lobectomy or trisegmentectomy). 9 (21%) had vascular reconstruction: each had reconstruction of the SMV and portal vein, while 1 had concomitant reconstruction of the SMA. There were no deaths, but 12 (28%) had complications. 18 (42%) were immediately disease-free and 5 recurred with follow-up leaving 14 (33%) long-term disease-free. The 10-year overall survival was 60%. Functional tumors had a better overall survival (p<0.0001), and liver metastases decreased overall survival (p<0.0001).
Conclusions
Aggressive surgery including superior mesenteric vein reconstruction, and liver resection can be done with acceptable morbidity and mortality rates for patients with advanced PETs. Although survival rates following surgery are excellent, most patients will develop recurrence. These findings suggest that surgical resection is indicated even in PETs with vascular invasion and nodal or distant metastases. Distant metastases decrease the probability of long-term survival, still 60% are alive at 10 years and one third remain disease-free.
Introduction
Malignant pancreatic endocrine tumors (PETs), if resectable, can have a good prognosis 1-7. Unfortunately, a proportion may present late with tumors that encase or invade adjacent major blood vessels 8-10. A number of studies have shown vascular invasion in both patients with pancreatic adenocarcinomas and advanced PETs is associated with decreased survival 2, 4, 11-15. The surgical approach to this group of patients is controversial. Based on analogies to pancreatic adenocarcinomas and limited experience with attempted surgical resection with this group of patients with advanced PETs, for many, involvement of the superior mesenteric vein (SMV), inferior vena cava (IVC), portal vein (PV), splenic vein with extensive varices (SV), superior mesenteric artery (SMA), aorta or heart is considered a contra-indication to surgery 11, 14, 16.
Recent surgical series in pancreatic adenocarcinoma suggest that there are reasons to question this approach 11, 14, 17-19. The operability of pancreatic tumors is usually defined by the results of CT or other imaging studies 9-11, 14, 20, 21. However, these studies may not always give an accurate image for determining operability 14. For example, in patients with adenocarcinoma of the pancreas, when preoperative CT suggests that the tumor involves the SMV, SMA or portal vein, in the past, most would say that the tumor is inoperable 11, 14, 17. However, recent studies dispute this thinking and suggest that these locally advanced tumors may be resectable for benefit 11, 14, 17, 22-24. Yet this is controversial because patients may not benefit in terms of disease-free and overall survival. We have recently demonstrated that vascular surgery techniques can be used to remove sarcomas that were previously thought to be inoperable with acceptable morbidity and good survival 25. Because PETs are rare, there has been no systematic studies of the ability to surgically resect malignant PETs thought to abut or involve major vascular structures, with most reports being isolated case reports or involving only a few PET patients 9, 10, 26-33. In this study we report our long-term results with PETs that abut or involve major vascular structures including the PV, SMV, SMA, inferior vena cava, splenic vein with large collaterals and the heart (interventricular septum). The findings suggest that possible/definite major vascular involvement on preoperative imaging studies should not be a contraindication to PET resection.
Methods
Since 1982 at the National Institutes of Health (NIH), and 2004 at Stanford University hospitals, 195 patients with Zollinger-Ellison syndrome (ZES) and 78 patients with either other functional or nonfunctional pancreatic PETs were involved in a prospective plan to perform surgical exploration for cure as described previously 34-41. All patients at the NIH, after confirming the diagnosis, underwent detailed imaging studies (CT scan with intravenous contrast, MRI with gadolinium, ultrasound, somatostatin receptor scintigraphy since 1994 [using [111In-DTPA-DPhe1]-octreotide (6 mCi) with whole body, planar, and SPECT views 42-45], and in selected cases selective abdominal angiography) to determine operability as described previously 34, 36, 46. All patients at Stanford underwent CT plus additional studies as deemed necessary. Patients were invited to undergo surgery to remove the tumor if they had no co-morbid medical condition markedly limiting life expectancy, had apparently operable tumor, and if MEN1 was present, had tumor ≥2.5 cm in diameter 35-37, 40, 47-49. Patients were also included with limited liver metastases, which were potentially completely resectable, as described previously 38, 39, 50. 46 (17%) patients described in detail here were identified on preoperative imaging either CT or MRI or both to have a PET that involved a major vascular structure. This included all patients, in which the PET by imaging studies involved the heart 51; IVC; either abutted (Figure 1) or involved the SMV or portal vein at or near the confluence (Figure 2) or the SMA; or invaded the splenic vein with large short gastric collateral vessels.
Figure 1.
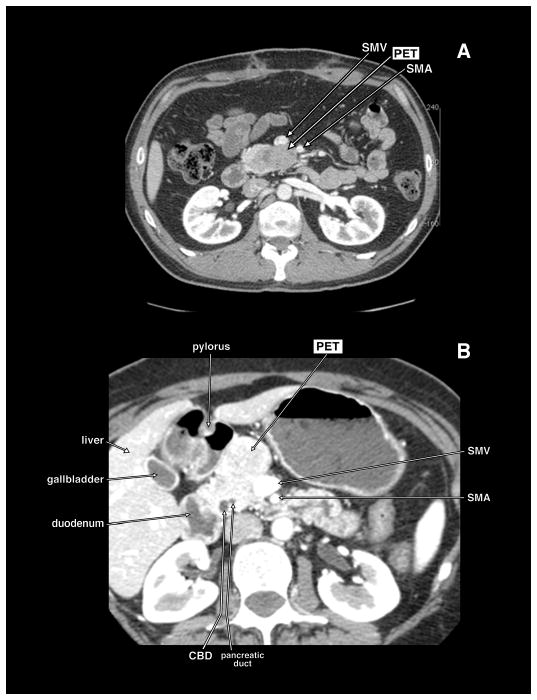
Computed tomography (CT) scans of 2 different patients with pancreatic endocrine tumor (PET) in the head of the pancreas abutting the mesenteric vessels. In panel A, the PET is in the uncinate portion of the pancreatic head and lies abutting the posterior surface of the superior mesenteric vein (SMV) and superior mesenteric artery (SMA). In panel B, the PET is in the anterior portion of the head of the pancreas abutting the anterior and lateral wall of the SMV. These patients could have the PET dissected off the SMV.
Figure 2.
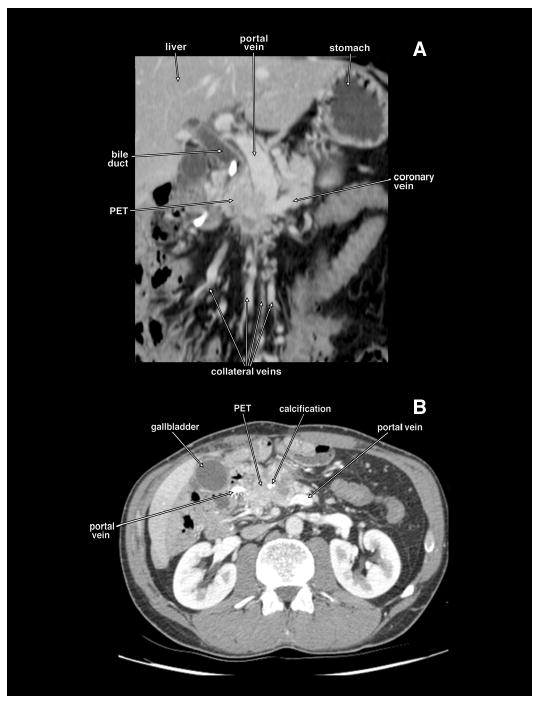
Coronal planar reformation (panel A) and axial tomogram (panel B) of a computed tomography of the same patient with a locally invasive non-metastatic pancreatic endocrine tumor (PET) obstructing the proximal portal vein. The PET has calcifications. There are extensive collateral veins because of the portal vein obstruction. This patient had the portal vein resected and reconstructed with autologous femoral vein.
The diagnosis of ZES was based on acid secretory studies, measurement of fasting serum level of gastrin as well as the results of secretin and calcium provocative tests 52-54. Basal and maximal acid output (BAO, MAO) was determined for each patient using methods described previously 54. Doses of oral gastric antisecretory drug were determined as described previously 55-57. The diagnosis of glucagonoma (the only other functional PET in this study) was based on the presence of a characteristic rash and elevated fasting plasma levels of glucagon 58.
A detailed past history of disease was taken at first admission including symptoms related to ZES and past medical/surgical procedures as described previously 53, 59. Time from onset of the disease to exploration was determined for all patients with ZES 35, 52, 52, 60, 60. The time of diagnosis of ZES was the time the diagnosis was first established by appropriate laboratory studies or when a physician established the diagnosis based on clinical presentation 35, 53.
