Abstract
The active components of a primary pyrogenic liver abscess (PLA) Klebsiella pneumoniae in stimulating cytokine expression in macrophages are still unclear. The capsular polysaccharide (CPS) of PLA K. pneumoniae is important in determining clinical manifestations, and we have shown that it consists of repeating units of the trisaccharide (→3)-β-d-Glc-(1→4)-[2,3-(S)-pyruvate]-β-d-GlcA-(1→4)-α-l-Fuc-(1→) and has the unusual feature of extensive pyruvation of glucuronic acid and acetylation of C2-OH or C3-OH of fucose. We demonstrated that PLA K. pneumoniae CPS induces secretion of tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) by macrophages through Toll-like receptor 4 (TLR4) and that this effect was lost when pyruvation and O-acetylation were chemically destroyed. Furthermore, expression of TNF-α and IL-6 in PLA K. pneumoniae CPS-stimulated macrophages was shown to be regulated by the TLR4/ROS/PKC-δ/NF-κB, TLR4/PI3-kinase/AKT/NF-κB, and TLR4/MAPK signaling pathways.
Keywords: Carbohydrate Structure, Inflammation, Innate Immunity, Pathogen-associated Molecular Pattern, Toll-like Receptors (TLR), Klebsiella pneumoniae, Capsular Polysaccharide
Introduction
Klebsiella pneumoniae is an opportunistic pathogen of the Enterobacteriaceae family and usually causes pneumonia or urinary tract infections. In the past 2 decades, a new type of invasive disease caused by Klebsiella pneumoniae has emerged. In Taiwan, Korea, North America, and Europe, the bacterium now causes primary pyrogenic liver abscess (PLA)3 in patients without biliary tract diseases or other intra-abdominal infections (1–4). Sepsis and bacteremia are common, although metastatic meningitis or septic endophthalmitis complicate the course in 10–12% of cases. Diabetes mellitus, which can be a predisposing factor, has been detected in about 50% of patients; the remainder, including hepatobiliary malignancy and newly diagnosed malignancy, has risk factors for non-K. pneumoniae liver abscess in nondiabetic patients (1, 4, 5–7).
Two major surface components that are essential for the virulence of K. pneumoniae are capsular polysaccharide (CPS; the K antigen) and lipopolysaccharide (LPS; the O antigen) (8, 9). CPS may be important for the establishment of pneumonia, because active immunization with purified CPS protects experimental rats against lethal pneumonia caused by K. pneumoniae (10, 11). To understand the molecular basis of bacterium-host interactions in liver abscess formation, the molecular architecture of the bacterial cell surface must be characterized, and how the bacterium modifies this architecture in response to its different environments must be understood, as variations in the microenvironment may lead to the need for the surface molecules to adapt to these conditions. The structure and the biological activities of purified CPS from the K. pneumoniae strain have been investigated (12–15); however, the detailed information in pathogenesis for PLA K. pneumoniae is still unclear.
Toll-like receptors (TLRs) are known to act as cell receptors for microbial components (16). Stimulation of TLR signaling increases the production of proinflammatory cytokines and the up-regulation of MHC and costimulatory molecules (17, 18), thus playing a role in the interface between innate and adaptive immunity (19). Importantly, the normal immune response that leads to abscess formation involves both types of immunity. After activation of TLRs, the cytoplasmic adaptor protein TIRAP (Toll/IL-1R domain-containing adaptor protein) and MyD88 (myeloid differentiation primary response gene 88) are recruited to the TLR complex, which results in the production of pro-inflammatory cytokines, including TNF-α and IL-6 (20, 21). It has been reported that TNF-α and IL-6 production, as well as neutrophil influxes in response to K. pneumoniae intratracheal inoculation are attenuated in TIRAP- and MyD88-deficient mice (22). In addition, compared with wild-type mice, impaired cytokine expression is seen in TLR4-deficient mice in response to K. pneumoniae infection (23).
Here, we determined the structure of CPS from a PLA K. pneumoniae, NTUH K-2044 isolated from a liver abscess patient and investigated the signaling pathways in murine macrophages incubated with PLA K. pneumoniae CPS.
EXPERIMENTAL PROCEDURES
Materials
Escherichia coli 0111:B4 LPS, Alexa-conjugated LPS (LPS-Alexa), Pam3Cys, anti-phospho-MAPK antibody, anti-phospho-AKT antibody, anti-actin antibody, NAC, LY294002, PD98059, SP600125, and SB203580 were purchased from Sigma. Anti-phospho-PKC-α antibody, anti-phospho-PKC-δ antibody, and anti-MAPK antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Kinase assay kits were purchased from Cell Signaling Technology (Beverly, MA). Human IL-8, mouse TNF-α, and mouse IL-6 enzyme-linked immunosorbent assay (ELISA) kits were purchased from R & D Systems (Minneapolis, MN). Rat anti-mouse TLR4/MD2 antibody (clone MTS510) and rat IgG2a isotype control antibody were obtained from eBioscience (San Diego).
Cell Cultures and Animals
Murine J774A.1 macrophages were obtained from ATCC (Manassas, VA). HeNC2 and GG2EE cells were kindly provided by Dr. Danuta Radzioch, McGill University, Montreal, Canada. RAW-BlueTM cells (RAW264.7 macrophages stably transfected with the NF-κB reporter gene), HEK293-mTLR4/MD2/CD14, and HEK293-null were purchased from InvivoGen (San Diego). TLR4-deficient mice (C57BL/10ScN) were obtained from The Jackson Laboratory. Peritoneal macrophages were isolated from wild-type (C57BL) and TLR4-deficient mice 3 days after intraperitoneal injection of thioglycollate, and the cells (5 × 105/ml) were cultured with various reagents for 24 h. Fresh spleen cells were harvested from wild-type and TLR4-deficient mice, treated with RBC lysis buffer to destroy red blood cells, and adjusted to 1 × 106 cells/ml. All cells were grown in RPMI 1640 medium supplemented with 10% heat-inactivated FCS and 2 mm l-glutamine (Invitrogen) at 37 °C under an atmosphere containing 5% CO2.
Purification and Identification of PLA K. pneumoniae CPS
To isolate crude bacterial CPS, the method of Zamze et al. (24) was followed and modified as needed. The PLA K. pneumoniae CPS was digested by ribonuclease and deoxyribonuclease I for 24 h at 37 °C and then with Pronase in 10 mm Tris-HCl, pH 7.4, containing 1 mm CaCl2 for 24 h. The sample was then dialyzed extensively against water using a 50-kDa cutoff membrane and lyophilized. The CPS was further purified on a TSK HW-65F column (1.6 diameter × 90 cm height) with elution with H2O and then on an anionic DEAE-chromatograph (1.2 diameter × 7 cm height) in fast protein liquid chromatography (FPLC) with elution at 0–2 m NaCl. High performance size-exclusion chromatography (BioBasic SEC-1000, 300 × 7.5 mm) was used for the final purification and determination of the molecular mass of the CPS. Fractions containing carbohydrates were concentrated by lyophilization and analyzed by GC-MS.
Sugar composition and linkages were determined by methanolysis and trimethylsilylation, followed by GC-MS analysis (25). Sugar linkages were also determined using Hakomori methylation analysis (26). The glucuronic acid linkage was determined using purified CPS converted and reduced to the corresponding neutral sugars with 1-cyclohexyl-3-(2-morpholinoethyl)-carbodiimide metho-p-toluenesulfonate and sodium borohydride (27).
The amount of pyruvylation in the CPS was determined by colorimetric analysis with dinitrophenylhydrazine under acidic conditions, with sodium pyruvate as the standard (28). The amount of O-acetylation was measured following the method of Hestrin (29). The position of pyruvation was determined as follows: the glucuronic acid derivative was reduced, then hydrolyzed with 5 mm H2SO4 at 80 °C for 30 min, and methylated following the Hakomori method for linkage analysis (30). The positions of O-acetylation were identified using the Prehm method (31).