All patients referred with a diagnosis of possible ZES underwent an evaluation to establish the diagnosis of ZES and to determine whether MEN1 was present 35, 37, 52, 53, 59 and studies to determine the suitability of surgical exploration for cure 35, 37, 38, 52. These latter studies included tumor localization studies, studies to determine the presence or absence of MEN1 35, 59, 61 and studies to determine the presence of other disease that might make surgery contraindicated. MEN1 was established by assessing plasma hormone levels (PTH [intact, mid-molecule], prolactin, insulin, proinsulin, glucagon), serum calcium (ionized, total) and glucose as well as from personal and family history 37, 48, 59, 61.
44 of 46 patients who fit this study for possible vascular involvement underwent a surgical exploration for potential cure. The operative techniques have been described previously 35, 36, 47, 62, 63. Tumors in the pancreatic head were enucleated. Tumors in the pancreatic body and tail were resected. If large pancreatic head tumors were present and could not be enucleated, a pancreaticoduodenectomy was performed 64. A detailed inspection for peripancreatic, periduodenal, or portohepatic lymph nodes was carried out, and these were routinely removed 34, 65. If liver metastases were present and localized, they were wedge-resected with a 1-cm margin, if possible; if this was not possible and they were localized, a segmental resection or lobectomy was performed 38, 39, 50. If the superior mesenteric vein was resected and reconstructed, it was done with either the proximal femoral vein or the jugular vein 25, 39. The superior mesenteric artery was reconstructed with the saphenous vein 25, 39. Postoperatively, patients underwent evaluation for disease-free status immediately after surgery (i.e., 2 weeks post-resection), within 3 to 6 months post-resection, and then yearly 35, 43, 52, 62. Yearly evaluations included conventional imaging studies (CT, ultrasound, MRI, and angiography, if necessary); somatostatin receptor scintigraphy (SRS) since 1994; assessment of functional disease status (in gastrinomas-acid secretory studies, fasting gastrin determinations, secretin provocative test; in glucagonomas-plasma glucagon levels); and assessment of endocrine status (parathyroid, pituitary, adrenal function) 35, 43, 52, 62. To compare the results with functional and nonfunctional PETs, disease-free status was defined as no evidence of tumor on conventional imaging studies usually CT. For patients with functional PETs (gastrinoma, glucagonoma), complete disease-free status assessed by plasma hormone levels and secretin provocative testing (gastrinomas) as described previously 35, 36, 52 and was defined as a normal fasting plasma gastrin/glucagon level, and negative secretin test (gastrinoma), and no tumor on imaging studies A recurrence post-resection was defined as occurring in a patient who was initially disease-free post-resection of the PET, but then lost disease-free status on follow-up evaluation by developing positive imaging studies (Nonfunctional-PETs, functional PETs) as well as recurrent fasting hormone/provocative test results with negative imaging in functioning PETs 35, 36, 52, 66. Recurrent disease was treated with chemotherapy or somatostatin analogues with or without alpha interferon as described previously 67-69.
The Fisher's exact test was used for two-group comparisons. All continuous variables were reported as mean ± standard error of the mean. The probabilities of survival were calculated and plotted according to the Kaplan-Meier method 70.
Results
Demographics
46 (17%) patients were identified on preoperative imaging studies to have pancreatic PETs either involving the IVC, heart, PV or abutting or encasing the SMV (Figures 1 and 2), SMA or splenic vein with extensive collateral veins (Tables 1 and 2). There were 21 men (45%) and 39 were Caucasian (85%) (Table1). The mean age was 42 years with a range of 24 to 76 years. 32 (57%) had functional tumors with 30 gastrinomas and 2 glucagonomas; the remainder (n=14) had nonfunctional PETs. 30 functional PET patients presented with symptoms related to ZES (peptic ulcer disease, GERD and diarrhea) and 2 glucagonoma patients presented with a rash that was later called necrolytic migratory erythema. While the nonfunctional tumor patients each presented with pain, some (n=6) functional PET patients also had pain as a presenting symptom (Table 1). 12 patients (26%) had MEN-1. For the patients with functional PETs, symptoms were present for approximately 5 years before the diagnosis was made. The median gastrin increase for the ZES patients was 12 fold (Table 1).
Table 1.
Patient demographics and clinical characteristics preoperatively.
| Characteristic | Number (%) |
|---|---|
| Total number | 46 |
| Male | 21 (45%) |
| Race (Caucasian) | 39 (85%) |
| Age Diagnosis (yrs) | |
| Mean ± SEM | 41.7 ± 2.1 |
| [range] | [24-76] |
| Type PET | |
| NF-PET | 14 (43%) |
| Functional PET | 32 (57%) |
| Gastrinoma (glucagonoma) | 30 (2)a |
| Presenting symptom | |
| Due to functional PET | 32 (70%)(b) |
| Due to pain | 20 (30%) |
| MEN1 present | 12 (26%) |
| Duration of symptoms at diagnosis (yrs) (Functional PETs) | |
| Mean ± SEM | 5.0 ± 0.9 |
| [range] | [0.25-17.9] |
| Hormone elevation (fold increase) | |
| median | 12(c) |
| Mean ± SEM | 292 ± 213 |
| [range] | [3-5500] |
Six patients with gastrinomas with MEN1 also had NF-PETs identified preoperatively. Two patients had glucagonoma.
All patients with functional PETs presented with symptoms due to hormone excess state.
Gastrin elevation in the 30 patients with gastrinomas preoperatively
Table 2.
Preoperative tumoral features assessed by imaging studies.
| Tumoral characteristic | Number (%) |
|---|---|
| Primary tumor | |
| Largest size | |
| Mean ± SEM | 5.8 ±0.5 |
| [range] | [2-13] |
| Number | |
| Mean ± SEM | 1.5 ± 0.2(a) |
| [range] | [1-5] |
| Preoperative primary location | |
| Pancreatic head/duodenum | 27 (59%)(b) |
| Pancreatic body | 9 (20%) |
| Pancreatic tail | 8 (17%) |
| Other | 2 (4%) |
| Metastases present | 35 (76%) |
| Lymph node metastases present | 27 (59%) |
| Liver metastases (limited) | 14 (30%)(c) |
| Vessels involved/abuts | 46(100%) |
| Portal vein or tributary | 20 (43%) |
| SMV/SMA | 16 (35%) |
| IVC | 4 (9%) |
| Splenic vein | 4 (9%) |
| Heart | 2 (4%) |
Six patients with gastrinomas with MEN1 had multiple primary PETs Identified preoperatively.
Imaging studies could not clearly differentiate whether in pancreatic head or duodenum.
Limited liver metastases refer to patients without diffuse liver metastases and liver metastatic disease thought completely resectable.
Abbreviations; SMV/SMA-superior mesenteric artery/vein; IVC-inferior vena cava;
Preoperative Imaging
The mean size for the primary pancreatic PET on preoperative CT was 5.8 cm (Table 2). Some patients had more than one primary so the mean number of tumors per patient was 1.5. 27 primary tumors were located within the head of the pancreas or the duodenum (59%). 17 were either in the pancreatic body (n=9, 20%) or tail (n=8, 17%). The other 3 were ectopic: two in the interventricular septum of the heart and the other within the wall of the right hepatic duct abutting the right portal vein (Figure 3). On preoperative imaging 35 patients were found to have metastases (76%) with 27 having lymph node metastases (59%) and 14 liver spread (30%). On the preoperative imaging studies the involved major vessels were as follows: portal vein (n=20, 43%), SMV or SMA (n=16, 35%), IVC (n=4, 9%), splenic vein (n=4, 9%) and heart (n=2, 4%) (Table 2).
Figure 3.
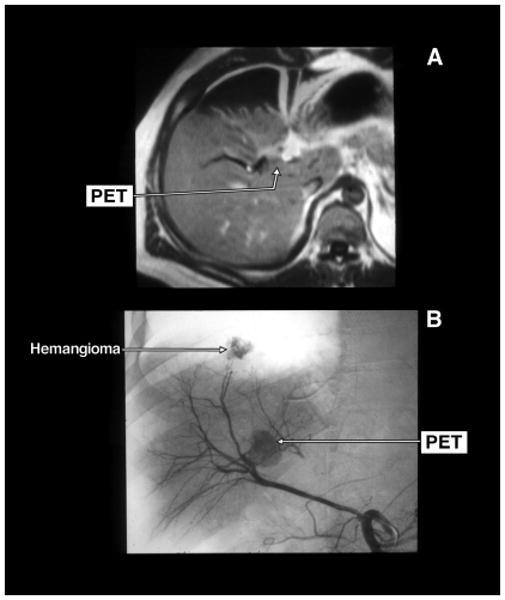
Gadolinium enhanced magnetic resonance imaging (MRI) (panel A) and selective arteriogram (panel B) of a pancreatic endocrine tumor (PET) that was in the wall of the right hepatic duct. The tumor was abutting the right portal vein. There is a second liver tumor shown on the hepatic arteriogram (panel B) as a liver hemangioma. The PET was locally resected with the right hepatic duct. The tumor was dissected off the portal vein.
Surgical Findings and Results
44 of the 46 patients underwent surgery (2 patients refused surgery) and in 42 patients the primary tumor was resected (91%) (Table 3). The mean age at the time of surgery was 49 years and it had been an average of 5 years since diagnosis. The primary PET was located within the pancreas in 30 (68%) patients, the duodenum in 12 (27%) and the remainder (n=5, 11%) had ectopic locations (bile duct n=1), liver n=2, heart n=1 and lymph node n=1) (Table 3). The average tumor size at surgery was 5 cm, and the largest tumor was 15 cm. At surgery, 31 (70%) patients had lymph node metastases and 18 (41%) had liver metastases. Eight patients had only a primary tumor found (18%), 31 had a primary tumor with lymph node involvement (70%), 20 had liver involvement (48%) and 15 had vascular encasement including the heart (n=1), cava (n=1), SMA (n=1) and the portal vein or SMV (n= 12) (Table 3).
Table 3.
Surgical findings
| Characteristic | Number (%) |
|---|---|
| Patient numbers | |
| Total number patients entered | 46 |
| Number undergoing surgery | 44 (100%)(a) |
| Number tumor resected | 42 (91%) (b) |
| Age surgery (yrs) | |
| Mean ± SEM | 49 ± 2 |
| [range] | [27.5-81) |
| Duration diagnosis to surgery (yrs) | |
| Mean ± SEM | 5.3 ± 1.1 |
| [range] | [0.1-18.8] |
| Primary PET location/size (surgery) | |
| Location | |
| Pancreas | 30 (68%) |
| Duodenum | 12 (27%) |
| Other | 5 (11%)(c) |
| Largest Primary size (cm) | |
| Mean ± SEM | 5 ± 0.6 |
| [range] | [0.4-15] |
| Metastases found at surgery | |
| Lymph node involvement | 31 (70%) |
| Liver metastases | 18 (41%)(d) |
| Tumor extent at surgery | |
| Primary only | 8 (18%) |
| Primary plus lymph node involvement | 31 (70%) |
| With liver involvement | 20 (48%)(d) |
| Invasion/encasement of major vessel | 15 (36%)(e) |
Two patients refused surgery.
Two patients with gastrinomas had unresectable disease. One with diffuse peritoneal implants/diffuse small liver metastases and the second with complete encasement of the IVC/portal vein with arterial invasion with bleeding. The number of patients undergoing surgery was used as 100% in this table.
Other refers to 1 patient with a primary gastrinoma in the bile duct (n=1), liver (n=2), heart (n=1) and lymph node (n=1).
Two patients had primary gastrinoma of the liver.
One patient had invasion of the heart by a gastrinoma, twelve encasement of the SMV or PV, one SMA and one involvement of the IVC.
42 patients had PETs removed: 12 (27%) a primary only, 30 (68%) with lymph node metastases, and 18 (41%) with liver metastases (Table 4). The pancreatic primary PET was removed by either enucleation n=5 (12%) or resection n=36 (86%). Resections included partial pancreatectomy either distal or subtotal pancreatectomy in 23 (55%), Whipple proximal pancreaticoduodenectomy in 10 (23%) and total pancreatectomy in 2 (5%). 19 (45%) patients had concomitant liver resection: 10 (23%) had wedge resections and 9 (21%) had anatomic resections (lobectomy or trisegmentectomy) (Table 4). 9 (21%) had vascular reconstruction: each had reconstruction of the SMV and portal vein, while 1 patient had concomitant reconstruction of the SMA. There were no deaths due to surgery, but 12 (28%) had complications including pancreatitis, abscess, wound infection, bile duct injury, leak at pancreaticojejunostomy and ischemic bowel.
Table 4.
Type and result of surgery, follow-up and complications
| Result | Number (%) |
|---|---|
| Tumor resected | 42 (95%)(a) |
| Primary only | 12 (27%) |
| With lymph node metastases | 30 (68%) |
| With liver metastases | 18 (41%) |
| Type Primary surgery | |
| Enucleation | 5 (12%) |
| Resection | 36 (86%) |
| Partial pancreatectomy | 23 (55%) |
| Whipple resection | 10 (23%) |
| Total pancreatectomy | 2 (5%) |
| Liver resection | 19 (45%) |
| Wedge resection | 10 (23%) |
| Lobectomy | 9 (21%)(b) |
| Vascular reconstruction | 9 (21%) |
| SMV-portal vein | 9 (21%) |
| SMA | 1 (2%) |
| Surgical complications | |
| Surgical death | 0 (0%) |
| Complications | 12(28%)(c) |
| Surgical result | |
| Immediate tumor free | 18 (42%)(d) |
| Recurrence | 5 (12%) (d) |
| Time to recurrence years | |
| Mean ± SEM | 1.7 ± 0.6 |
| [range] | [0.5-3] |
| Long term disease-free | 14 (33%)(d) |
| Status Last follow-up | |
| Alive | 34 (77%) |
| Dead | 10 (23%) |
| Years surgery to death | |
| Mean ± SEM | 5.5 ± 0.3 |
| [range] | [3-8] |
| Duration of follow-up (yrs) | |
| Time from surgery | |
| Mean ± SEM | 5.7 ± 1.0 |
| [range] | [1.3-21.2] |
| Time from diagnosis | |
| Mean ± SEM | 12.7 ± 1.2 |
| [range] | [3.9-25.8] |
| Other anti-tumor treatment | 18 (43%) |
| Chemotherapy | 12 (28%) |
| Other | 10 (23%)(e) |
Percentage based on 24 patients who underwent surgical exploration. Two patients had unresectable disease. See Table 3 footnote.
Include a trisegmentectomy (n=1), l hepatic lobe resection(n=4), and segmentectomy (n=4)
Complications include postoperative pancreatitis (n=1), abscess (n=4), wound infection (n=3), bile duct injury (n=1), leak at pancreaticojejunostomy (n=2) and ischemic bowel (n=1).
Disease free status and recurrence based on serial imaging studies as described in Methods
Patients treated with alpha-interferon (n=2), somatostatin analogues (n=9) or PRRT (n=1). Two that received alpha-interferon also got somatostatin analogues
Follow-up and Outcome
18 (42%) were immediately disease-free and 5 recurred with follow-up leaving 14 (33%) long-term disease-free assessed by serial imaging studies (Table 4). 7 patients with gastrinomas immediately postoperatively and 6 on follow-up had normal imaging studies but an elevated fasting gastrin and/or provocative test. The long-term 10 year overall survival is 60% and the disease-free survival is 33% (Figure 4). Possible prognostic factors were examined to determine if they affected long-term and disease-free survival. Most variables did not affect disease-free survival (Table 5). Patients with functional tumors had a greater long-term overall survival than nonfunctional tumors, but the disease-free survival was similar (p=0.0001) (Figure 5). The presence of lymph node metastases did not decrease disease-free survival. However, the presence of liver metastases decreased disease-free survival from 66% to 20% (p= 0.002), and the use of liver resection decreased disease-free survival from 66% to 25% (p=0.007). The use of vascular reconstruction did not affect disease-free survival (Table 5). The use of other anti-tumor treatment following surgery did not affect disease-free survival (Table 5).
Figure 4.