To determine the α/β configuration of the glycosidic linkages, purified CPS was degraded into smaller fragments by incubation with 0.1 m TFA for 1 h at 90 °C (32), and then small fragments with similar molecular weights were purified and collected for structural analysis, including configuration, linkage, and sequences. The NMR spectra of small CPS fragments in D2O were recorded on a Bruker AVANCE 500 spectrometer (equipped with the CryoprobeTM) at 300 K using XINNMR 3.5 software (33). All two-dimensional NMR experiments were carried out with standard pulse sequences provided by the Bruker. One-dimensional TOCSY spectra were recorded with mixing times that allowed the assignments of proton resonances. NMR data were processed using XWINMR 3.1 and AURELIA 3.1 software packages (Karlsruhe, Germany). Free induction decay was multiplied by skewed phase-shifted sine bell window functions prior to further transformation. The cross-peaks taken along the ω2 axis were subjected to an inverse Fourier transformation zero-filled to 8092 data points and Fourier transformed to give a digital resolution of 0.8 Hz/point.
Construction of Acetyltransferase and Pyruvyltransferase Mutants of PLA K. pneumoniae
Deletion mutants were generated using a pKO3-kanamycin vector modified from a pKO3 vector that contains a temperature-sensitive origin of replication and markers for positive and negative selection for chromosomal integration and excision (34, 35). In brief, a kanamycin resistance gene (Km) from the pUC4K plasmid was enzymatically digested with AccI and ligated into an AccI site of the pKO3 plasmid, resulting in the pKO3-Km plasmid. The flanking regions of mutant genes were cloned into the pKO3-Km plasmid separately, and the resulting construct was then electroporated into wild-type strain NTUH K-2044, and the transformant was then cultured at 43 °C. Candidate colonies were cultured in 1 ml of LB broth, followed by serial dilution and plating on LB plates containing 5% sucrose and cultured at 30 °C. Colonies sensitive to kanamycin were screened and confirmed by PCR using appropriate combinations of primers. The putative acetyltransferase gene and its flanking regions were amplified by PCR using the forward primer 5′-GCACTCTTCACTTCGATTGC-3′ and the reverse primer 5′-TCGCAGAGTTTTATACCGGC-3′ and cloned into a pGEM-T easy plasmid. Inverse PCR using the forward primer 5′-GATTATCTTGTTTGCGTGAC-3′ and the reverse primer 5′-GTGGGGTACCTGCAAAAGTA-3′ was used to delete the acetyltransferase gene, and the acetyltransferase gene deletion fragment was then cloned into the pKO3-Km plasmid (36). The resultant plasmid was transformed into K. pneumoniae wild-type strain to generate the acetyltransferase deletion mutant as described previously (36). The forward primer 5′-GAAACGCAATCAGTGTAGCG-3′ and the reverse primer 5′-GCAATGGCCATTTGCGTTAG-3′ for cloning the pyruvyltransferase and its flanking regions and the forward primer 5′-ATTAAATTTCTCGTTCCCGTAAATAG-3′ and reverse primer 5′-ATTTTAATATCTCATAAAGGGATGAAAAAC-3′ for inverse PCR were adopted for construction of the pyruvyltransferase deletion mutant.
String Test and K1 Serotyping
The mucoviscosity of K. pneumoniae was determined by a string test as described previously (21). K1 serotyping was performed by the double immunodiffusion assay as described previously (35).
Serum Resistance Assay and Animal Inoculation
The serum resistances of K. pneumoniae strains were determined as described previously (21). Female 5-week-old BALB/cByl mice were inoculated intraperitoneally with K. pneumoniae as described previously (21).
Western Blotting
Whole cell lysates were separated by SDS-PAGE and electrotransferred to a PVDF membrane. The membranes were incubated at room temperature for 1 h in blocking solution (5% nonfat milk in phosphate-buffered saline (PBS) containing 0.1% Tween 20) and then at room temperature for 2 h with specific primary antibody. After three washes in PBS with 0.1% Tween 20, the membrane was incubated with an HRP-conjugated secondary antibody. The membrane was developed using an enhanced chemiluminescence Western blot detection system.
In Vitro MAPK Activity Assay
Activated ERK1/2, JNK1/2, or p38 was immunoprecipitated by immobilized phospho-ERK1/2, phospho-JNK1/2, or phospho-p38 antibodies, respectively, and the immune complexes were incubated with Elk-1 fusion protein (for ERK1/2), c-Jun fusion protein (for JNK1/2), or ATF-2 fusion protein (for p38) in kinase assay buffer in the presence of unlabeled ATP at 30 °C for 30 min. The phosphorylation level of the Elk-1, c-Jun, and ATF-2 fusion proteins was then analyzed by Western blotting.
LPS Binding Assay
To measure the binding of LPS-Alexa to the cell surface, 2% paraformaldehyde-fixed J774A.1 macrophages were incubated with PLA K. pneumoniae CPS (20 μg/ml), LPS (20 μg/ml), or Pam3Cys (20 μg/ml) for 30 min at 4 °C, then with LPS-Alexa (2 μg/ml) for 30 min at 4 °C. After washing, the cells were analyzed by flow cytometry on a FACSCalibur using CellQuest software from BD Biosciences.
Measurement of Intracellular ROS Production
Stimulation of intracellular ROS production by PLA K. pneumoniae CPS was measured by detecting the fluorescent intensity of carboxyl-2′,7′-dichlorofluorescein, the peroxide-oxidized product of carboxyl-2′,7′-dichlorofluorescein diacetate (Molecular Probes, Eugene, OR). In short, J774A.1 macrophages, HEK293-mTLR4/MD2/CD14 or HEK293-null, were preincubated with carboxyl-2′,7′-dichlorofluorescein diacetate (2 μm) for 30 min in the dark, followed by PLA K. pneumoniae CPS (3 μg/ml) or LPS (1 μg/ml) treatment for the indicated periods. The relative fluorescent intensity of the carboxyl-2′,7′-dichlorofluorescein was detected at an excitation wavelength of 485 nm and an emission wavelength of 530 nm on a Cytofluor 2300 fluorometer (Millipore, Bedford, MA).
NF-κB Reporter Assay
RAW-BlueTM cells (RAW264.7 macrophages stably expressed a secreted embryonic alkaline phosphatase gene inducible by NF-κB and resistant to the selectable marker Zeocin) were seeded in 60-mm dishes at a density of 4 × 105 cells/ml and grown overnight in a 5% CO2 incubator at 37 °C. After treatment as indicated, the medium was harvested, and 20-μl samples were mixed with QUANTI-BlueTM (InvivoGen) medium (200 μl) in 96-well plates at 37 °C for 15 min, and then the optical density at 655 nm was measured on an ELISA reader.
Molecular Modeling
The model of PLA K. pneumoniae CPS in complex with TLR4 was constructed by docking the PLA K. pneumoniae CPS (4-sugar fragment of PLA K. pneumoniae CPS) to the crystallographic structure of TLR4 (Protein Data Bank code 2Z64) (64). The three-dimensional structure of PLA K. pneumoniae CPS was built using the SYBYL 7.3 program (SYBYL 7.3; Tripos Associates, St. Louis). GOLD 3.2 was used to dock PLA K. pneumoniae CPS onto the protein with the flexible docking option turned on (37, 38). Kollmann-all atom charges were assigned to the protein atoms (39), and Gasteiger-Hückel charges were assigned to ligand atoms using the SYBYL 7.3 program (40–42). During the following docking procedure, initially 1000 independent genetic algorithm cycles of computation were carried out with ligand torsion angles varying between −180 and 180°. The search efficiency was set at 200% to ensure the most meticulous search for the docking conformational space. All other parameters were the default settings. The docking processes were distributed to a 40-processor Linux cluster with an Intel® Xeon® CPU 3.00 GHz CPU. Resultant PLA K. pneumoniae CPS-TLR4 complex structures were ranked using the CHEMSCORE scoring function to determine the top 1000 hits.
Statistical Analysis
All values are given as the mean ± S.D. Data were analyzed by one-way analysis of variance with a subsequent Scheffé test.