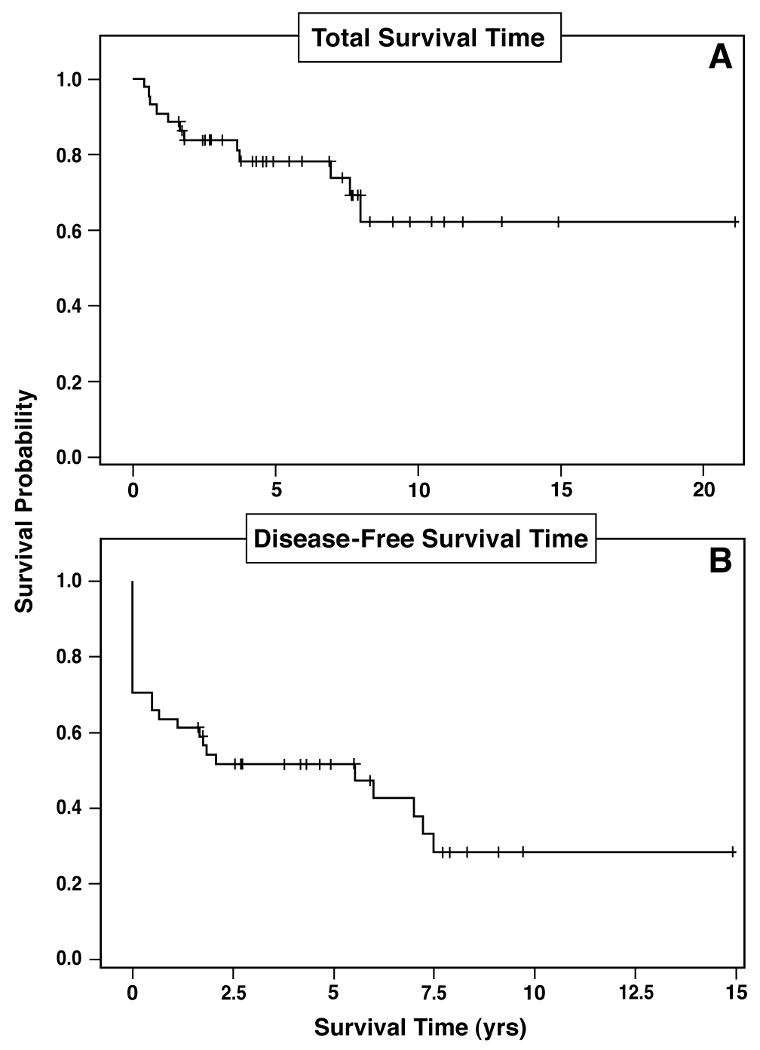
Kaplan Meier plot of total survival (upper panel) and disease-free survival (lower panel) of the 44 patients with pancreatic endocrine tumors (PET) involving major vascular structures who had the tumor removed surgically. The 10-year total survival was 60 % and the 10-year disease-free survival was 33%.
Table 5.
Possible prognostic factors for prolonged disease-free survival postresection
| Variable present | Number (%) | Significance | |
|---|---|---|---|
| Disease-free last follow-up | |||
| Yes (n=20) | No (n=24) | ||
| General features | |||
| Male gender | 10/20 (50%) | 10/24 (41%)a | ns |
| Caucasian race | 19/20 (95%) | 19/24 (79%) | ns |
| NF-PET present | 9/20 (45%) | 7/24 (29%) | ns |
| MEN-1 present | 5/20 (25%) | 7/24 (29%) | ns |
| Hormone elevation >11.6 fold | 8/20 (40%) | 8/24 (33%) | ns |
| Pre-operative imaging | |||
| Primary>3.7 cm | 11/20 (55%) | 17/24 (71%) | ns |
| Primary: pancreatic head/duodenum | 13/20 (65%) | 17/24 (71%) | ns |
| Possible Portal vein involvement | 14/20 (70%) | 20/24(83%) | ns |
| Possible liver metastases present | 6/20 (30%) | 12/24 (50%) | ns |
| Surgical findings | |||
| Age surgery>47 yrs | 14/20 (70%) | 11/24 (46%) | ns |
| Primary tumor: pancreas | 14/20 (70%) | 16/24 (66%) | ns |
| Primary size>3 cm | 11/20 (55%) | 15/24 (63%) | ns |
| Lymph node metastases present | 14/20 (70%) | 19/24 (79%) | ns |
| Liver metastases found | 2/20 (10%) | 16/24 (66%) | <0.0001 |
| Invasion/encasement of vessel | 7/20 (35%) | 8/24 (33%) | ns |
| Surgical treatment/result | |||
| Liver resection | 3/20 (15%) | 16/24 (66%) | <0.001 |
| Vascular reconstruction | 5/20 (25%) | 4/24 (17%) | ns |
| Immediate postoperative = tumor free | 20/20 (100%) | 11/24 (46%) | 0.0001 |
| Surgical followup | |||
| Alive last follow-up | 20/20 100%) | 14/24 (58%) | <0.0001 |
| Last follow-up>7.5 yrs from surgery | 6/20 (30%) | 11/24 (45%) | ns |
| Other antitumor treatment postoperative | 0/20 (0%) | 16/24 (66%) | <0.0001 |
Proportions compared by Fisher's exact test.
Figure 5.
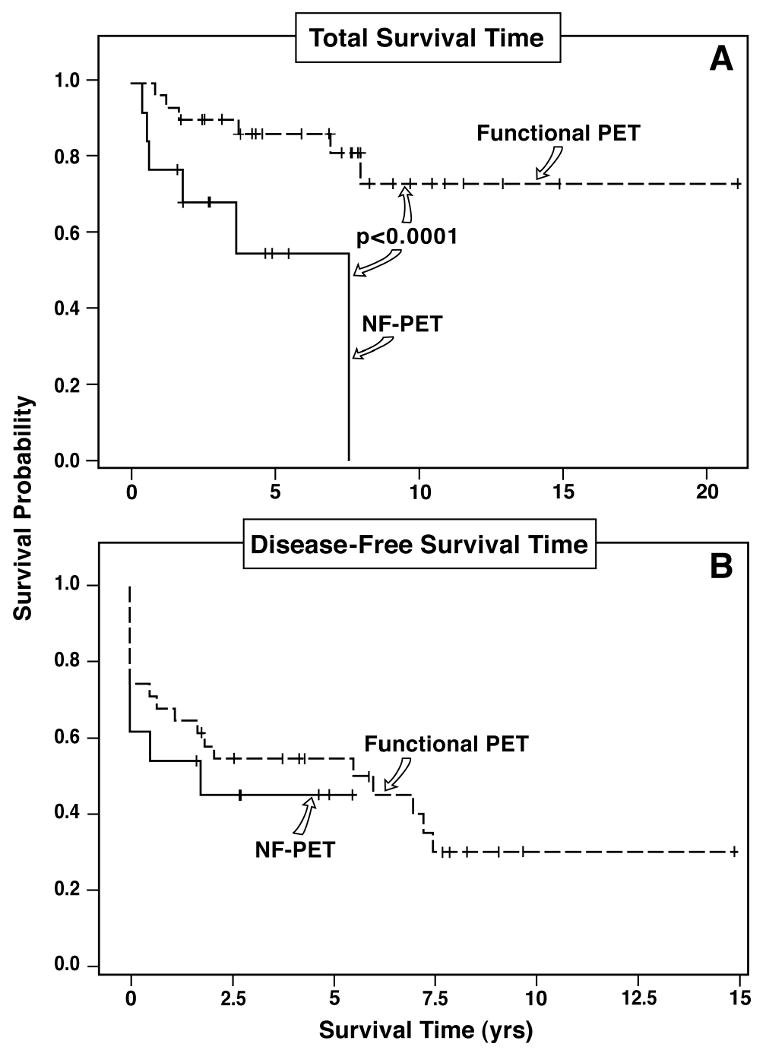
Kaplan Meier plot of total survival (upper panel) and disease-free survival (lower panel) based on the clinical production of a hormone (gastrin and glucagon); that is, defined as functional vs non-functional pancreatic endocrine tumor (NF-PET). Functional PET had a significantly better total survival than NF-PET (p<0.001, upper panel), but there was no difference in disease-free survival (lower panel).
Discussion
PETs are uncommon neoplasms with a clinical incidence of approximately 1-3 per million 71. The patients described here are those with the PET on extensive preoperative imaging studies likely or actually invading major vascular structures either by abutting and/or encasing the vascular structure or thought to invade a major vascular structure. We found that such patients comprised 17% (44 patients) of our total surgical population of 273 patients with PETs. This is the first series of PET patients that has systemically investigated such a large group of such patients. Our frequency of preoperative vascular involvement is similar to results in smaller series of patients with PETs where a 13-16% frequency of likely vascular involvement was reported in two radiologic studies 8, 10 of patients with PETs and 26% reported in the radiological study of 30 PET patients 9 with large, malignant PETs. These results demonstrate that possible vascular involvement on preoperative imaging studies is not an uncommon occurrence in patients with PETs, although it is less common than the 44% (mean -13 series, 891 patients) seen with pancreatic adenocarcinoma 24. These results support the importance of the present study, because they demonstrate that if vascular involvement is used as a contra-indication to surgical resection for PET patients, approximately 20% of all PET patients will be excluded from surgery.