RESULTS
Characterization of PLA K. pneumoniae CPS
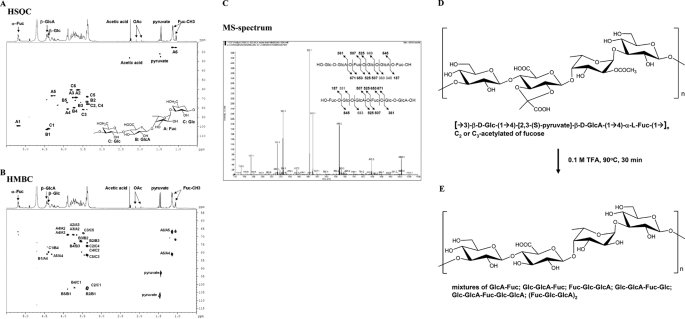
The CPS of the liver abscess strain K. pneumoniae NTUH-K2044 was purified and its sugar composition was determined. To rule out the possibility of LPS contamination during isolation, we constructed an LPS (O-antigen)-deficient mutant, named the wbbO mutant, which is unable to produce complete LPS.4 Using size-exclusion chromatography, the lower molecular weight incomplete LPS could be separated from the CPS. LPS amounts could be measured by 2-keto-3-deoxy-d-manno-octonic acid-thiobarbituric acid method (43), but not the Limulus assay, because it could cross-react with glucuronic acid in K. pneumoniae CPS (44). We also used an alternative sensitive method using gas chromatography and mass analysis (GC-MS) to detect galactose in the polysaccharide fraction, as K. pneumoniae LPS is rich in galactose (45). The CPS of the wild-type NTUH K-2044 and the wbbO mutant are identical, consisting of fucose, glucose, and glucuronic acid in a ratio of 1:1:1. Chemical analyses, mass fragmentation, and NMR assignment of partial acid-treated CPS fragments revealed the chemical structure of CPS to be a trisaccharide repeating unit (→3)-β-d-Glc-(1→4)-[2,3-(S)-pyruvate]-β-d-GlcA-(1→4)-α-l-Fuc-(1→) (Fig. 1, A–C, and Table 1). Thus, the structure of the CPS of PLA K. pneumoniae strain NTUH-K2044 is similar to that of K. pneumoniae strain K1 (24). In addition to pyruvation modification of both K1 strains and NTUH K-2044, the PLA K. pneumoniae CPS of strain NTUH-K2044 is highly acetylated (Fig. 1D). The glucuronic acid residue of the trisaccharide repeating unit was pyruvylated to 48–54%, which suggests that every other trisaccharide repeating unit is modified by pyruvylation. The position of O-acetylation was determined from the reduced CPS derivatives and further analyzed by GC-MS, showing that 6% of the fucosidic linkages were 1,4-linked, as estimated on the basis of molar equivalence; the amount of 1,2,4-fucosidic linkage was almost equal to that of 1,3,4-fucosidic linkage, although no 1,2,3,4-fucosidic linkage was found. These results show that either the C2-OH or the C3-OH of fucose, but not both, is acetylated and that the total level of acetylation is ∼94%, i.e. nearly stoichiometric.
FIGURE 1.
Structural determination of PLA K. pneumoniae CPS. NMR and MS spectra of the fragmented trisaccharide repeat unit after partial acid hydrolysis of purified PLA K. pneumoniae CPS are shown. HSQC (A) and HMBC (B) NMR spectra were used to assign the inter-residue linkages, sequences, and configurations. The MS/MS analysis is shown in C. D and E, chemical structure of PLA K. pneumoniae CPS (D) and after TFA treatment (E). Glc, glucose; GlcA, glucuronic acid; Fuc, fucose; OAc, O-acetyl.
TABLE 1.
HSQC-NMR spectrum data in D2O at 25 °C for CPS from K. pneumoniae NTUH-K2044
Data are given in ppm. 1H (400 MHz) and 13C (100 MHz) NMR data are for CPS in D2O.
| Fucose | GluA | Glc | ||||
|---|---|---|---|---|---|---|
| 1 | 5.18, d, J = 4.0 Hz | 98.9 | 4.44, d, J = 7.16 Hz | 102.8 | 4.40, d, J = 7.03 Hz | 102.1 |
| 2 | 3.68, m | 68.5 | 3.39, m | 73.3 | 3.38, m | 75.6 |
| 3 | 3.76, m | 68.7 | 3.55, t, J = 9.40 Hz | 73.8 | 3.48, m | 81.4 |
| 4 | 3.89, m | 80.9 | 3.72, m | 79.5 | 3.38, m | 75.6 |
| 5 | 4.30, m | 66.7 | 3.90, m | 74.0 | 3.38, m | 67.8 |
| 6 | 1.14, d, J = 5.7 Hz | 15.0 | 172.8 | 3.62, m | 60.4 | |
| Pyruvate | 1.46, s | 25.0 | 3.79, m | |||
| 92.5 | ||||||
| 175.3 | ||||||
| 1.47, s | 21.9 | |||||
| 106.7 | ||||||
| 175.6 | ||||||
| OAc | 2.27, s | 26.2 | ||||
| 169.1 |
Deletion of Acetyltransferase and Pyruvyltransferase
The two modifications seen in PLA K. pneumoniae NTUH K-2044 CPS were acetylation and pyruvation. The ORF11 and ORF8 encoding acetyltransferase and pyruvyltransferase located in the PLA K. pneumoniae NTUH K-2044 capsular polysaccharide synthesis region were deleted (35), and the mucoviscosity and serotype of the deletion mutants were analyzed using the string test and double immunodiffusion. Deletion of either ORF11 or ORF8 resulted in loss of mucoviscosity and a serotype K1-negative bacterium. Production of normal capsular polysaccharide was aborted, because no fucose was seen in the sugar composition analysis. In addition, LPS became the major population of the collected surface carbohydrates in both mutants. In particular, the ORF11 acetyltransferase and ORF8 pyruvyltransferase deletion mutants remained resistant to normal human serum, but virulence in mice was significantly decreased (LD50 >107 in intraperitoneal infection).
Pyruvation and Acetylation of PLA K. pneumoniae CPS Are Essential for Cytokine Induction
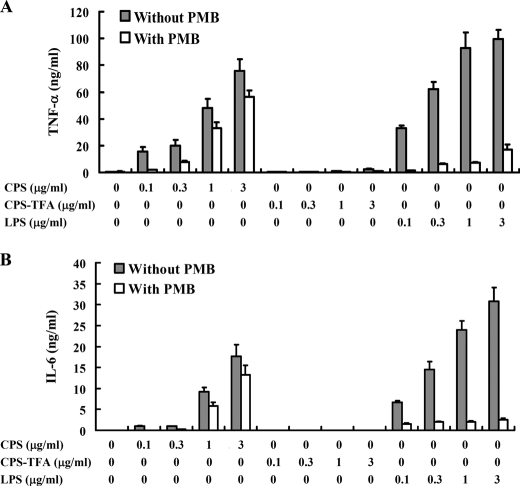
TNF-α and IL-6 are important pro-inflammatory cytokines produced mainly by activated macrophages and mediate multiple biological effects, including activation of immune responses. To test whether the purified CPS could stimulate TNF-α and IL-6 secretion in macrophages, we used ELISA to characterize the TNF-α and IL-6 secretion dose-response in PLA K. pneumoniae CPS-stimulated cells and found that secretion of TNF-α (Fig. 2A, left) and IL-6 (Fig. 2B, left) was induced in a dose-dependent manner. We tried to cleave the high molecular weight CPS into small oligosaccharide fragments using glucosidase and fucosidase, but we were not successful, possibly because of spatial modification of sugars, including acetylation and pyruvylation, which hinder the enzymatic reaction. To determine the effect of the structure and function of PLA K. pneumoniae CPS on cytokine induction activity, the O-acetyl and O-pyruvyl groups of PLA K. pneumoniae CPS were removed by trifluoroacetic acid treatment (Fig. 1E), and this resulted in a significant reduction in cytokine induction (Fig. 2, A and B, central region). LPS, a cell wall component of Gram-negative bacteria, is a potent stimulator of cytokine gene expression in macrophages (46). The effect of polymyxin B, an antibiotic used to neutralize the activity of LPS (47), was also examined and was found to significantly inhibited E. coli LPS-induced TNF-α and IL-6 secretion but not PLA K. pneumoniae CPS-stimulated secretion (Fig. 2, A and B, right).
FIGURE 2.
Pyruvylation and acetylation of PLA K. pneumoniae CPS are essential for its cytokine induction activity. J774A.1 cells (1 × 106/ml) were stimulated for 6 h with untreated PLA K. pneumoniae CPS (CPS), TFA-treated PLA K. pneumoniae CPS (CPS-TFA), or E. coli LPS in the presence or absence of polymyxin B (PMB) (10 μg/ml), and then TNF-α (A) or IL-6 (B) in the culture medium was measured by ELISA. The data are expressed as the mean ± S.D. for three separate experiments.
TLR4 Is the Major Receptor for PLA K. pneumoniae CPS
K. pneumoniae infection induces TLR4 up-regulation (15, 48), suggesting that TLR4 is involved in K. pneumoniae-mediated signaling. Bengoechea and co-workers (15) showed that CPS of pneumoniae K. pneumoniae 52145 (serotype O1:K2) up-regulates TLR2 and TLR4 expression in human airway epithelial A549 cells. However, the receptors responsible for the effect of purified CPS from liver abscess K. pneumoniae NTUH K-2044 (serotype O1:K1) are not known, and we therefore examined whether TLR4 participates in the host response to PLA K. pneumoniae CPS.
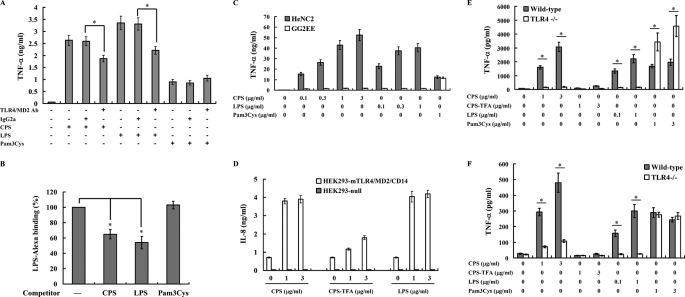
First, we examined a possible interaction between TLR4 and PLA K. pneumoniae CPS, leading to induction of TNF-α and IL-6 secretion. Macrophages were preincubated with an anti-TLR4/MD2 neutralizing antibody, known to specifically inhibit TLR4-mediated responses (49). As shown in Fig. 3A, the antibody reduced TNF-α secretion induced by PLA K. pneumoniae CPS or LPS but did not inhibit cytokine secretion induced by the TLR2 ligand Pam3Cys.
FIGURE 3.
TLR4 is the major receptor for PLA K. pneumoniae CPS. A, J774A.1 cells (1 × 105/ml) were incubated for 6 h with PLA K. pneumoniae CPS (3 μg/ml; left of panel), E. coli LPS (1 μg/ml; center of panel), or Pam3Cys (1 μg/ml; right of panel) in the presence or absence of an anti-TLR4/MD2 neutralizing antibody or control IgG2a (both 10 μg/ml), and then TNF-α in the culture medium was measured by ELISA. B, PLA K. pneumoniae CPS competes with fluorescent LPS (LPS-Alexa) for binding to the cell surface. Paraformaldehyde-fixed J774A.1 macrophages were preincubated for 30 min at 4 °C with PLA K. pneumoniae CPS, E. coli LPS, or Pam3Cys (all 20 μg/ml) and then incubated for 30 min at 4 °C with LPS-Alexa (2 μg/ml), and cell surface-bound LPS-Alexa was analyzed by flow cytometry. C, impaired cytokine production in PLA K. pneumoniae CPS-stimulated TLR4-deficient macrophages. HeNC2 and GG2EE cells (1 × 106/ml) were stimulated for 6 h with PLA K. pneumoniae CPS, E. coli LPS, or Pam3Cys, and then TNF-α in the culture medium was measured by ELISA. D, HEK293-mTLR4/MD2/CD14 and HEK293-null cells (1 × 106/ml) were stimulated for 24 h with PLA K. pneumoniae CPS (CPS), TFA-treated PLA K. pneumoniae CPS (CPS-TFA), or E. coli LPS, and then IL-8 in the culture medium was measured by ELISA. E, peritoneal macrophages (5 × 105/ml); F, splenocytes (1 × 106/ml) isolated from wild-type and TLR4-deficient mice were stimulated for 24 h with PLA K. pneumoniae CPS (CPS), TFA-treated PLA K. pneumoniae CPS (CPS-TFA), E. coli LPS, or Pam3Cys, and then TNF-α in the culture medium was determined by ELISA. In all figures, the data are expressed as the mean ± S.D. for three separate experiments. * indicates a significant difference at the level of p < 0.05.
Furthermore, we examined whether PLA K. pneumoniae CPS could compete with LPS in binding to CD14/TLR4 at the cell surface and found that PLA K. pneumoniae CPS, but not Pam3Cys, was able to compete with fluorescent Alexa-conjugated LPS in binding to the cell surface (Fig. 3B). Competition between nonconjugated LPS and fluorescent LPS was used as a positive control.
In addition, to further study the role of TLR4 in PLA K. pneumoniae CPS-mediated cytokine expression, two murine macrophage cell lines, HeNC2 (with a functional TLR4) and GG2EE (lacking a functional TLR4), were used to examine the role of TLR4 (50). As shown in Fig. 3C, PLA K. pneumoniae CPS induced TNF-α secretion in a dose-dependent fashion in HeNC2 cells, but not in GG2EE, whereas Pam3Cys stimulated both HeNC2 cells and GG2EE.
To confirm the role of TLR4 in PLA K. pneumoniae CPS-mediated cytokine expression, we compared the cytokine induction activity of PLA K. pneumoniae CPS in mouse TLR4-, MD2-, and CD14-transfected human embryonic kidney 293 cells (HEK293-mTLR4/MD2/CD14) and in vector-transfected control cells (HEK293-null), and we found that both PLA K. pneumoniae CPS and LPS induced IL-8 expression in HEK293-mTLR4/MD2/CD14 cells, but not in HEK293-null cells, and that TFA-treated PLA K. pneumoniae CPS had a reduced effect (Fig. 3D).
In addition, we used peritoneal macrophages from wild-type and TLR4-deficient mice (C57BL/10ScN) to further confirm the role of TLR4 in PLA K. pneumoniae CPS-mediated cytokine expression. Both the CPS and E. coli LPS induced TNF-α secretion in macrophages from wild-type mice but not in TLR4-deficient mice, whereas TFA-treated PLA K. pneumoniae CPS had little effect in either cell type (Fig. 3E). Pam3Cys-induced TNF-α production was not impaired in macrophages from TLR4-deficient mice (Fig. 3E). In addition, PLA K. pneumoniae CPS induced TNF-α secretion in splenocytes from wild-type mice, but not from TLR4-deficient mice (Fig. 3F). These results strongly indicated that TLR4 is one of the cellular receptors for PLA K. pneumoniae CPS and that pyruvylation and acetylation of PLA K. pneumoniae CPS are essential for the cytokine induction activity.
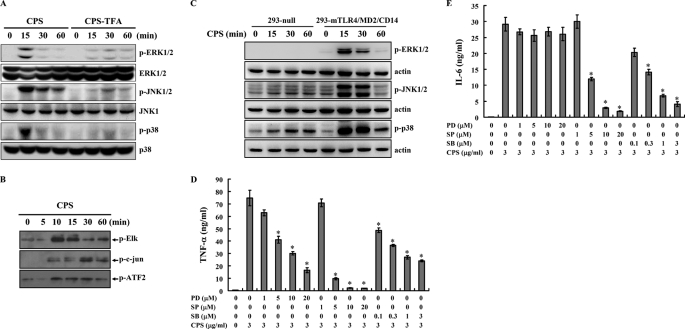
PLA K. pneumoniae CPS Induces Cytokine Expression through MAPK
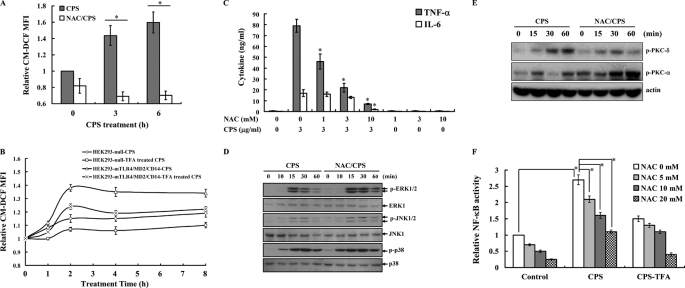
Activation of TLR4 through many signaling pathways, including the MAPK signaling pathways, leads to pro-inflammatory cytokine expression (46). To examine whether PLA K. pneumoniae CPS-induced cytokine expression was associated with MAPK signaling cascades, phosphorylation levels of the MAPK p38, JNK1/2, and ERK1/2 in PLA K. pneumoniae CPS-stimulated macrophages were measured by Western blot analysis, and the results showed that phosphorylation levels of ERK1/2, JNK1/2, and p38 were increased compared with control cells and that little effect was seen with TFA-treated PLA K. pneumoniae CPS (Fig. 4A). In addition, kinase activities of ERK1/2, JNK1/2, and p38 induced by PLA K. pneumoniae CPS were also demonstrated using an in vitro kinase assay (Fig. 4B). The role of TLR4 in PLA K. pneumoniae CPS-mediated MAPK activation was investigated using HEK293-mTLR4/MD2/CD14 and HEK293-null cells. Phosphorylation of ERK1/2, JNK1/2, and p38 was induced by PLA K. pneumoniae CPS in HEK293-mTLR4/MD2/CD14 cells, but little effect was seen in HEK293-null cells (Fig. 4C). These results show that PLA K. pneumoniae CPS activates the MAPK signaling cascades in macrophages mainly through TLR4.