This paper focuses on the role of surgery to remove PETs abutting, invading or encasing a major blood vessel, usually the superior mesenteric vein or portal vein (Figures 1 and 2), as well as removing the primary, lymph nodes and any limited, liver metastases in these patients. This study was undertaken for a number of reasons. First, a proportion of PETs show aggressive, malignant growth, which is associated with decreased survival, and the medical treatment of these large, advanced tumors is generally only marginally effective 67, 71-73. Second, a number of studies have shown vascular invasion in both patients with pancreatic adenocarcinomas and advanced PETs is associated with decreased survival 2, 4, 11-15. Third, there are a number of surgical reports by different groups that resection of distant metastatic PETs, including within the liver, may improve survival, improve symptom control and even render some patients disease-free 38, 50, 74-77. However, there has never been a controlled study establishing this, because these patients are uncommon, most believe that this type of surgery is helpful when it can be done in patients with limited liver metastases, and in a recent consensus statement it is recommended it be performed whenever possible 3, 38, 50, 71, 74, 75, 78]. Fourth, similar to PETs, until recently most studies with pancreatic adenocarcinoma, considered involvement of major vascular structures a contra-indication to surgery 11, 14, 16. However, recent studies in patients with exocrine adenocarcinoma of the pancreas suggest that these tumors, even when invading venous structures, may be resected with acceptable morbidity and this may be of benefit 11, 14, 17-19. Similarly, we have recently reported that this aggressive approach to resection of invasive sarcomas of the extremity and retroperitoneum that involved or replaced major blood vessels, results in some cases, in the complete removal of tumors thought previously to be inoperable 27. Furthermore, numerous case reports and small series suggested a similar approach might be feasible and perhaps beneficial in patients with advanced functional 9, 28, 79-81 and nonfunctional PETs 9, 29, 30, 33, 82-84, although no study has systematically examined this issue until the present study. Fifth, despite the fact that PETs can result in death and shorten the life-expectancy of an individual patient, an aggressive PET can also cause dramatic life-threatening complications like massive bleeding due to the formation of vascular shunts 27 or from the development of short gastric varices in the setting of splenic vein occlusion by tumor 80, 83-86.
In our study we found that the PETs were resectable in 91% of our patients, even though the tumors were usually large (mean size-5.5 cm) and associated with advanced disease in most cases, with metastases present in three-fourths of the patients, including to lymph nodes (68%) and the liver (41%). Even though preoperative imaging studies suggested vascular involvement in all patients, at surgery, the PET was found to have invaded and/or encased a major vascular structure in less than one-half the patients (40%), and in the remaining cases, the PET showed either only partial vascular involvement or vascular abutment without encasement and/or invasion of a major vascular structure. This result is similar to those in a study 9 of patients with advanced, large PETs in which 50% of the patients thought to have vascular involvement from preoperative studies, were found at surgery, to not have vascular encasement and/or invasion of major vessels. CT and MRI scanning are reported to have excellent sensitivity for detecting vascular involvement and are the standard imaging modalities used to determine vascular involvement in patients with either pancreatic exocrine adenocarcinomas or and PETs 3 9-11, 14. In the present study both modalities were used in the gastrinoma patients and CT in the nonfunctional PET patients. With pancreatic adenocarcinomas, preoperative CT is reported to have excellent sensitivity for identifying involvement of major vessels, however, similar to our results in PETs, it can also have false positives in pancreatic adenocarcinoma, with specificity as low as 50% in some studies 11, 14. The difference between our surgical results and the preoperative imaging results in PET patients is likely explained by our surgical findings and suggests that PETs differ from pancreatic adenocarcinomas in this regard. Our results demonstrate that with PETs, radiological abutment or even possible vascular involvement is not frequently synonymous with vascular involvement at surgery. The PET was found frequently to be encroaching, abutting, or distorting the major vascular structure, without encasing it and/or invading it. That CT may falsely suggest vessel involvement, has been reported in a small number of patients with PETs in other studies 9, 87. In pancreatic adenocarcinoma false positives imaging results for vascular involvement occur, especially in the portal vein, where in some studies, up to 50% of the tumors thought to involve the portal vein, on histological evaluation showed no tumor involvement, but the radiological changes were due to inflammatory adhesions 14.
In our study only 9 of the 42 patients (15%) undergoing PET resections required vascular reconstruction demonstrating that in most PET patients, even if the radiological evaluation suggests vascular involvement and at surgery, the PET is found to partially encase or involve the vessel, the PET can be removed with careful dissection without requiring vascular reconstruction. These results differ from pancreatic adenocarcinoma where venous resection is usually required with tumor involvement and where the percentage of the patients with potentially resectable disease having major vascular involvement is much lower 14, 17, 23, 24.
In our study, despite the extensive tumor involvement, post surgical resection, both the long-term survival rate and the disease-free survival rates at 5-years were high (77% and 50%, respectively). An important prognostic factor associated with a decreased disease-free survival was the presence of liver metastases, which is in agreement with the results a number of studies that show the presence of liver metastases are one of the most important factors in patients with PETS associated with decreased either disease-free or total survival 4-7, 71, 88. In contrast, disease-free survival was not affected by either the extent or type of vascular involvement (i.e. by the presence of vascular invasion or encasement of a major vessel by the PET, the need for vascular reconstruction at surgery, the type of vascular involvement) or by the presence of other factors which have prognostic significance for disease-free or total survival in other studies of PET patients [i.e. lymph node metastases, the presence of MEN1, high tumor marker levels or the presence of a large primary PET (i.e. >3 cm)]2, 4, 6, 7, 71, 88. In our study patients with nonfunctional PETs with possible vascular involvement on preoperative imaging studies had a significantly decreased (p<0.001) survival compared to similar patients with functional PETs. In previous reports patients with nonfunctional PETs have a poorer survival than patients with functional PETs in many studies 1, 4, 5, 7, 89 but not all 2, 6. The decreased survival of patients with nonfunctional PETs is reported to be likely due to their more aggressive behavior or the fact that these patients characteristically present later in their disease course with more advanced disease than patients with functional PETs 1, 7 In our study the extent of disease in the patients with functional and nonfunctional tumors was comparable, so this supports the proposal that these patients have more aggressive tumors. 1, 7
Unfortunately, our study, like many other surgical studies of patients with advanced PETs including those with liver metastases, because of the low patient numbers available, the study design does not clearly establish the value of the surgical approach taken 76-78, 90. Nevertheless, a number of findings in our study are suggestive of surgical benefit and encouraging for future studies. First, despite the decreased survival of patients with vascular involvement and/or liver metastases with malignant PETs 2, 4, 12, 13, 71, 72, in our series, the results suggest that the long-term survival rate was 77% at 5 years and our results suggest surgery was beneficial as 42% were immediately disease-free and 33% long-term disease-free. These data are encouraging, because in historic controls of patients with metastatic PETs who remained untreated and with potentially resectable disease, as is our group because of their vascular involvement, the five-year survival rates were 30-40% 89, 91. Second, in our study these results were obtained in the setting of major pancreatic resection in most patients (86%) plus liver resection in 45%. Furthermore, tumor removal required dissection an/or removal of PETs adjacent or partially involving major vessels in all patients, and vascular reconstruction in 21%. Despite this extent of surgical resection, there was no surgical mortality and the surgical complication rate (28%) was well within the range of that reported in studies with PET resections, usually involving less extensive resections than in these patients with and without vascular involvement 1, 92, 93. Third, in the present study five patients presented with upper gastrointestinal hemorrhage from gastric varices secondary to splenic vein occlusion by the PET and the bleeding was totally ameliorated by removal of the tumor with the spleen. This experience is similar to that reported in a small number of case reports 8, 83, 85-87, 94-96. These results suggest the resection of the PET despite vascular involvement is of particular benefit in this group of PET patients. Fourth, medical therapies of advanced PETs have provided only modest benefits, with many studies reporting short term disease stabilization and a small percentage of partial responses 71-73, 97. Therefore, it has generally not been possible to downsize extensive disease in a patient with a malignant PET to make it surgically resectable, as has been done in some other tumors 71-73, 98, 99. However, one patient on a novel chemotherapy protocol with capecitabine, oxaliplatin and bevacizumab became operable after his upfront chemotherapy treatment suggesting that this and other protocols may increase operability in the future. Therefore, at present, surgical resection is recommended for these patients, even by consensus conference, whenever it is possible. Our data suggests that major vascular involvement should not be a contra-indication to surgery, thus increases the possible number of patients in whom surgical resection should be considered, because this occurs in 20% of patients with advanced PETs.
Acknowledgments
This study was partially supported by intramural funds of NIDDK, NIH.
Reference List
- 1.Phan GQ, Yeo CJ, Hruban RH, Lillemoe KD, Pitt HA, Cameron JL. Surgical experience with pancreatic and peripancreatic neuroendocrine tumors: review of 125 patients. J Gastrointest Surg. 1998 September;2(5):472–82. [PubMed] [Google Scholar]
- 2.Hochwald SN, Zee S, Conlon KC, et al. Prognostic factors in pancreatic endocrine neoplasms: an analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002 June 1;20(11):2633–42. doi: 10.1200/JCO.2002.10.030. [DOI] [PubMed] [Google Scholar]
- 3.Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg. 2004;240(5):757–73. doi: 10.1097/01.sla.0000143252.02142.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen RT. Natural history of digestive endocrine tumors. In: Mignon M, Colombel JF, editors. Recent advances in pathophysiology and management of inflammatory bowel diseases and digestive endocrine tumors. Paris, France: John Libbey Eurotext Publishing Co.; 1999. pp. 192–219. [Google Scholar]
- 5.Madeira I, Terris B, Voss M, et al. Prognostic factors in patients with endocrine tumours of the duodenopancreatic area. Gut. 1998;43:422–7. doi: 10.1136/gut.43.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panzuto F, Nasoni S, Falconi M, et al. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer. 2005 December;12(4):1083–92. doi: 10.1677/erc.1.01017. [DOI] [PubMed] [Google Scholar]
- 7.Ekeblad S, Skogseid B, Dunder K, Oberg K, Eriksson B. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res. 2008 December 1;14(23):7798–803. doi: 10.1158/1078-0432.CCR-08-0734. [DOI] [PubMed] [Google Scholar]
- 8.Bok EJ, Cho KJ, Williams DM, Brady TM, Weiss CA, Forrest ME. Venous involvement in islet cell tumors of the pancreas. AJR Am J Roentgenol. 1984 February;142(2):319–22. doi: 10.2214/ajr.142.2.319. [DOI] [PubMed] [Google Scholar]
- 9.Hellman P, Andersson M, Rastad J, et al. Surgical strategy for large or malignant endocrine pancreatic tumors. World J Surg. 2000;24:1353–60. doi: 10.1007/s002680010224. [DOI] [PubMed] [Google Scholar]
- 10.Johnson DBS, Francis IR, Eckhauser FE, Knol JA, Chang AE. Dual-phase helical CT of nonfunctioning islet cell tumors. J Comput Assist Tomogr. 1998 January;22(1):59–63. doi: 10.1097/00004728-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Wray CJ, Ahmad SA, Matthews JB, Lowy AM. Surgery for pancreatic cancer: recent controversies and current practice. Gastroenterology. 2005 May;128(6):1626–41. doi: 10.1053/j.gastro.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 12.La Rosa S, Rigoli E, Uccella S, Novario R, Capella C. Prognostic and biological significance of cytokeratin 19 in pancreatic endocrine tumours. Histopathology. 2007 April;50(5):597–606. doi: 10.1111/j.1365-2559.2007.02662.x. [DOI] [PubMed] [Google Scholar]
- 13.Deshpande V, Fernandez-Del Castillo C, Muzikansky A, et al. Cytokeratin 19 is a powerful predictor of survival in pancreatic endocrine tumors. Am J Surg Pathol. 2004;28(9):1145–53. doi: 10.1097/01.pas.0000135525.11566.b4. [DOI] [PubMed] [Google Scholar]
- 14.Buchs NC, Chilcott M, Poletti PA, Buhler LH, Morel P. Vascular invasion in pancreatic cancer: Imaging modalities, preoperative diagnosis and surgical management. World J Gastroenterol. 2010 February 21;16(7):818–31. doi: 10.3748/wjg.v16.i7.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballian N, Loeffler AG, Rajamanickam V, Norstedt PA, Weber SM, Cho CS. A simplified prognostic system for resected pancreatic neuroendocrine neoplasms. HPB (Oxford) 2009;11(5):422–8. doi: 10.1111/j.1477-2574.2009.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans DB, Skibber JM, Lee JE, et al. Nonfunctioning islet cell carcinoma of the pancreas. Surgery. 1993 December;114(6):1175–81. [PubMed] [Google Scholar]
- 17.Martin RC, Scoggins CR, Egnatashvili V, Staley CA, McMasters KM, Kooby DA. Arterial and venous resection for pancreatic adenocarcinoma: operative and long-term outcomes. Arch Surg. 2009 February;144(2):154–9. doi: 10.1001/archsurg.2008.547. [DOI] [PubMed] [Google Scholar]
- 18.Glanemann M, Shi B, Liang F, et al. Surgical strategies for treatment of malignant pancreatic tumors: extended, standard or local surgery? World J Surg Oncol. 2008;6:123. doi: 10.1186/1477-7819-6-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siriwardana HP, Siriwardena AK. Systematic review of outcome of synchronous portal-superior mesenteric vein resection during pancreatectomy for cancer. Br J Surg. 2006 June;93(6):662–73. doi: 10.1002/bjs.5368. [DOI] [PubMed] [Google Scholar]
- 20.Zhao WY, Luo M, Sun YW, et al. Computed tomography in diagnosing vascular invasion in pancreatic and periampullary cancers: a systematic review and meta-analysis. Hepatobiliary Pancreat Dis Int. 2009 October;8(5):457–64. [PubMed] [Google Scholar]
- 21.Noone TC, Hosey J, Firat Z, Semelka RC. Imaging and localization of islet-cell tumours of the pancreas on CT and MRI. Best Pract Res Clin Endocrinol Metab. 2005 June;19(2):195–211. doi: 10.1016/j.beem.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Harrison LE, Klimstra DS, Brennan MF. Isolated portal vein involvement in pancreatic adenocarcinoma. A contraindication for resection. Ann Surg. 1996 September;224(3):342–7. doi: 10.1097/00000658-199609000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng JF, Tamm EP, Lee JE, Pisters PW, Evans DB. Venous resection in pancreatic cancer surgery. Best Pract Res Clin Gastroenterol. 2006 April;20(2):349–64. doi: 10.1016/j.bpg.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Ramacciato G, Mercantini P, Petrucciani N, et al. Does portal-superior mesenteric vein invasion still indicate irresectability for pancreatic carcinoma. Ann Surg Oncol. 2009 April;16(4):817–25. doi: 10.1245/s10434-008-0281-8. [DOI] [PubMed] [Google Scholar]
- 25.Song TK, Harris EJ, Jr, Raghavan S, Norton JA. Major blood vessel reconstruction during sarcoma surgery. Arch Surg. 2009 September;144(9):817–22. doi: 10.1001/archsurg.2009.149. [DOI] [PubMed] [Google Scholar]
- 26.Obuz F, Bora S, Sarioglu S. Malignant islet cell tumor of the pancreas associated with portal venous thrombus. Eur Radiol. 2001;11(9):1642–4. doi: 10.1007/s003300000799. [DOI] [PubMed] [Google Scholar]
- 27.Handa M, Nakada T, Kajitsuka S, Hirose M, Sato Y. Portosystemic A-V fistula and portal hypertension associated with islet-cell tumor of the pancreas. Nippon Geka Gakkai Zasshi. 1985 August;86(8):953–8. [PubMed] [Google Scholar]
- 28.Rusher AH, Henson K. Metastatic malignant insulinoma: debulking for palliation of symptoms. J Ark Med Soc. 1991 November;88(6):267–9. [PubMed] [Google Scholar]
- 29.Sakamoto E, Hasegawa H, Ogiso S, et al. Curative resection for a pancreatic endocrine carcinoma involving the portal vein. Hepatogastroenterology. 2004 November;51(60):1849–51. [PubMed] [Google Scholar]
- 30.Kawakami H, Kuwatani M, Hirano S, et al. Pancreatic endocrine tumors with intraductal growth into the main pancreatic duct and tumor thrombus within the portal vein: a case report and review of the literature. Intern Med. 2007;46(6):273–7. doi: 10.2169/internalmedicine.46.6314. [DOI] [PubMed] [Google Scholar]
- 31.Akatsu T, Aiura K, Shimazu M, et al. Successful pancreatectomy with en-bloc resection of the celiac artery and portal vein for pancreatic endocrine carcinoma. Hepatogastroenterology. 2007 June;54(76):1269–71. [PubMed] [Google Scholar]
- 32.Yamato H, Kawakami H, Kuwatani M, et al. Pancreatic carcinoma associated with portal vein tumor thrombus: three case reports. Intern Med. 2009;48(3):143–50. doi: 10.2169/internalmedicine.48.1049. [DOI] [PubMed] [Google Scholar]