FIGURE 4.
PLA K. pneumoniae CPS induces activation of MAPK. A, cells were incubated for the indicated time with PLA K. pneumoniae CPS (CPS) or TFA-treated PLA K. pneumoniae CPS (CPS-TFA) (both 3 μg/ml), and then phosphorylated and total MAPKs were analyzed by Western blotting. B, PLA K. pneumoniae CPS (3 μg/ml)-induced kinase activity of ERK1/2, JNK1/2, or p38 was measured by measuring phosphorylation of Elk, c-Jun, and ATF-2, respectively, in an in vitro kinase assay. C, HEK293-mTLR4/MD2/CD14 and HEK293-null cells were incubated with PLA K. pneumoniae CPS (3 μg/ml) for the indicated time, and then phosphorylated MAPK was measured by Western blotting. A–C, results presented are representative of those obtained in three different experiments. D and E, J774A.1 macrophages (1 × 106/ml) were incubated for 6 h with PLA K. pneumoniae CPS (3 μg/ml) in the presence or absence of PD98059 (PD), SP600125 (SP), or SB203580 (SB), and then TNF-α (D) or IL-6 (E) in the culture medium was measured by ELISA. The data are expressed as the mean ± S.D. for three separate experiments. * indicates a significant difference at the level of p < 0.05 compared with K. pneumoniae CPS alone.
To elucidate the role of MAPK in the regulation of TNF-α and IL-6 secretion in PLA K. pneumoniae CPS-stimulated macrophages, we used the specific pharmacological antagonists PD98059, SP600125, and SB203580, which, respectively, inhibit the activation of MEK1, JNK1/2, or p38. The effect of the specific protein-kinase inhibitors on MAPK kinase activity in macrophages was assayed. Working concentrations of the protein kinase inhibitors that did not affect the viability of the cells were determined (data not shown). We found that that the MEK1 inhibitor PD98059 inhibited PLA K. pneumoniae CP- induced TNF-α secretion in a dose-dependent manner (Fig. 4D), but it had no significant effect on PLA K. pneumoniae CPS-induced IL-6 secretion (Fig. 4E). In contrast, the JNK1/2 inhibitor SP600125 significantly inhibited PLA K. pneumoniae CPS-induced TNF-α (Fig. 4D) and IL-6 (Fig. 4E) secretion, as did the p38 inhibitor SB203580 (Fig. 4, D and E). Taken together, these results demonstrate that both the JNK1/2- and p38-dependent signaling pathways are involved in PLA K. pneumoniae CPS-induced TNF-α and IL-6 secretion in macrophages and that the ERK1/2-dependent signaling pathway is involved in PLA K. pneumoniae CPS-induced TNF-α secretion but not IL-6 secretion.
PLA K. pneumoniae CPS Induces Cytokine Expression through ROS
It has been reported that ROS are involved in signaling transduction and cytokine expression in activated macrophages (46, 47). To examine whether PLA K. pneumoniae CPS-mediated signaling and cytokine expression were regulated by ROS, the fluorescent oxidative product of carboxyl-2′,7′-dichlorofluorescein diacetate was measured, and PLA K. pneumoniae CPS was found to induce ROS production in macrophages. This effect was decreased by the antioxidant N-acetylcysteine (NAC) (Fig. 5A). The role of TLR4 in PLA K. pneumoniae CPS-mediated ROS production was investigated using HEK293-mTLR4/MD2/CD14 and HEK293-null cells. ROS production in response to PLA K. pneumoniae CPS stimulation was higher in HEK293-mTLR4/MD2/CD14 cells than in HEK293-null cells, although TFA-treated CPS had less effect in both cells (Fig. 5B). These results demonstrate that TLR4 is one of the receptors mediating ROS production in response to PLA K. pneumoniae CPS. Furthermore, we tested whether PLA K. pneumoniae CPS-induced ROS production regulates TNF-α and IL-6 secretion in macrophages using NAC and found that the antioxidant elicited a dose-dependent inhibition of TNF-α and IL-6 secretion (Fig. 5C), indicating that ROS is involved in TNF-α and IL-6 secretion in PLA K. pneumoniae CPS-stimulated macrophages. As it has been reported that ROS are involved in TLR4-mediated MAPK activation (46), we tested whether ROS were involved in PLA K. pneumoniae CPS-induced MAPK activation and found that PLA K. pneumoniae CPS-mediated phosphorylation of ERK1/2, JNK1/2, and p38 was not affected by NAC (Fig. 5D), suggesting that the PLA K. pneumoniae CPS-mediated activation of ERK1/2, JNK1/2, and p38 is independent of ROS. These results indicate that ROS plays a different role in MAPK activation in PLA K. pneumoniae CPS- and E. coli LPS-activated macrophages. To investigate which pathways involved in cytokine secretion are affected by ROS, we tested the effect of NAC on PKC and NF-κB, two important signaling pathways regulated TLR4 signaling (51, 52) in PLA K. pneumoniae CPS-activated macrophages and found that PLA K. pneumoniae CPS-induced phosphorylation of PKC-δ, but not PKC-α, was inhibited by NAC (Fig. 5E). NF-κB transcriptional activity induced by PLA K. pneumoniae CPS was also inhibited by NAC (Fig. 5F). As with its cytokine induction activity, PLA K. pneumoniae CPS lost its NF-κB transcriptional induction activity after TFA treatment (Fig. 5F). The effects of PLA K. pneumoniae CPS and TFA-treated CPS on NF-κB activity were confirmed by detection of IκB phosphorylation (data not shown).
FIGURE 5.
PLA K. pneumoniae CPS induces TNF-α and IL-6 secretion through ROS. A–C and F, data are expressed as the mean ± S.D. for three separate experiments, although in D and E, the results presented are representative of those obtained in three different experiments. A, J774A.1 macrophages were incubated with PLA K. pneumoniae CPS (3 μg/ml) in the presence or absence of NAC (10 mm) for the indicated time, and then ROS levels were measured by detection of the fluorescence intensity of the fluorophore carboxyl-2′,7′-dichlorofluorescein and expressed relative to those at time 0. * indicates a significant difference at the level of p < 0.05. B, HEK293-mTLR4/MD2/CD14 and HEK293-null cells were incubated for the indicated times with PLA K. pneumoniae CPS (CPS) or TFA-treated PLA K. pneumoniae CPS (CPS-TFA) (both 3 μg/ml), and then ROS levels were measured as in A. C, J774A.1 macrophages (1 × 106/ml) were incubated for 6 h with PLA K. pneumoniae CPS (3 μg/ml) in the presence or absence of NAC, and then TNF-α or IL-6 in the culture medium was measured by ELISA. * indicates a significant difference at the level of p < 0.05 compared with PLA K. pneumoniae CPS alone. D, J774A.1 macrophages were incubated for the indicated time with PLA K. pneumoniae CPS (3 μg/ml) in the presence or absence of NAC (10 mm), and phosphorylation of MAPK was measured by Western blotting. E, J774A.1 macrophages were incubated for the indicated time with PLA K. pneumoniae CPS (3 μg/ml) in the presence or absence of NAC (10 mm), and then phosphorylation of PKC-δ and PKC-α was measured by Western blotting. F, RAW-BlueTM cells were incubated for 24 h with PLA K. pneumoniae CPS or TFA-treated PLA K. pneumoniae CPS (CPS-TFA) (both 3 μg/ml) in the presence or absence of NAC (10 mm), and then secreted embryonic alkaline phosphatase activity was measured by QUANTI-BlueTM. * indicates a significant difference at the level of p < 0.05.