- 33.Bedirli A, Patiroglu TE, Sakrak O, Aritas Y. Portal vein resection for a portal vein thrombus caused by nonfunctioning islet cell carcinoma: report of a case. Surg Today. 2004;34(9):802–4. doi: 10.1007/s00595-004-2806-5. [DOI] [PubMed] [Google Scholar]
- 34.Norton JA, Alexander HA, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Possible primary lymph node gastrinomas: occurrence, natural history and predictive factors: A prospective study. Ann Surg. 2003;237(5):650–9. doi: 10.1097/01.SLA.0000064375.51939.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norton JA, Fraker DL, Alexander HR, et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341(9):635–44. doi: 10.1056/NEJM199908263410902. [DOI] [PubMed] [Google Scholar]
- 36.Norton JA, Alexander HR, Fraker DL, Venzon DJ, Jensen RT. Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases or survival in patients with Zollinger-Ellison syndrome (ZES)? Ann Surg. 2004;239(5):617–26. doi: 10.1097/01.sla.0000124290.05524.5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norton JA, Alexander HR, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Ann Surg. 2001;234(4):495–506. doi: 10.1097/00000658-200110000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norton JA, Doherty GD, Fraker DL, et al. Surgical treatment of localized gastrinoma within the liver: A prospective study. Surgery. 1998;124(6):1145–52. doi: 10.1067/msy.1998.93110. [DOI] [PubMed] [Google Scholar]
- 39.Norton JA, Kirlen MA, Li M, Schreiber D, Chuter T, Jensen RT. Morbidity and mortality of aggressive resections in patients with advanced neuroendocrine tumors. Arch Surg. 2003;138:859–66. doi: 10.1001/archsurg.138.8.859. [DOI] [PubMed] [Google Scholar]
- 40.Fraker DL, Norton JA, Alexander HR, Venzon DJ, Jensen RT. Surgery in Zollinger-Ellison syndrome alters the natural history of gastrinoma. Ann Surg. 1994;220:320–30. doi: 10.1097/00000658-199409000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norton JA. Surgical treatment and prognosis of gastrinoma. Best Pract Res Clin Gastroenterol. 2005 October;19(5):799–805. doi: 10.1016/j.bpg.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Termanini B, Gibril F, Reynolds JC, et al. Value of somatostatin receptor scintigraphy: A prospective study in gastrinoma of its effect on clinical management. Gastroenterology. 1997;112:335–47. doi: 10.1053/gast.1997.v112.pm9024287. [DOI] [PubMed] [Google Scholar]
- 43.Alexander HR, Fraker DL, Norton JA, et al. Prospective study of somatostatin receptor scintigraphy and its effect on operative outcome in patients with Zollinger-Ellison syndrome. Ann Surg. 1998;228(2):228–38. doi: 10.1097/00000658-199808000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gibril F, Reynolds JC, Chen CC, et al. Specificity of somatostatin receptor scintigraphy: a prospective study and the effects of false positive localizations on management in patients with gastrinomas. J Nucl Med. 1999;40:539–53. [PubMed] [Google Scholar]
- 45.Krudy AG, Doppman JL, Jensen RT, et al. Localization of islet cell tumors by dynamic CT: Comparison with plain CT, arteriography, sonography and venous sampling. Am J Roentgenol. 1984;143:585–9. doi: 10.2214/ajr.143.3.585. [DOI] [PubMed] [Google Scholar]
- 46.Termanini B, Gibril F, Doppman JL, et al. Distinguishing small hepatic hemangiomas from vascular liver metastases in patients with gastrinoma: Use of a somatostatin-receptor scintigraphic agent. Radiology. 1997;202:151–8. doi: 10.1148/radiology.202.1.8988205. [DOI] [PubMed] [Google Scholar]
- 47.Sugg SL, Norton JA, Fraker DL, et al. A prospective study of intraoperative methods to diagnose and resect duodenal gastrinomas. Ann Surg. 1993;218:138–44. doi: 10.1097/00000658-199308000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacFarlane MP, Fraker DL, Alexander HR, Norton JA, Jensen RT. A prospective study of surgical resection of duodenal and pancreatic gastrinomas in multiple endocrine neoplasia-Type 1. Surgery. 1995;118:973–80. doi: 10.1016/s0039-6060(05)80102-3. [DOI] [PubMed] [Google Scholar]
- 49.Norton JA, Fraker DL, Alexander HR, et al. Surgery increases survival in patients with gastrinoma. Ann Surg. 2006;244(3):410–9. doi: 10.1097/01.sla.0000234802.44320.a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norton JA, Warren RS, Kelly MG, Zurek MB, Jensen RT. Aggressive surgery for metastatic liver neuroendocrine tumors. Surgery. 2003;134(6):1057–65. doi: 10.1016/j.surg.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 51.Noda S, Norton JA, Jensen RT, Gay WA., Jr Surgical resection of intracardiac gastrinoma. Ann Thorac Surg. 1999;67:532–3. doi: 10.1016/s0003-4975(98)01205-3. [DOI] [PubMed] [Google Scholar]
- 52.Fishbeyn VA, Norton JA, Benya RV, et al. Assessment and prediction of long-term cure in patients with Zollinger-Ellison syndrome: the best approach. Ann Intern Med. 1993;119:199–206. doi: 10.7326/0003-4819-119-3-199308010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roy P, Venzon DJ, Shojamanesh H, et al. Zollinger-Ellison syndrome: clinical presentation in 261 patients. Medicine (Baltimore) 2000;79(6):379–411. doi: 10.1097/00005792-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Roy P, Venzon DJ, Feigenbaum KM, et al. Gastric secretion in Zollinger-Ellison syndrome: correlation with clinical expression, tumor extent and role in diagnosis - A prospective NIH study of 235 patients and review of the literature in 984 cases. Medicine(Baltimore) 2001;80(3):189–222. doi: 10.1097/00005792-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Raufman JP, Collins SM, Pandol SJ, et al. Reliability of symptoms in assessing control of gastric acid secretion in patients with Zollinger-Ellison syndrome. Gastroenterology. 1983;84:108–13. [PubMed] [Google Scholar]
- 56.Jensen RT, Niederle B, Mitry E, et al. Gastrinoma (duodenal and pancreatic) Neuroendocrinology. 2006;84(3):173–82. doi: 10.1159/000098009. [DOI] [PubMed] [Google Scholar]
- 57.Termanini B, Gibril F, Sutliff VE, III, Yu F, Venzon DJ, Jensen RT. Effect of long-term gastric acid suppressive therapy on serum vitamin B12levels in patients with Zollinger-Ellison syndrome. Am J Med. 1998;104:422–30. doi: 10.1016/s0002-9343(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 58.Norton JA, Kahn CR, Schiebinger R, Gorschboth C, Brennan MF. Amino acid deficiency and the skin rash associated with glucagonoma. Ann Intern Med. 1979;91:213–5. doi: 10.7326/0003-4819-91-2-213. [DOI] [PubMed] [Google Scholar]
- 59.Gibril F, Schumann M, Pace A, Jensen RT. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. A prospective study of 107 cases and comparison with 1009 patients from the literature. Medicine (Baltimore) 2004;83(1):43–83. doi: 10.1097/01.md.0000112297.72510.32. [DOI] [PubMed] [Google Scholar]
- 60.Weber HC, Venzon DJ, Lin JT, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108:1637–49. doi: 10.1016/0016-5085(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 61.Benya RV, Metz DC, Venzon DJ, et al. Zollinger-Ellison syndrome can be the initial endocrine manifestation in patients with multiple endocrine neoplasia-type 1. Am J Med. 1994;97:436–44. doi: 10.1016/0002-9343(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 62.Norton JA, Doppman JL, Jensen RT. Curative resection in Zollinger-Ellison syndrome: Results of a 10-year prospective study. Ann Surg. 1992;215:8–18. doi: 10.1097/00000658-199201000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Norton JA. Intraoperative methods to stage and localize pancreatic and duodenal tumors. Ann Oncol. 1999;10:182–4. [PubMed] [Google Scholar]
- 64.Norton JA. Surgical treatment of neuroendocrine metastases. Best Pract Res Clin Gastroenterol. 2005 August;19(4):577–83. doi: 10.1016/j.bpg.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 65.Arnold WS, Fraker DL, Alexander HR, Weber HC, Jensen RT. Apparent lymph node primary gastrinoma. Surgery. 1994;116:1123–30. [PubMed] [Google Scholar]