PLA K. pneumoniae CPS Induces Cytokine Expression through PI3K
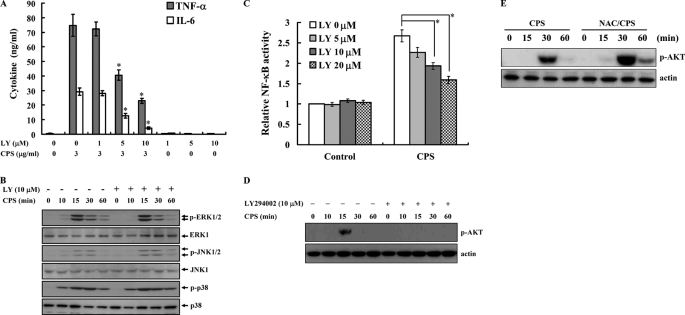
It has been reported that PI3K is an important regulator of TLR4-mediated signaling in macrophages (53). To explore its role in the regulation of TNF-α and IL-6 secretion in PLA K. pneumoniae CPS-stimulated macrophages, we tested the effect of LY294002, a specific inhibitor of PI3K, on TNF-α and IL-6 secretion and found that it inhibited TNF-α and IL-6 secretion in PLA K. pneumoniae CPS-stimulated macrophages in a dose-dependent manner (Fig. 6A). In addition, we tested its effect on PLA K. pneumoniae CPS-mediated signaling and found that it had no effect on PLA K. pneumoniae CPS-induced phosphorylation of ERK1/2, JNK1/2, and p38 (Fig. 6B), indicating that PI3K does not act upstream of ERK1/2, JNK1/2, and p38 in PLA K. pneumoniae CPS-stimulated macrophages. In contrast, LY294002 inhibited the increase in NF-κB transcriptional activity (Fig. 6C) and AKT phosphorylation (Fig. 6D) in PLA K. pneumoniae CPS-activated macrophages. Because our results showed that ROS regulated NF-κB transcriptional activity (Fig. 5F), we examined whether ROS acted upstream of AKT and found that the ROS scavenger NAC did not inhibit AKT phosphorylation in PLA K. pneumoniae CPS-activated macrophages but in fact increased it (Fig. 6E), showing that PLA K. pneumoniae CPS-induced AKT activation is independent of ROS.
FIGURE 6.
PLA K. pneumoniae CPS induces TNF-α and IL-6 secretion through PI3K. A and C, data are expressed as the mean ± S.D. for three separate experiments, although in B, D, and E, the results presented are representative of those obtained in three different experiments. A, J774A.1 macrophages (1 × 106/ml) were incubated for 6 h with PLA K. pneumoniae CPS (3 μg/ml) in the presence or absence of LY294002 (LY), and then TNF-α or IL-6 in the culture medium was measured by ELISA. * indicates a significant difference at the level of p < 0.05 compared with PLA K. pneumoniae CPS alone. B, J774A.1 macrophages (1 × 106/ml) were incubated for the indicated time with PLA K. pneumoniae CPS (3 μg/ml) in the presence or absence of LY294002 (LY), and then phosphorylation of MAPK was measured by Western blotting. C, RAW-BlueTM cells were incubated for 24 h with PLA K. pneumoniae CPS (3 μg/ml) in the presence or absence of LY294002 (LY), and then SEAP activity was measured by QUANTI-BlueTM. * indicates a significant difference at the level of p < 0.05. D, J774A.1 macrophages were incubated for the indicated time with PLA K. pneumoniae CPS (3 μg/ml) in the presence or absence of LY294002 (LY), and phosphorylation of AKT was measured by Western blotting. E, J774A.1 macrophages were incubated for the indicated time with PLA K. pneumoniae CPS (3 μg/ml) in the presence or absence of NAC, and then phosphorylation of AKT was measured by Western blotting.
DISCUSSION
The incidence of pyrogenic liver abscess and even splenic abscess caused by K. pneumoniae is higher than that caused by other bacteria, and infection is more common in diabetic than nondiabetic patients in Taiwan (54, 55). How Klebsiella survives and replicates within the host while resisting the innate immune system, nitrous oxide oxidation, and antimicrobial factors and how it expresses virulence genes at the appropriate time during systemic infection are poorly understood (56, 57). Using microarrays of transcription profiling, it has been shown that more than 4% of the entire Salmonella genome is required to infect the mouse (58). The same technique and genome-wide scanning would be further applied for understanding the pathogenesis of liver abscess K. pneumoniae. Recently, we used phosphoproteomics to study the metabolic regulation of capsular polysaccharide biosynthesis in K. pneumoniae NTUH K-2044 and found that 81 proteins were related to the regulation of capsular biosynthesis (59).
For surface polysaccharides of bacteria, LPS and CPS play important roles in the infection and survival of pathogenic bacteria within host animals and are therefore important virulence factors. Previous studies have shown that the mucoviscosity of CPS from a liver abscess strain of K. pneumoniae is important for bacterial virulence (21, 60). Here, we determined the complete structure and immunological responses of PLA K. pneumoniae NTUH K-2044 CPS, which is similar to that of CPS from a K1 serotype strain (24). Clinical data show that the K1 serotype is predominant (60–70%) among K. pneumoniae isolates causing liver abscesses in Korea and Taiwan (5, 35). The K1 capsular polysaccharide per se is considered one of the essential virulence determinants for the development of invasive liver abscess (35, 61). However, unlike other CPS structures, in this present study we found that either the C2 or C3 position of fucose in PLA K. pneumoniae CPS, but not both, was modified with an O-acetyl group. We speculate that the O-acetyl group could provide an immunogenic epitope of PLA K. pneumoniae CPS in patients, either by causing steric hindrance or a conformational change, thus allowing the pathogen to escape immune surveillance or resulting in less functionally active antibodies. This may be similar to the case of meningococcal CPS, in which the O-acetyl group provides an epitope of misdirected immunogenicity for meningococcal polysaccharide capsules, enabling escape from immune surveillance (62).
O-Acetylation and O-pyruvylation of PLA K. pneumoniae CPS biosynthesis are important steps, and because of the deletion of putative acetyltransferase and pyruvyltransferase, the complete CPS could not be synthesized. O-Acetylation and O-pyruvation are also important for immunological activities, because PLA K. pneumoniae CPS lacking these groups was not active (Fig. 2, A and B). These groups are epitopes for immune responses and have the potential to influence the host-pathogen interaction at several levels, although the detailed mechanism needs to be further investigated. A previous study showed that liver abscess K. pneumoniae could be killed by an anti-CPS monoclonal antibody (clone 10F8G4), and this antibody could protect mice from magA+ K. pneumoniae-induced death (63).
We believe that the correct length of oligosaccharide on CPS and both modifications are important for immunological responses and recognition by cellular receptors. Fucosidase and glucosidase were used to cleave the polysaccharides into oligosaccharides; however, it was not successful. Computer modeling helps us to explain the interaction between CPS moiety and TLR4. How does TLR4 activation bring a carbohydrate moiety to the macrophage surface? What is the molecular arrangement holding the CPS at the surface and presenting it to induce an immune response? Sites in mouse TLR4 interacting with a short fragment (Glc-Fuc-GlcA-Glc) of PLA K. pneumoniae CPS were suggested by computer modeling (see the supplemental material) (64, 65). Hydrogen bond interactions suggested by modeling include those between the guanidinium group of Arg-90 in TLR4 and the carboxylate anion of the pyruvate acetal substituent, between the hydroxyl group of tyrosine Tyr-102 in TLR4 and the carbonyl group of the acetyl group of fucose (when C3-OH is acetylated), and between the main chain amide-NH group of serine Ser-120 and the CO group of proline (Pro-118) and glucose. The proposed hydrophobic interactions include those between Phe-121 and glucose, between Phe-121 and the CH3 of the pyruvate acetal substituent of glucuronic acid, and between leucine Leu-94 and the CH3 of the acetyl group of fucose. Arg-90 and lysines 89, 91, 122, 125, 128, and 132 are reported to be essential for LPS binding and NF-κB activation (66). Ionic interactions may play the major role in the binding of TLR4 to its ligand. Charged groups, including the acetyl group of fucose and the carboxylate anion of the pyruvate acetal substituent, of PLA K. pneumoniae CPS may interact with TLR4 and trigger the signal to transit, whereas the deacetylated and depyruvylated oligosaccharides seem to be deficient in the binding of TLR4.
ROS generated from oxidative bursts during phagocytosis are important in destroying pathogens in innate immunity responses (67). In this study, we found that PLA K. pneumoniae CPS-induced ROS production was dependent on the presence of TLR4 in transfection studies using HEK293 cells that do not normally express this receptor, although PLA K. pneumoniae CPS was also able to induce a small amount of ROS in non-TLR4-expressing HEK293 cells (Fig. 5B). These results suggest that TLR4 is the critical receptor but not the only receptor for PLA K. pneumoniae CPS. It has been reported that ROS regulate TLR4-mediated activation of NF-κB (68). We also demonstrated that PLA K. pneumoniae CPS activated NF-κB through ROS (Fig. 5F). The PKC-dependent signaling pathway is reported to regulate NF-κB activation in LPS-stimulated macrophages (69). In our study, PLA K. pneumoniae CPS-induced PKC-δ activation was also shown to be dependent on the presence of ROS (Fig. 5E). Taken together, these results suggest that PLA K. pneumoniae CPS induces TNF-α and IL-6 secretion, at least in part, through TLR4/ROS/PKC-δ/NF-κB dependent pathways.
An earlier study in HepG2 cells showed that PLA K. pneumoniae infections activate two MAPK pathways, ERK1/2 and p38, but not the JNK1/2 pathway and induce TLR2 and TLR4 expression (48). We demonstrated that PLA K. pneumoniae CPS activated ERK1/2, JNK1/2, and p38 through TLR4 (Fig. 4C) and that this phenomenon was independent of ROS (Fig. 5D). These results are different from those in LPS-activated macrophages, in which activation of ERK1/2, JNK1/2, and p38 was dependent on ROS (46, 68).
The PI3K-dependent pathway provides negative feedback inhibition in NF-κB-dependent gene transcription in response to LPS stimulation of macrophages (70, 71). In contrast, in our study, inhibition of PI3K by LY294002 reduced TNF-α and IL-6 secretion (Fig. 6A) and NF-κB activation (Fig. 6C) in PLA K. pneumoniae CPS-stimulated macrophages, indicating that PI3K positively regulates PLA K. pneumoniae CPS-mediated cytokine expression through NF-κB. Inhibition of PI3K by LY294002 did not affect the phosphorylation of ERK1/2, JNK1/2, and p38 (Fig. 6B), indicating that PLA K. pneumoniae CPS-induced activation of ERK1/2, JNK1/2, and p38 are independent of PI3K. In contrast, inhibition of PI3K by LY294002 reduced the activation of JNK1/2 and p38 in LPS-activated macrophages (46). These results indicated that although both PLA K. pneumoniae CPS and LPS use TLR4 as their receptor to activate macrophages, the detailed signaling pathways are different.
Our results provide structural information for PLA K. pneumoniae CPS, which activates macrophages to produce cytokines. The pyruvation and O-acetylation of PLA K. pneumoniae CPS are essential for its cytokine induction activity. We also demonstrate that PLA K. pneumoniae CPS induced-cytokine production occurs through the TLR4/ROS/PKC-δ/NF-κB, TLR4/PI3K/AKT/NF-κB, and TLR4/MAPK signaling pathways.
Supplementary Material
This work was supported by the National Science Council of Taiwan Grants NSC 92-2321-B-001-019, 94-2311-B-001-045, 98-2320-B-197-003-MY2, 098-2811-B-197-001, and NRPGM 96IDP003-2.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
P.-F. Hsieh, T.-L. Lin, M.-C. Wu, Y.-J. Pan, F.-L. Yang, S.-H. Wu, and J.-T. Wang, unpublished data.
- PLA
- pyrogenic liver abscess
- CPS
- capsular polysaccharide
- ROS
- reactive oxygen species
- TLR
- Toll-like receptor
- NAC
- N-acetylcysteine.
REFERENCES
- 1. Ko W. C., Paterson D. L., Sagnimeni A. J., Hansen D. S., Von Gottberg A., Mohapatra S., Casellas J. M., Goossens H., Mulazimoglu L., Trenholme G., Klugman K. P., McCormack J. G., Yu V. L. (2002) Emerg. Infect. Dis. 8, 160–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang C. C., Yen C. H., Ho M. W., Wang J. H. (2004) J. Microbiol. Immunol. Infect. 37, 176–184 [PubMed] [Google Scholar]
- 3. Lederman E. R., Crum N. F. (2005) Am. J. Gastroenterol. 100, 322–331 [DOI] [PubMed] [Google Scholar]
- 4. Chung D. R., Lee S. S., Lee H. R., Kim H. B., Choi H. J., Eom J. S., Kim J. S., Choi Y. H., Lee J. S., Chung M. H., Kim Y. S., Lee H., Lee M. S., Park C. K. (2007) J. Infect. 54, 578–583 [DOI] [PubMed] [Google Scholar]
- 5. Fung C. P., Chang F. Y., Lee S. C., Hu B. S., Kuo B. I., Liu C. Y., Ho M., Siu L. K. (2002) Gut 50, 420–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomsen R. W., Jepsen P., Sørensen H. T. (2007) Clin. Infect. Dis. 44, 1194–1201 [DOI] [PubMed] [Google Scholar]
- 7. Chuang H. C., Chen T. L., Chiang D. H., Lee Y. T., Huang L. J., Wang F. D., Fung C. P., Liu C. Y. (2009) J. Microbiol. Immunol. Infect. 42, 385–392 [PubMed] [Google Scholar]
- 8. Williams P., Tomás J. M. (1990) Rev. Med. Microbiol. 1, 196–204 [Google Scholar]
- 9. Tomás J. M., Camprubi S., Merino S., Davey M. R., Williams P. (1991) Infect. Immun. 59, 2006–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cryz S. J., Jr., Fürer E., Germanier R. (1986) Infect. Immun. 54, 403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fang C. T., Chuang Y. P., Shun C. T., Chang S. C., Wang J. T. (2004) J. Exp. Med. 199, 697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choy Y. M., Tsang S. F., Kong S. K., Leung K. N., Parolis H., Lee C. Y., Fung K. P. (1996) Life Sci. 58, PL153–PL158 [DOI] [PubMed] [Google Scholar]
- 13. Ho C. Y., Lo T. W., Leung K. N., Fung K. P., Choy Y. M. (2000) Immunopharmacology 46, 1–13 [DOI] [PubMed] [Google Scholar]
- 14. Wu J. H., Wu A. M., Tsai C. G., Chang X. Y., Tsai S. F., Wu T. S. (2008) Exp. Biol. Med. 233, 64–70 [DOI] [PubMed] [Google Scholar]
- 15. Regueiro V., Moranta D., Campos M. A., Margareto J., Garmendia J., Bengoechea J. A. (2009) Infect. Immun. 77, 714–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee M. S., Kim Y. J. (2007) Annu. Rev. Biochem. 76, 447–480 [DOI] [PubMed] [Google Scholar]
- 17. Werling D., Jungi T. W. (2003) Vet. Immunol. Immunopathol. 91, 1–12 [DOI] [PubMed] [Google Scholar]
- 18. Takeda K., Akira S. (2004) Semin. Immunol. 16, 3–9 [DOI] [PubMed] [Google Scholar]
- 19. Pasare C., Medzhitov R. (2005) Adv. Exp. Med. Biol. 560, 11–18 [DOI] [PubMed] [Google Scholar]
- 20. Kawai T., Adachi O., Ogawa T., Takeda K., Akira S. (1999) Immunity 11, 115–122 [DOI] [PubMed] [Google Scholar]
- 21. Yamamoto M., Sato S., Hemmi H., Sanjo H., Uematsu S., Kaisho T., Hoshino K., Takeuchi O., Kobayashi M., Fujita T., Takeda K., Akira S. (2002) Nature 420, 324–329 [DOI] [PubMed] [Google Scholar]
- 22. Jeyaseelan S., Young S. K., Yamamoto M., Arndt P. G., Akira S., Kolls J. K., Worthen G. S. (2006) J. Immunol. 177, 538–547 [DOI] [PubMed] [Google Scholar]