- 66.Jaskowiak NT, Fraker DL, Alexander HR, Norton JA, Doppman JL, Jensen RT. Is reoperation for gastrinoma excision in Zollinger-Ellison syndrome (ZES) indicated? Surgery. 1996;120(6):1057–63. doi: 10.1016/s0039-6060(96)80055-9. [DOI] [PubMed] [Google Scholar]
- 67.von Schrenck T, Howard JM, Doppman JL, et al. Prospective study of chemotherapy in patients with metastatic gastrinoma. Gastroenterology. 1988;94:1326–34. doi: 10.1016/0016-5085(88)90670-1. [DOI] [PubMed] [Google Scholar]
- 68.Shojamanesh H, Gibril F, Louie A, et al. Prospective study of the anti-tumor efficacy of long-term octreotide treatment in patients with progressive metastatic gastrinomas. Cancer. 2002;94:331–43. doi: 10.1002/cncr.10195. [DOI] [PubMed] [Google Scholar]
- 69.Pisegna JR, Slimak GG, Doppman JL, et al. An evaluation of human recombinant alpha interferon in patients with metastatic gastrinoma. Gastroenterology. 1993;105:1179–83. doi: 10.1016/0016-5085(93)90965-f. [DOI] [PubMed] [Google Scholar]
- 70.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 71.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors:; Pancreatic endocrine tumors. Gastroenterology. 2008;135:1469–92. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu F, Venzon DJ, Serrano J, et al. Prospective study of the clinical course, prognostic factors and survival in patients with longstanding Zollinger-Ellison syndrome. J Clin Oncol. 1999;17:615–30. doi: 10.1200/JCO.1999.17.2.615. [DOI] [PubMed] [Google Scholar]
- 73.Strosberg JR, Kvols LK. A review of the current clinical trials for gastroenteropancreatic neuroendocrine tumours. Expert Opin Investig Drugs. 2007 February;16(2):219–24. doi: 10.1517/13543784.16.2.219. [DOI] [PubMed] [Google Scholar]
- 74.Eriksson J, Stalberg P, Nilsson A, et al. Surgery and radiofrequency ablation for treatment of liver metastases from midgut and foregut carcinoids and endocrine pancreatic tumors. World J Surg. 2008 May;32(5):930–8. doi: 10.1007/s00268-008-9510-3. [DOI] [PubMed] [Google Scholar]
- 75.Sarmiento JM, Que FG. Hepatic surgery for metastases from neuroendocrine tumors. Surg Oncol Clin North Am. 2003;12(1):231–42. doi: 10.1016/s1055-3207(02)00076-5. [DOI] [PubMed] [Google Scholar]
- 76.Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197(1):29–37. doi: 10.1016/S1072-7515(03)00230-8. [DOI] [PubMed] [Google Scholar]
- 77.Steinmuller T, Kianmanesh R, Falconi M, et al. Consensus guidelines for the management of patients with liver metastases from digestive (neuro)endocrine tumors: foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2008;87(1):47–62. doi: 10.1159/000111037. [DOI] [PubMed] [Google Scholar]
- 78.Gurusamy KS, Ramamoorthy R, Sharma D, Davidson BR. Liver resection versus other treatments for neuroendocrine tumours in patients with resectable liver metastases. Cochrane Database Syst Rev. 2009;(2):CD007060. doi: 10.1002/14651858.CD007060.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pachera S, Yokoyama Y, Nishio H, et al. A rare surgical case of multiple liver resections for recurrent liver metastases from pancreatic gastrinoma: liver and vena cava resection. J Hepatobiliary Pancreat Surg. 2009;16(5):692–8. doi: 10.1007/s00534-009-0055-0. [DOI] [PubMed] [Google Scholar]
- 80.Geehan DM, Kapcala LP, Saberinia M, Scovill WA. Intravascular tumor: a previously unreported finding of glucagonoma. South Med J. 1997 July;90(7):743–7. doi: 10.1097/00007611-199707000-00020. [DOI] [PubMed] [Google Scholar]
- 81.Gold RP, Black TJ, Rotterdam H, Casarella WJ. Radiologic and pathologic characteristics of the WDHA syndrome. AJR Am J Roentgenol. 1976 September;127(3):397–401. doi: 10.2214/ajr.127.3.397. [DOI] [PubMed] [Google Scholar]
- 82.Fortner JG, Kim DK, Cubilla A, Turnbull A, Pahnke LD, Shils ME. Regional pancreatectomy: en bloc pancreatic, portal vein and lymph node resection. Ann Surg. 1977 July;186(1):42–50. doi: 10.1097/00000658-197707000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Metz DC, Benjamin SB. Islet cell carcinoma of the pancreas presenting as bleeding from isolated gastric varices. Report of a case and review of the literature. Dig Dis Sci. 1991;36:241–4. doi: 10.1007/BF01300765. [DOI] [PubMed] [Google Scholar]
- 84.Ait-Ali A, Sall I, El-Kaoui H, et al. Medial pancreatectomy for a neuroendocrine tumor invading the splenic artery and vein. JOP. 2010;11(1):75–7. [PubMed] [Google Scholar]
- 85.Okuno M, Sakaguchi S, Nagayama M, et al. Nonfunctioning islet cell carcinoma presenting bleeding gastric varices and splenomegaly. Jpn J Surg. 1984 May;14(3):244–7. doi: 10.1007/BF02469576. [DOI] [PubMed] [Google Scholar]
- 86.Yamaguchi T, Takahashi H, Kagawa R, et al. Nonfunctioning pancreatic endocrine tumor presenting with hemorrhage from isolated gastric varices. Am Surg. 2005 December;71(12):1027–30. doi: 10.1177/000313480507101208. [DOI] [PubMed] [Google Scholar]
- 87.Chellappa M, Yee CK, Gill DS. Left-sided portal hypertension from malignant islet cell tumour of the pancrease: review with a case report. J R Coll Surg Edinb. 1986 August;31(4):251–2. [PubMed] [Google Scholar]
- 88.Bettini R, Boninsegna L, Mantovani W, et al. Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumours. Ann Oncol. 2008 May;19(5):903–8. doi: 10.1093/annonc/mdm552. [DOI] [PubMed] [Google Scholar]
- 89.Thompson GB, Van Heerden JA, Grant CS, Carney JA, Ilstrup DM. Islet cell carcinomas of the pancreas: a twenty-year experience. Surgery. 1988;104:1011–7. [PubMed] [Google Scholar]
- 90.Fendrich V, Waldmann J, Bartsch DK, Langer P. Surgical management of pancreatic endocrine tumors. Nat Rev Clin Oncol. 2009 July;6(7):419–28. doi: 10.1038/nrclinonc.2009.82. [DOI] [PubMed] [Google Scholar]
- 91.Moertel CG. Karnofsky memorial lecture. An odyssey in the land of small tumors. J Clin Oncol. 1987;5:1502–22. doi: 10.1200/JCO.1987.5.10.1502. [DOI] [PubMed] [Google Scholar]
- 92.Fendrich V, Langer P, Celik I, et al. An Aggressive Surgical Approach Leads to Long-term Survival in Patients With Pancreatic Endocrine Tumors. Ann Surg. 2006 December;244(6):845–53. doi: 10.1097/01.sla.0000246951.21252.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kazanjian KK, Reber HA, Hines OJ. Resection of pancreatic neuroendocrine tumors: results of 70 cases. Arch Surg. 2006 August;141(8):765–9. doi: 10.1001/archsurg.141.8.765. [DOI] [PubMed] [Google Scholar]
- 94.Wolf JH, Long RJ, Miller FJ, Jr, Jeffries GH. Pancreatic islet cell tumor presenting as bleeding gastric varices secondary to splenic vein occlusion. Am J Dig Dis. 1977 July;22(7):652–5. doi: 10.1007/BF01073088. [DOI] [PubMed] [Google Scholar]
- 95.Maclean N, Falconer CW, Gilmour IE, Webb FN. Islet cell tumours of the pancreas with portal varices and gastrointestinal haemorrhage. J R Coll Surg Edinb. 1970 July;15(4):206–12. [PubMed] [Google Scholar]
- 96.Watase M, Sakon M, Monden M, et al. A case of splenic vein occlusion caused by the intravenous tumor thrombus of nonfunctioning islet cell carcinoma. Surg Today. 1992;22(1):62–5. doi: 10.1007/BF00326127. [DOI] [PubMed] [Google Scholar]
- 97.Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 2008 January;9(1):61–72. doi: 10.1016/S1470-2045(07)70410-2. [DOI] [PubMed] [Google Scholar]
- 98.Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010 January;11(1):38–47. doi: 10.1016/S1470-2045(09)70330-4. [DOI] [PubMed] [Google Scholar]
- 99.Thomas AA, Rini BI, Stephenson AJ, et al. Surgical resection of renal cell carcinoma after targeted therapy. J Urol. 2009 September;182(3):881–6. doi: 10.1016/j.juro.2009.05.014. [DOI] [PubMed] [Google Scholar]