- 23. Happel K. I., Zheng M., Young E., Quinton L. J., Lockhart E., Ramsay A. J., Shellito J. E., Schurr J. R., Bagby G. J., Nelson S., Kolls J. K. (2003) J. Immunol. 170, 4432–4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zamze S., Martinez-Pomares L., Jones H., Taylor P. R., Stillion R. J., Gordon S., Wong S. Y. (2002) J. Biol. Chem. 277, 41613–41623 [DOI] [PubMed] [Google Scholar]
- 25. Yang F. L., Lu C. P., Chen C. S., Chen M. Y., Hsiao H. L., Su Y., Tsay S. S., Zou W., Wu S. H. (2004) Eur. J. Biochem. 271, 4545–4551 [DOI] [PubMed] [Google Scholar]
- 26. Waeghe T. J., Darvill A. G., McNeil M., Albersheim P. (1983) Carbohydr. Res. 123, 281–304 [Google Scholar]
- 27. Ray A. K., Roy A., Roy N. (1987) Carbohydr. Res. 165, 77–86 [DOI] [PubMed] [Google Scholar]
- 28. Anthon G. E., Barrett D. M. (2003) J. Sci. Food Agric. 83, 1210–1213 [Google Scholar]
- 29. Hestrin H. (1949) J. Biol. Chem. 180, 249–261 [PubMed] [Google Scholar]
- 30. Hakomori S. (1964) J. Biochem. 55, 205–208 [PubMed] [Google Scholar]
- 31. Prehm P. (1980) Carbohydr. Res. 78, 372–374 [Google Scholar]
- 32. Verhoef R., de Waard P., Schols H. A., Rättö M., Siika-aho M., Voragen A. G. J. (2002) Carbohydr. Res. 337, 1821–1831 [DOI] [PubMed] [Google Scholar]
- 33. Kelly A. E., Ou H. D., Withers R., Dötsch V. (2002) J. Am. Chem. Soc. 124, 12013–12019 [DOI] [PubMed] [Google Scholar]
- 34. Link A. J., Phillips D., Church G. M. (1997) J. Bacteriol. 179, 6228–6237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chuang Y. P., Fang C. T., Lai S. Y., Chang S. C., Wang J. T. (2006) J. Infect. Dis. 193, 645–654 [DOI] [PubMed] [Google Scholar]
- 36. Hsieh P. F., Lin T. L., Lee C. Z., Tsai S. F., Wang J. T. (2008) J. Infect. Dis. 197, 1717–1727 [DOI] [PubMed] [Google Scholar]
- 37. Jones G., Willett P., Glen R. C. (1995) J. Mol. Biol. 245, 43–53 [DOI] [PubMed] [Google Scholar]
- 38. Jones G., Willett P., Glen R. C., Leach A. R., Taylor R. (1997) J. Mol. Biol. 267, 727–748 [DOI] [PubMed] [Google Scholar]
- 39. Cornell W. D., Cieplak P., Bayly C. I., Gould I. R., Merz K. M., Jr., Ferguson D. M., Spellmeyer D. C., Fox T., Caldwell J. W., Kollman P. A. (1995) J. Am. Chem. Soc. 117, 5179–5197 [Google Scholar]
- 40. Gasteiger J., Marsili M. (1980) Tetrahedron 36, 3219–3228 [Google Scholar]
- 41. Marsili M., Gasteiger J. (1980) Croat. Chem. Acta 53, 601–614 [Google Scholar]
- 42. Purcell W. P., Singer J. A. (1967) J. Chem. Eng. Data 12, 235–246 [Google Scholar]
- 43. Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. (1978) Anal. Biochem. 85, 595–601 [DOI] [PubMed] [Google Scholar]
- 44. Nowak T. P., Barondes S. H. (1975) Biochim. Biophys. Acta 393, 115–123 [PubMed] [Google Scholar]
- 45. Whitfield C., Richards J. C., Perry M. B., Clarke B. R., MacLean L. L. (1991) J. Bacteriol. 173, 1420–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hsu H. Y., Wen M. H. (2002) J. Biol. Chem. 277, 22131–22139 [DOI] [PubMed] [Google Scholar]
- 47. Hsu H. Y., Hua K. F., Lin C. C., Lin C. H., Hsu J., Wong C. H. (2004) J. Immunol. 173, 5989–5999 [DOI] [PubMed] [Google Scholar]
- 48. Wu J. H., Hong L. C., Tsai Y. Y., Chen H. W., Chen W. X., Wu T. S. (2006) Cell. Microbiol. 8, 1467–1474 [DOI] [PubMed] [Google Scholar]
- 49. Cook C. H., Trgovcich J., Zimmerman P. D., Zhang Y., Sedmak D. D. (2006) J. Virol. 80, 9151–9158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Poltorak A., He X., Smirnova I., Liu M. Y., Van Huffel C., Du X., Birdwell D., Alejos E., Silva M., Galanos C., Freudenberg M., Ricciardi-Castagnoli P., Layton B., Beutler B. (1998) Science 282, 2085–2088 [DOI] [PubMed] [Google Scholar]
- 51. Spitaler M., Cantrell D. A. (2004) Nat. Immunol. 5, 785–790 [DOI] [PubMed] [Google Scholar]
- 52. Baeuerle P. A. (1998) Cell 95, 729–731 [DOI] [PubMed] [Google Scholar]
- 53. Kuo C. C., Lin W. T., Liang C. M., Liang S. M. (2006) J. Immunol. 176, 5943–5949 [DOI] [PubMed] [Google Scholar]
- 54. Cheng D. L., Liu Y. C., Yen M. Y., Liu C. Y., Wang R. S. (1991) Arch. Intern. Med. 151, 1557–1559 [PubMed] [Google Scholar]
- 55. Lee C. H., Hu T. H., Liu J. W. (2005) Scand. J. Infect. Dis. 37, 905–909 [DOI] [PubMed] [Google Scholar]
- 56. Mastroeni P., Vazquez-Torres A., Fang F. C., Xu Y., Khan S., Hormaeche C. E., Dougan G. (2000) J. Exp. Med. 192, 237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vazquez-Torres A., Jones-Carson J., Mastroeni P., Ischiropoulos H., Fang F. C. (2000) J. Exp. Med. 192, 227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bowe F., Lipps C. J., Tsolis R. M., Groisman E., Heffron F., Kusters J. G. (1998) Infect. Immun. 66, 3372–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lin M. H., Hsu T. L., Lin S. Y., Pan Y. J., Jan J. T., Wang J. T., Khoo K. H., Wu S. H. (2009) Mol. Cell. Proteomics 8, 2613–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cortés G., Borrell N., de Astorza B., Gómez C., Sauleda J., Albertí S. (2002) Infect. Immun. 70, 2583–2590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yeh K. M., Chang F. Y., Fung C. P., Lin J. C., Siu L. K. (2006) J. Infect. Dis. 194, 403–405 [DOI] [PubMed] [Google Scholar]
- 62. Fusco P. C., Farley E. K., Huang C. H., Moore S., Michon F. (2007) Clin. Vaccine Immunol. 14, 577–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu M. F., Yang C. Y., Lin T. L., Wang J. T., Yang F. L., Wu S. H., Hu B. S., Chou T. Y., Tsai M. D., Lin C. H., Hsieh S. L. (2009) Infect. Immun. 77, 615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim H. M., Park B. S., Kim J. I., Kim S. E., Lee J., Oh S. C., Enkhbayar P., Matsushima N., Lee H., Yoo O. J., Lee J. O. (2007) Cell 130, 906–917 [DOI] [PubMed] [Google Scholar]
- 65. Park B. S., Song D. H., Kim H. M., Choi B. S., Lee H., Lee J. O. (2009) Nature 458, 1191–1195 [DOI] [PubMed] [Google Scholar]
- 66. Gruber A., Mancek M., Wagner H., Kirschning C. J., Jerala R. (2004) J. Biol. Chem. 279, 28475–28482 [DOI] [PubMed] [Google Scholar]
- 67. Forman H. J., Torres M. (2001) Mol. Aspects Med. 22, 189–216 [DOI] [PubMed] [Google Scholar]
- 68. Asehnoune K., Strassheim D., Mitra S., Kim J. Y., Abraham E. (2004) J. Immunol. 172, 2522–2529 [DOI] [PubMed] [Google Scholar]
- 69. Zhou X., Yang W., Li J. (2006) J. Biol. Chem. 281, 31337–31347 [DOI] [PubMed] [Google Scholar]
- 70. Keck S., Freudenberg M., Huber M. J. (2010) J. Immunol. 184, 5809–5818 [DOI] [PubMed] [Google Scholar]
- 71. Chaurasia B., Mauer J., Koch L., Goldau J., Kock A. S., Brüning J. C. (2010) Mol. Cell. Biol. 30, 4354–4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.